Abstract
Natal dispersal, the one-way movement between birth site and first breeding site, is an important determinant of species gene-flow and invasion potential. While dispersing in unfamiliar habitat, individuals may adjust their movement based on possible costs and benefits of moving, termed context-dependent dispersal. The role of factors, such as population density or spatial organisation of habitats, is well studied in the departure, transfer and settlement phases of dispersal. However, the role of predators for context-dependent dispersal remains less studied, particularly for the transfer phase and settlement phase of natal dispersal. We studied natal dispersal of radio-collared Eurasian red squirrels, Sciurus vulgaris, in relation to nest site locations of their main predator, the goshawk, Accipiter gentilis, in Finland. The locations of nest sites of goshawk had no influence on movement made during the transfer phase or on the location of settlement sites of juvenile red squirrels. Limited data on squirrel response to indices of predator presence (call playback of goshawk and faecal odours of mammalian predators) appeared to support the conclusion that predators had limited role in explaining movements of dispersers. We suggest that predators do not modify context-dependent dispersal among red squirrels in our boreal study area. This finding may be due to the low density of squirrel predators in northern boreal forests but may not hold true for species in which dispersers frequently encounter predators. Our study supports the conclusion that the resource and habitat availability are more important factors than predator presence for context-dependent dispersal among red squirrels.
Significance statement
Context-dependent dispersal means that individuals rely on a set of external cues, such as local population density and habitat quality, to adjust their movement tactics. One potentially important but little studied factor for context-dependent dispersal is the presence of predators. We studied movements of radio-collared dispersing Eurasian red squirrels in relation to nest site locations of their main predator, the goshawk. Movements of dispersing red squirrels were not influenced by the presence of predators. Thus, it seems likely that food and habitat availability, which were previously observed to shape dispersal patterns of red squirrels, determine where and how far Eurasian red squirrels disperse in boreal forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal, the movement between birth place and first breeding site or between two successive breeding sites, is one of the most important processes in the life history of animals (Clobert et al. 2009). Dispersal patterns of the species are determined by the costs and benefits of movement through unfamiliar habitats (Bonte et al. 2012). To adjust their movement in unfamiliar habitat, individuals may rely on a set of external cues, termed context-dependent dispersal (Verhulst et al. 1997; Ims and Hjermann 2001; Matthysen 2005; Fronhofer et al. 2015). The roles of population density and factors related to spatial organisation of habitats and resources are well-studied for context-dependent dispersal, but some factors remain less studied (Ronce 2007; Le Galliard et al. 2012; Cote et al. 2017). One such potentially important factor is the presence of predators that may affect movement during the different phases of dispersal, i.e. departure, transfer and settlement (Bonte et al. 2012).
It is well known that the presence of predators may increase the likelihood of dispersal departures (Hakkarainen et al. 2001; McCauley and Rowe 2010; Cote et al. 2013; Bestion et al. 2014) and increase costs of crossing unfamiliar habitats during the transfer phase of dispersal (Hiddink et al. 2002; Bonte et al. 2012). For example, in Tengmalm’s owls (Aegolius funereus), the short-term experimental introduction of the main nest predator (the pine marten, Martes martes) near a nest-box induced more extensive dispersal in the following breeding season (Hakkarainen et al. 2001). Similarly among insects, predators are observed to increase dispersal departures (Bernstein 1984; McIntosh et al. 2002; McCauley and Rowe 2010). However, it is still poorly understood how predator presence modifies movement decisions during the transfer and settlement phases of dispersal (Le Galliard et al. 2012). The costs and benefits experienced during the transfer and settlement are the prime determinants of context-dependent dispersal.
Here, we studied the responses of dispersing juvenile Eurasian red squirrels (Sciurus vulgaris; hereafter called red squirrels) to predator presence in a boreal forest environment. We used data on 30 dispersing juveniles and analysed how their movements are related to locations of nests of the main predator, the goshawk (Accipiter gentilis), over a 1000 km2 study area. Our hypothesis is that red squirrels avoid proximity of goshawk nest sites both during the transfer phase of dispersal and in the sites used for settlement. We hypothesise that individuals can evaluate the predator presence and make decisions about the suitable settlement location. If the predator presence does not change movement patterns of dispersers, then predators have a limited role in the context-dependent dispersal of red squirrels. We predict that (a) red squirrels would be found further away from goshawk nests than random locations in the study area both during the transfer phase of dispersal and the settlement. In addition, settlement sites could be further away from goshawk nests than locations recorded during the transfer phase of dispersal. (b) If dispersers aim to move away from areas with predators, increased proximity to goshawk nests is positively related to dispersal distance and movement step lengths of dispersers. Alternatively, movement steps might shorten if dispersers near predator decrease their movement activity (see, e.g., Gendron and Staddon 1984). Finally, in a small experiment, we seek further evidence on response of red squirrel to predator presence by spraying mammalian predator odour or playing a playback of a goshawk call for sample of juvenile squirrels to see whether they move away from the proximity of these signals of predator presence.
Methods
Study species and study area
The red squirrel is an arboreal rodent widely distributed in temperate and boreal forest ecosystems in Eurasia (Lurz et al. 2005). Red squirrels feed on conifer seeds but can utilise varying food items. Adult home range size varies from 5 to 50 ha (Lurz et al. 2005). Females give birth to the first litter in spring and sometimes have a second litter in summer, and the average litter size is 4 ± 1 juveniles (Lurz et al. 2005; Selonen et al. 2016b). The main dispersal of red squirrels occurs when juveniles in first autumn after birth move away from their natal territory. Dispersal distances observed in rural areas are typically between 1 and 2 km, but can be up to 16 km (Wauters et al. 2010; Selonen and Hanski 2015). In urban areas dispersal distances may be shorter, on average only half a kilometre in a study conducted in city of Turku Finland (Hämäläinen et al. 2018).
The main study was carried out in Kauhava in western Finland (63°06′05″N, 23°03′5″E), during years 2012, 2013 and 2015. The Kauhava study area is a rural forested area ca. 1300 km2 in size. The landscape is dominated by coniferous forest (70%) that is fragmented due to forest management, but also consists of agricultural land (20%) and peatland bogs (Hakkarainen et al. 2003). For the experiment where we sprayed odour of mammalian faeces, we also used individuals from urban Turku (see Fey et al. 2016; Hämäläinen et al. 2018), in SW Finland (60°27′05″N, 22°16′00″E). The city of Turku has 180,000 inhabitants, and the urban landscape consists of several parks, 1–10 ha in size. Solitary trees grow outside of parks, between buildings.
The main predator of red squirrels in Kauhava is the diurnal goshawk (Selonen et al. 2010). Other predators include large nocturnal owls (the Ural owl Strix uralensis and the eagle owl Bubo bubo) and the pine marten (Selonen et al. 2010; VS and SH personal observation). The pine marten is not very common in the Kauhava region based on snow track records (Selonen et al. 2016a) but nevertheless is the main mammalian predator of red squirrels in boreal forest areas (Lurz et al. 2005) and in our study area (VS and SH personal observation). The red fox (Vulpes vulpes) rarely preys on red squirrels in Kauhava (Dell'Arte et al. 2007). Instead, in urban Turku, pine martens are absent and red foxes and cats (Felis catus) are the main mammalian predators of red squirrels, because red squirrels spend more time on the ground than they do in rural areas (Fey et al. 2016; see also Lurz et al. 2005).
Radio-tracking and dispersal data
Between late May and late June of each year of the study, we captured juvenile red squirrels at the age of approximately 6–8 weeks and fitted them with radio-collars (Biotrack or Wildlife Materials; 5 or 8 g in weight). Twenty-four tracked individuals were captured from nest boxes and 6 individuals were trapped from ground with baited traps (9 in 2012, 16 in 2013 and 5 in 2015; all individuals that we could capture; originating from 24 litters). Sixteen of the juveniles were females and 14 males, but the dispersal distance does not differ between males and females in red squirrels in our sample (SH et al. unpublished) or in other studies (Wauters et al. 2008; Fey et al. 2016), and we combined the data for sexes. We collared all captured individuals that were suitable sized (100–150 g). The aim was to capture full litters, but in the end, this was not possible, because when juveniles were suitable-sized for collaring, all juveniles from the litter were not always in the same nest (juveniles might also be in twig dreys). In addition, individuals were difficult to capture from nest boxes, and often, juveniles escaped from the nest box. However, sometimes, we lost the first out coming individual and sometimes the one that came last. In addition, due to problems in collar attachment, the collars of many juveniles fell off soon after collaring and could not be tracked. In the end, the sample of used individuals (n = 30) seemed to be quite random, without clear indication of favouring of bold/large or shy/small individuals, but we cannot rule out that some biases may have occurred (see, e.g., Biro and Dingemanse 2009; Biro 2013). Thus, it remains unclear how representative sample our juveniles were from the population of dispersers. Body mass at capture or number of external parasites (fleas) encountered from trapped individuals were not related to dispersal distance in our dataset (analyses not shown).
Captured red squirrels weighed on average (± standard deviation, SD) 133 ± 44 g and based on previous analysis, the juveniles of this age move only in proximity of site of birth (Wauters et al. 2010; Fey et al. 2016; Hämäläinen et al. 2018). After collaring, the juveniles were immediately released at the place of their capture. We followed the radio-tagged red squirrels (using a Biotrack flexible Yagi antenna and a Biotrack Sika receiver) until either the animal died or the battery transmitter failed (typically in December–January in next winter). We recorded individual locations about five times a week during the 2–4 summer months after capture; individuals still alive in autumn were checked every second week throughout autumn (for more information on radio-tracking and dispersal of red squirrels, see Selonen and Hanski 2015; Fey et al. 2016; Hämäläinen et al. 2018).
In Kauhava, we used data for natal site (location of capture), movements during the transfer phase of dispersal, and settlement sites from 30 juvenile red squirrels. All locations outside of a 300-m buffer from natal nest (which was previously selected as a cut-off distance to describe when a juvenile is outside of its mother’s territory: Fey et al. 2016; Hämäläinen et al. 2018) and not in the forest patch used for settlement were classified being transfer phase of dispersal (Fig. 1, on average (± SD) 16 ± 13 transfer phase locations per individual, multiple locations from the same coordinates omitted). The settlement occurred when the individual stayed in a forest patch (i.e. the movement activity clearly dropped) where it finally settled before the next winter. The last observation of the individual in autumn/winter was used as the location of the settlement site. Eight out of the 30 individuals died or disappeared during or soon after the transfer phase (4 died and 4 disappeared due to transmitter failure). Thus, it was uncertain if they had truly settled, because sometimes, a disperser may stop moving for several days before they continue dispersing. However, the goshawk index (see below) in the last recorded location did not differ between individuals with uncertain settlement (goshawk index 0.41 ± 0.42 (SD)) and those with certain settlement (goshawk index 0.38 ± 0.43, t28 = 0.1, p = 0.92), and all individuals were used in further analysis, except for analysis including dispersal distance.
The goshawk kernels
Long-term studies of birds of prey have been carried out in the Kauhava region (e.g. Korpimäki and Sulkava 1987; Korpimäki and Hakkarainen 2012; Morosinotto et al. 2017), so the reproductive success and locations of nests of birds of prey are well known in this study area. The density of goshawks was approximately 1 pair per 10 km2 in the study area (Korpimäki and Hakkarainen 2012; EK and M. Hänninen, unpublished data). Goshawk presence was modelled by calculating flat-top bivariate Gaussian kernels around nests of the hawks (Björklund et al. 2016). The flat-top part represents the area where the impact of the goshawks is strongest, beyond which it declines following the Gaussian distribution until a cut-off distance of 10 km. The height of the kernel at each red squirrel location was used as a proxy for predation pressure (hereafter referred to as goshawk presence index). The goshawk presence index was calculated for each red squirrel location and for 1000 random points laid in the landscape. Random points were created inside the convex polygon surrounding all tracking locations of all tracked red squirrels during the entire study period. Using a landscape map, random points were not equally created throughout the whole polygon, but only in natural red squirrel and goshawk habitats, i.e. in or in close proximity to (< 100 m buffer) old/mature coniferous forests. Kernels with an SD of 1, 2, 3 or 4 and flat-tops of 500, 1000, 1500, 2000 or 2500 m, as well as all possible combinations, were compared, and the one that explained the presence/absence of red squirrels most parsimoniously (as measured with model AIC) was used (SD of 1 and flat-top of 1000 m).
In addition to habitat and predation pressure, food availability (conifer seed mast) may affect settlement patterns in red squirrels (Wauters and Dhondt 1993). We did not have data on the availability of red squirrels’ main food, spruce cones, in the chosen forest sites. However, there was a clear difference in spruce cone production between 2012 and 2013 in the Kauhava region (Selonen et al. 2015). This was not reflected in dispersal distances observed in this study (2012 2860 ± 1200 m (average ± SD); 2013 2960 ± 3330 m, sex controlled; F1, 18 = 0.45, p = 0.51). Similarly, year had no effect on dispersal distance in our data (n: 9 in 2012, 16 in 2013 and 5 in 2015; F1, 28 = 0.1, p = 0.8) and to simplify models for settlement site and dispersal distance, we combined data for different years. However, year was included in the model for movement steps.
Mammalian predator odour and goshawk call playbacks
We sprayed odour of mammalian predators or played goshawk calls for 13 radio-tracked individuals to simulate the situation where a juvenile red squirrel is within a habitat intensively used by a predator. We used the odour of the main mammalian predator of red squirrels in each area, that is, the red fox in Turku and the pine marten in Kauhava (Dell'Arte et al. 2007; Kauhala et al. 2016). The odours were obtained from red fox faeces collected close to known nest sites and from pine marten faeces from Ähtäri Zoo, Central Finland. About 50–100 g of faeces was mixed with 1 l water, left for about 2 h and then filtered through cloth to remove solid material (Fülling and Halle 2004; Fey et al. 2010). With a spray bottle, the extract was distributed in equal doses at the field sites. The extract was sprayed in two consecutive evenings on the trunk of a nest tree in which a red squirrel had been localised to sleep and to trees and/or stumps and stones within 10 m from this nest tree. We treated nest trees of 10 individuals: six in Turku with fox odour and four in Kauhava with pine marten odour. With this setup, we attempted to simulate odours left behind by predators foraging in the site. As a control, water was sprayed in equal doses on the trunks of nest trees for eight red squirrels (three in Kauhava and five in Turku). For three individuals (not the same individuals as above), we played a recording of a goshawk territorial call in Kauhava study area. A playback device was set up 20–30 m from the nest used by the red squirrel. One playback session consisted of repetitious series of 2 min of predator calling followed by 10 min of silence, lasted 6 h, and was repeated the next day. As a control, we played the call of nightingale, Luscinia luscinia, for two individuals, using the same protocol as with the goshawk playbacks.
We compared movement distances between the location of the red squirrel at the start of the treatment and the location 1 day after the end of the treatment. We also analysed whether the individual changed its sleeping nest site or whether the treatment had affected the final dispersal distance at the end of dispersal period (i.e. late autumn, Fey et al. 2016; Hämäläinen et al. 2018). The average distances moved were similar between odour and playback treatments (distance moved next day: playback 86 ± 160 m (average ± SD), predator odour 90 ± 130 m; nest site change: playback 92 ± 170 m, predator odour 73 ± 140 m). Thus, due to small sample size, we combined the data for the analysis. The data gathering for this experiment was difficult, because we selected only individuals not in contact with each other (moving in separate areas and no same individual could be used for odour or playback treatment or as a control) from the short dispersal period that usually lasts only for 1–3 weeks. Consequently, the number of study individuals remained low and can only give suggestive evidence on red squirrel response to goshawk calls or mammalian scent.
Analysis
We analysed whether dispersing red squirrels were located non-randomly in relation to the goshawk presence index in the Kauhava study area. First, we built binomial models (Glimmix SAS 9.3.) for red squirrel locations (coded as 1) and random points (coded as 0). Locations of natal sites, settlement sites and transfer phase of dispersal (locations outside natal and settlement sites) were analysed in separate models. The goshawk presence index of the location was set as an explanatory variable. For analysis of settlement sites (coded as 0) versus transfer locations (coded as 1), random locations were omitted and goshawk presence index was set as an explanatory variable. In models including the transfer phase locations, year was included as class variable and individual identity was set as a random factor using generalised estimation equations (Glimmix SAS). All individuals in this study dispersed in different directions and settled in separate areas. Thus, dispersers were considered independent in relation to presence of goshawk nests in the landscape.
Next (prediction (b) in aims), we analysed whether a high goshawk presence index in a location increased or decreased the subsequent step length taken by a disperser. We built two log-normal models where step length between locations during the transfer phase was the dependent variable. Goshawk index and duration of step (in days, on average for all steps 3 ± 5 days) were explanatory variables, and individual identity was a random factor. In the first model, step length was based on all locations during the transfer phase (on average (± SD) 288 ± 806 m step, 16 ± 13 steps per individual). In the second model, only the steps where an individual changed forest patches during dispersal (on average 1360 ± 1810 m, 3 ± 2.5 steps per individual) were included. With this model, we took into account the possibility that response to predator presence index appeared only in a scale larger than movement steps made within a forest patch. For the first model (model including all steps), we also included the forest patch of locations as a random factor (using Kenward-Roger degrees of freedom, Glimmix, SAS). This was done because the data included small-scale moves within a forest patch. Finally, we built a lognormal model where dispersal distance (straight-line distance between natal site and settlement site) was explained by the average goshawk index calculated over all transfer phase locations. From this analysis, we deleted eight individuals whose settlement was uncertain (used n = 22 individuals).
For mammalian odour and goshawk call playback treatment (n = 23), we built lognormal models for distance moved 1 day after the treatment, distance between sleeping site observations before and after treatment and final dispersal distance of the individual. For the first two models, we included time since treatment as a covariate (on average (± SD) 21 ± 19 and 88 ± 99 h for distance moved and sleeping site change, respectively). For the model for dispersal distance, the study area was included as a covariate. Treatment (1 treatment, 2 control) was included as a class variable in the models.
It was not possible to record data blind because our study involved focal animals in the field.
Data availability
The dataset analysed during the current study is available from the corresponding author on reasonable request.
Results
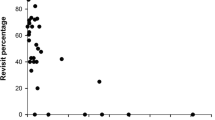
Goshawk presence index did not differ between random locations (average ± SD for goshawk presence index 0.32 ± 0.35) and natal sites (goshawk presence index 0.42 ± 0.44) or settlement sites (goshawk presence index 0.39 ± 0.42; Table 1) of dispersing red squirrels. The same was true for locations during the transfer phase of dispersal (goshawk presence index 0.41 ± 0.41; Table 1). The goshawk index did not either differ between the transfer phase locations and settlement sites (Table 1). The average goshawk presence index during the transfer phase was not related to the dispersal distance of red squirrels or step length moved from a previous location (Table 1; Fig. 2). The shortest distance between a red squirrel location and a goshawk nest was 250 m, and 13% of all red squirrel locations (86/533) were within a kilometre from a goshawk nest (considered as the area of greatest predation pressure).
Individuals treated (n = 13) with either the predator odour or the goshawk call playback did not move further the next day, did not change nest site and did not disperse further than control individuals (n = 10; Table 2).
Discussion
The locations of nest sites of the main avian predator, the goshawk, did not affect movements during the transfer phase of dispersal or the settlement patterns of juvenile red squirrels in our study area. In other words, we did not observe our predator presence index to affect context-dependent dispersal. Previous studies of context-dependent dispersal have concluded that factors such as local population density (De Meester and Bonte 2010), territory availability (Larsen and Boutin 1994) and food availability (Kuefler et al. 2012; Terraube et al. 2015) can affect movement patterns. Furthermore, public information on performance of local con- or heterospecifics can be used to indicate conditions in potential target patches for making settlement decisions (Doligez et al. 2002; Cote and Clobert 2007). For arboreal squirrels, previous studies indicate that habitat quality and food availability (Wauters and Dhondt 1993; Lurz et al. 1997; Selonen et al. 2007; Selonen and Hanski 2012), as well as territory availability (Wauters and Dhondt 1993; Larsen and Boutin 1994; Selonen and Wistbacka 2017), are important determinants of dispersal distances and settlement patterns (but see also Cooper et al. 2017; Merrick and Koprowski 2017). Because we did not observe predator presence index to explain movements during the transfer and settlement phases of dispersal, it seems likely that the previously found habitat, territory availability and food-related factors are the main determinants of dispersal in this species. Reacting to cues of predator presence might compromise locating good-quality habitats and time allocated to feeding and exploration, creating additional costs for dispersal.
Densities of the main predator of red squirrels remain low in boreal forests (one to 10 goshawk nests per 100 km2, Tornberg et al. 2006, present study), but dispersing red squirrels move very long distances, up to 16 km in our data (Selonen and Hanski 2015; Hämäläinen et al. 2018). Thus, dispersers will move in areas with varying predator presence (Fig. 1), but even in unfamiliar habitat, the likelihood that a dispersing squirrel ends up killed by a predator may remain relatively low (Hanski and Selonen 2009; Fey et al. 2016). Dispersers obviously do encounter predators occasionally, as is shown by the four observed mortality events (4 cases; 2 probably by a cat/pine marten and 2 by a goshawk) during our study period. In addition, we are not suggesting that an individual red squirrel would not try to flee when encountering a predator chasing prey. It is obvious that the response to risk of predation depends on the type of signal received (Hakkarainen and Korpimäki 1994; Carlson et al. 2017). However, for context-dependent dispersal, the individual may need to rely on indirect cues that predict predator presence in potential settlement sites. For this, we did not find support. It is possible that dispersers simply were unaware of the goshawk presence. It should be noted, however, that our point location data provided only a hint of the true exploration movements performed by the dispersers. In other words, red squirrels have necessarily been much closer to predator nests than we recorded.
In the small experiment with odour of mammalian faeces and goshawk playbacks, we combined indices of presence for three different predator species. The sample size for this experiment remained too low for final conclusions. For example, we could not analyse each predator species separately, and it remains possible that red squirrels would have reacted to only one of the used predator presence indexes and not to others. In particular, the red squirrel response to goshawk calls will require further study, but the three individuals used in the experiment did not seem to pay any attention to our goshawk playback. For mammalian predators, we are unaware whether the red squirrel can detect odour signals from faeces. The same technique that we used has been previously used to successfully deliver predator odours to voles (Fülling and Halle 2004; Fey et al. 2010) and birds (Forsman et al. 2013), but not to red squirrels. In any case, dispersers being unresponsive to signal they detected or being unable to read the signal produce the same outcome: predator odour from faeces did not adjust context-dependent dispersal of red squirrels. Our predator presence experiment is in line with the results from our main analysis with the goshawk nest locations. However, it is clear that the experiment provided only suggestive evidence on red squirrel response to mammalian scent signals and goshawk calls. Thus, this topic requires further study.
Predator presence has been observed to affect several behaviours of the prey individuals, such as habitat selection (Morosinotto et al. 2010; Forsman et al. 2013), foraging movements (Kotler et al. 1991; Laundré et al. 2001), stress reactions (Boonstra et al. 1998; Thomson et al. 2010) and reproductive success (Lima 2009; Morosinotto et al. 2017). For example, predator odour treatment affects home-range use and reproduction in voles (Fuelling and Halle 2004; Fey et al. 2010). Similarly, red squirrels might react to our predator presence index during stages of the life cycle other than the natal dispersal, but this remains unstudied. It is known that the location of goshawk nests has a negative effect on occupancy patterns of red squirrels (Turkia et al. 2018; see also Selonen et al. 2016a). Based on the current results, this negative association may result from direct predation or avoidance of predators during the breeding stage, and not during the natal dispersal period. Goshawks prefer to nest in old-growth spruce-dominated forests (Tornberg et al. 2006), and these sites are also where food availability (spruce cone crop) is highest for red squirrels. Thus, it remains possible that red squirrel settlement patterns are related to balancing the risk of starvation with the risk of predation. On the other hand, predators may use prey resource distributions to locate in high prey areas (predator-prey spatial games, e.g. Hammond et al. 2007). From previous studies, we know that variation in food availability largely determines population fluctuations and the reproductive behaviour of arboreal squirrels (Gurnell 1983; Petty et al. 2003; Descamps et al. 2008; Wauters et al. 2008; Selonen et al. 2015, 2016b; Hämäläinen et al. 2017).
Theoretical studies indicate that predation is an important force behind evolution of dispersal (Poethke et al. 2010; Bonte et al. 2012). However, the relative role of predation compared to other factors behind costs of dispersal, such as difficulty to locate resources and habitats (Wauters and Dhondt 1993; Lurz et al. 1997), determines which cues should be used to adjust context-dependent dispersal (Clobert et al. 2009; Bonte et al. 2012). Based on our results, predators are not behind context-dependent dispersal in red squirrels in the boreal forests. This pattern may be more general for the transfer phase and settlement of dispersal in animals in regions with low density of predators but is likely not the case in regions where dispersers frequently encounter predators.
References
Bernstein C (1984) Prey and predator emigration responses in the acarine system Tetranychus urticae Phytoseiulus persimilis. Oecologia 61:134–142
Bestion E, Teyssier A, Aubret F, Clobert J, Cote J (2014) Maternal exposure to predator scents: offspring phenotypic adjustment and dispersal. Proc R Soc B 281:20140701
Biro PA (2013) Are most samples of animals systematically biased? Consistent individual trait differences bias samples despite random sampling. Oecologia 171:339–345
Biro PA, Dingemanse NJ (2009) Sampling bias resulting from animal personality. Trends Ecol Evol 24:66–67
Björklund H, Santangeli AF, Blanchet G, Huitu O, Lehtoranta H, Lindén H, Valkama J, Laaksonen T (2016) Intraguild predation and competition impacts on a subordinate predator. Oecologia 181:257–269
Bonte D, van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, Lehouck V, Matthysen E, Mustin K, Saastamoinen M, Schtickzelle N, Stevens VM, Vandewoestijne S, Baguette M, Barton K, Benton TG, Chaput-Bardy A, Clobert J, Dytham C, Hovestadt T, Meier CM, Palmer SCF, Turlure C, Travis JMJ (2012) Costs of dispersal. Biol Rev 87:290–312
Boonstra R, Hik D, Singleton GR, Tinnikov A (1998) The impact of predator-induced stress on the snowshoe hare cycle. Ecol Monogr 68:371–394
Carlson NV, Pargeter HM, Templeton CN (2017) Sparrowhawk movement, calling, and presence of dead conspecifics differentially impact blue tit (Cyanistes caeruleus) vocal and behavioral mobbing responses. Behav Ecol Sociobiol 71:133
Clobert J, Le Galliard J-F, Cote J, Meylan S, Massot M (2009) Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett 12:197–209
Cooper EB, Taylor RW, Kelley AD, Martinig AR, Boutin S, Humphries MM, Dantzer B, Lane JE, McAdam AG (2017) Personality is correlated with natal dispersal in north American red squirrels. Behaviour 154:939–961
Cote J, Bestion E, Jacob S, Travis J, Legrand D, Baguette M (2017) Evolution of dispersal strategies and dispersal syndromes in fragmented landscapes. Ecography 40:56–73
Cote J, Clobert J (2007) Social information and emigration: lessons from immigrants. Ecol Lett 10:411–441
Cote J, Fogarty S, Tymen B, Sih A, Brodin T (2013) Personality dependent dispersal cancelled under predation risk. Proc R Soc B 280:20132349
De Meester N, Bonte D (2010) Information use and density-dependent emigration in an agrobiont spider. Behav Ecol 21:992–998
Dell'Arte GL, Laaksonen T, Norrdahl K, Korpimäki E (2007) Variation in the diet composition of a generalist predator, the red fox, in relation to season and density of main prey. Acta Oecol 31:276–281
Descamps S, Boutin S, Berteaux D, McAdam AG, Jean-Michel G (2008) Cohort effects in red squirrels: the influence of density, food abundance and temperature on future survival and reproductive success. J Anim Ecol 77:305–314
Doligez B, Danchin E, Clobert J (2002) Public information and breeding habitat selection in a wild bird population. Science 297:1168–1170
Fey K, Banks PB, Ylönen H, Korpimäki E (2010) Behavioural responses of voles to simulated risk of predation by a native and an alien mustelid: an odour manipulation experiment. Wildlife Res 37:273–282
Fey K, Hämäläinen S, Selonen V (2016) Roads are no barrier for dispersing red squirrels in an urban environment. Behav Ecol 27:741–747
Forsman JT, Mönkkönen M, Korpimäki E, Thomson RL (2013) Mammalian nest predator feces as a cue in avian habitat selection decisions. Behav Ecol 24:262–266
Fronhofer EA, Klecka J, Melián CJ, Altermatt F (2015) Condition-dependent movement and dispersal in experimental metacommunities. Ecol Lett 18:954–963
Fülling O, Halle S (2004) Breeding suppression in free-ranging grey-sided voles under the influence of predator odour. Oecologia 138:151–159
Gendron RP, Staddon JER (1984) A laboratory simulation of foraging behavior: the effect of search rate on the probability of detecting prey. Am Nat 124:407–415
Gurnell J (1983) Squirrel numbers and the abundance of tree seeds. Mammal Rev 13:133–148
Hakkarainen H, Ilmonen P, Koivunen V, Korpimäki E (2001) Experimental increase of predation risk induces breeding dispersal of Tengmalm’s owl. Oecologia 126:355–359
Hakkarainen H, Korpimäki E (1994) Nest defence of Tengmalm’s owls reflects offspring survival prospects under fluctuating food conditions. Anim Behav 48:843–849
Hakkarainen H, Mykra S, Kurki S, Korpimäki E, Nikula A, Koivunen V (2003) Habitat composition as a determinant of reproductive success of Tengmalm’s owls under fluctuating food conditions. Oikos 100:162–171
Hammond JI, Luttbeg B, Sih A (2007) Predator and prey space use: dragonflies and tadpoles in an interactive game. Ecology 88:1525–1535
Hanski IK, Selonen V (2009) Female-biased natal dispersal in the Siberian flying squirrel. Behav Ecol 20:60–67
Hämäläinen A, McAdam AG, Dantzer B, Lane JE, Haines JA, Humphries MM, Boutin S (2017) Fitness consequences of peak reproductive effort in a resource pulse system. Sci Rep 7:9335
Hämäläinen S, Fey K, Selonen V (2018) Habitat and nest use during natal dispersal of the urban red squirrel (Sciurus vulgaris). Landscape Urban Plan 169:269–275
Hiddink JG, Kock RP, Wolff WJ (2002) Active pelagic migrations of the bivalve Macoma balthica are dangerous. Mar Biol 140:1149–1156
Ims RA, Hjermann DØ (2001) Condition-dependent dispersal. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) Dispersal. Oxford University Press, Oxford, pp 203–216
Kauhala K, Talvitie K, Vuorisalo T (2016) Encounters between medium-sized carnivores and humans in the city of Turku, SW Finland, with special reference to the red fox. Mammal Res 61:25–33
Korpimäki E, Hakkarainen H (2012) The boreal owl: ecology, behaviour and conservation of a forest-dwelling predator. Cambridge University Press, Cambridge
Korpimäki E, Sulkava S (1987) Diet and breeding performance of Ural owls Strix uralensis under fluctuating food conditions. Ornis Fenn 64:57–66
Kotler BP, Brown JS, Hasson O (1991) Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72:2249–2260
Kuefler D, Avgar T, Fryxell JM (2012) Rotifer population spread in relation to food, density and predation risk in an experimental system. J Anim Ecol 81:323–329
Larsen KW, Boutin S (1994) Movements, survival, and settlement of red squirrel (Tamiasciurus hudsonicus) offspring. Ecology 75:214–223
Laundré JW, Hernández L, Altendorf KB (2001) Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, USA. Can J Zool 79:1401–1409
Le Galliard J-F, Remy A, Ims RA, Lambin X (2012) Patterns and processes of dispersal behaviour in arvicoline rodents. Mol Ecol 21:505–523
Lima SL (2009) Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol Rev 84:485–513
Lurz PWW, Garson PJ, Wauters LA (1997) Effects of temporal and spatial variation in habitat quality on red squirrel dispersal behaviour. Anim Behav 54:427–435
Lurz P, Gurnell J, Magris L (2005) Sciurus vulgaris. Mamm Spec 769:1–10
Matthysen E (2005) Density-dependent dispersal in birds and mammals. Ecography 28:403–416
McCauley SJ, Rowe L (2010) Notonecta exhibit threat-sensitive, predator-induced dispersal. Biol Lett 6:449–452
McIntosh AR, Peckarsky BL, Taylor BW (2002) The influence of predatory fish on mayfly drift: extrapolating from experiments to nature. Freshw Biol 47:1497–1513
Merrick MJ, Koprowski JL (2017) Altered natal dispersal at the range periphery: the role of behavior, resources, and maternal condition. Ecol Evol 7:58–72
Morosinotto C, Thomson RL, Korpimäki E (2010) Habitat selection as an antipredator behaviour in a multi-predator landscape: all enemies are not equal. J Anim Ecol 79:327–333
Morosinotto C, Villers A, Thomson RL, Varjonen R, Korpimäki E (2017) Competitors and predators alter settlement patterns and reproductive success of an intraguild prey. Ecol Monogr 87:4–20
Petty SJ, Lurz PWW, Rushton SP (2003) Predation of red squirrels by northern goshawks in a conifer forest in northern England: can this limit squirrel numbers and create a conservation dilemma? Biol Conserv 111:105–114
Poethke HJ, Weisser WW, Hovestadt T (2010) Predator-induced dispersal and the evolution of conditional dispersal in correlated environments. Am Nat 175:577–586
Ronce O (2007) How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol S 38:231–253
Selonen V, Hanski IK (2012) Dispersing Siberian flying squirrels (Pteromys volans) locate preferred habitats in fragmented landscapes. Can J Zool 90:885–892
Selonen V, Hanski IK (2015) Occurrence and dispersal of the red squirrel and the Siberian flying squirrel in fragmented landscapes. In: Shuttleworth CM, Lurz P, Hayward MW (eds) Red squirrels: ecology. Conservation & Management in Europe. European Squirrel Initiative, Suffolk, pp 67–82
Selonen V, Hanski IK, Desrochers A (2007) Natal habitat-biased dispersal in the Siberian flying squirrel. Proc R Soc Lond B 274:2063–2068
Selonen V, Sulkava P, Sulkava R, Sulkava S, Korpimäki E (2010) Decline of flying and red squirrels in boreal forests revealed by long-term diet analyses of avian predators. Anim Conserv 13:579–585
Selonen V, Varjonen R, Korpimäki E (2015) Immediate or lagged responses of a red squirrel population to pulsed resources. Oecologia 177:401–411
Selonen V, Varjonen R, Korpimäki E (2016a) Predator presence, but not food supplementation, affects forest red squirrels in winter. Ann Zool Fenn 53:183–193
Selonen V, Wistbacka R (2017) Role of breeding and natal movements in lifetime dispersal of a forest-dwelling rodent. Ecol and Evol 7:2204–2213
Selonen V, Wistbacka R, Korpimäki E (2016b) Food abundance and weather modify reproduction of two arboreal squirrel species. J Mamm 97:1376–1384
Terraube J, Vasko V, Korpimäki E (2015) Mechanisms and reproductive consequences of breeding dispersal in a specialist predator under temporally varying food conditions. Oikos 124:762–771
Thomson RL, Tomas G, Forsman JT, Broggi J, Mönkkönen M (2010) Predator proximity as a stressor in breeding flycatchers: mass loss, stress protein induction, and elevated provisioning. Ecology 91:1832–1840
Tornberg R, Korpimäki E, Byholm P (2006) Ecology of the northern goshawk in Fennoscandia. Stud Avian Biol-Ser 31:141–157
Turkia T, Korpimäki E, Villers A, Selonen V (2018) Predation risk landscape modifies flying and red squirrel nest site occupancy independently of habitat amount. PLoS One 13:e0194624
Verhulst S, Perrins CM, Riddington R (1997) Natal dispersal of great tits in a patchy environment. Ecology 78:864–872
Wauters L, Dhondt AA (1993) Immigration pattern and success in red squirrels. Behav Ecol Sociobiol 33:159–167
Wauters LA, Githiru M, Bertolino S, Molinari A, Tosi G, Lens L (2008) Demography of alpine red squirrel populations in relation to fluctuations in seed crop size. Ecography 31:104–114
Wauters LA, Verbeylen G, Preatoni D, Martinoli A, Matthysen E (2010) Dispersal and habitat cuing of Eurasian red squirrels in fragmented habitats. Popul Ecol 52:527–536
Acknowledgments
We thank Mikko Hänninen, Jorma Nurmi, Rauno Varjonen and Tanja Hannola for great help in the field. Constructive comments by two referees were valuable to improve the manuscript.
Funding
The study was financially supported by the Academy of Finland (no. 259562 to VS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. No ethical approval was required from an institutional or national ethics review board.
Additional information
Communicated by A. I. Schulte-Hostedde
Rights and permissions
About this article
Cite this article
Selonen, V., Fey, K., Hämäläinen, S. et al. Do predators modify context-dependent dispersal of red squirrels?. Behav Ecol Sociobiol 72, 136 (2018). https://doi.org/10.1007/s00265-018-2554-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2554-y