Abstract
Turbidity (a measure of the cloudiness of water) decreases the visual range of organisms, altering interactions within and between species. For species that visually assess mates, turbidity may affect mating interactions and mate choice. A central question, then, is to what degree organisms plastically adjust mating behaviors to cope with visually altered environments. Here, we investigate the effect of turbidity on the mating behavior of guppies (Poecilia reticulata) in Trinidad, where some streams are increasingly turbid due to upstream rock quarrying. We collected fish from two highly turbid streams (with upstream rock quarries) and two pristine streams (no upstream quarries) in the same drainages. We then observed male mating behaviors with females from the same populations in both turbid and clear assays, recording displays and sneak copulation attempts. Males from turbid streams showed greater individual consistency in mating behaviors. But regardless of provenance, male guppies spent less time associating with females in turbid water overall. When males and females did interact, however, males greatly increased the rate of all mating behaviors in turbid as compared to clear water. Thus, even when lacking a long-term evolutionary history with high turbidity, guppies compensate for lost time when mating in a visually altered environment.
Significance statement
Given the global nature of increases in turbidity, understanding behavioral responses to this aquatic anthropogenic change is critical. Here, we investigate the degree to which Trinidadian guppy males in affected areas are able to plastically compensate in turbid water by adjusting mating behaviors. We also test the degree to which previous chronic exposure to turbidity alters this plasticity. In contrast to predictions, we did not find population differences in plasticity; across all populations, however, we found significant plasticity in the guppy mating system in response to anthropogenically increased turbidity. While males spent less time associating with females in turbid water overall, males increased mating effort during periods of association, compensating for lost time in turbid water. We discuss this plasticity and the implications for guppy sexual selection and secondary sexual characteristics such as coloration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One common source of human-induced rapid environmental change (HIREC) in aquatic environments is the sedimentation of water bodies and associated increases in turbidity (Wilkinson 1999; Davies-Colley and Smith 2001). While turbidity caused by suspended sediments can be physiologically costly by hindering respiration at extremely high levels (e.g., Horkel and Pearson 1976), turbidity is more often a visual stressor, decreasing the visual range for organisms that would otherwise rely on vision (Gardner 1981; Abrahams and Kattenfeld 1997; Leahy et al. 2011; Ranåker et al. 2012).
A turbidity-induced decrease in visual range or acuity can influence mate choice in visually mediated mating systems. Many freshwater fish, for instance, rely on brightly colored pigmentation and conspicuous displays to attract mates (e.g., Houde 1987; Milinski and Bakker 1990; Seehausen and van Alphen 1998). Increases in turbidity can impair these mating systems, influencing the strength or direction of sexual selection (Järvenpää and Lindström 2004; Heubel and Schlupp 2006; Wong et al. 2007; Heuschele et al. 2009; Sundin et al. 2010, 2016). In populations of haplochromine cichlids in Lake Victoria, for example, sister species have been found to interbreed in turbid but not clear conditions (Seehausen et al. 1997), and female cichlids originating from turbid conditions show a decreased preference for male coloration (Maan et al. 2010). In threespine stickleback (Gasterosteus aculeatus), while females spent more time with males in clear vs. turbid conditions, males in turbid conditions partially compensate by courting more (Engström-Öst and Candolin 2007).
Populations of guppies in Trinidad are exposed to varying levels of turbidity (Luyten and Liley 1985; SME unpublished data). In the Northern Range mountains, large amounts of sediment from rock quarrying operations cause chronically high turbidity in some streams, while other streams in the same drainage are unaffected. In some of these localities (e.g., Arima River), quarries have been operating for nearly seven decades since World War II, representing ~ 115 guppy generations (Reznick et al. 1997). High turbidity has been shown to affect social dynamics in guppies: guppies in turbid water spend less time schooling with conspecifics (Borner et al. 2015; Kimbell and Morrell 2015). While this behavioral change may be due to a potential reduction in predation risk in turbid water (e.g., Gregory and Levings 1998; Lehtiniemi et al. 2005), decreased schooling may also result simply from an inability of individuals to relocate schoolmates in a fission/fusion schooling system (Borner et al. 2015). Turbidity has also been found to affect guppy activity levels, and has developmental effects on guppy visual systems (Ehlman et al. 2015). And while guppy mating behaviors from lowland populations in Trinidad with naturally high turbidity levels differ from those in the headwaters (Luyten and Liley 1985, 1991), these differences are somewhat confounded by differences in predation pressure and food availability between these localities, which are known to affect the guppy mating system (Endler 1980, 1987; Magurran 2005; Grether 2005; Kolluru et al. 2006). Still unknown, then, is how human-induced turbidity affects the guppy mating system across relatively recently impacted headwater streams.
Male guppies rely on two main strategies for securing mates. Males can either display bright ornamentation along their sides as well as their dorsal and caudal fins by performing conspicuous mating displays (i.e., sigmoid displays), or males can attempt to sneak copulate, whereby males approach females from behind and below and thrust with their modified anal fin, or gonopodium, in order to inseminate females (Clark and Aronson 1951; Baerends et al. 1955; Houde 1997). Populations differ in their use of these two mating strategies (Luyten and Liley 1985, 1991), and within populations, ambient light conditions (Endler 1987; Archard et al. 2009; Chapman et al. 2009), risk of predation (Godin 1995; Evans et al. 2002), or food availability (Kolluru and Grether 2005), among other things, are known to affect male mating behaviors. Of interest here is the degree to which males plastically adjust mating behaviors (e.g., the proportion of displays vs sneaks) in turbid vs clear water conditions. Such behavioral plasticity in male mating behaviors is particularly important since turbidity levels can fluctuate over short timescales (e.g., before and immediately following heavy rains; Lowe-McConnell 1987; Dearing and Jones 2003). Thus, we examine the degree to which guppies plastically adjust their mating tactics in response to human-induced turbidity and ask whether or not these adjustments differ across headland stream populations that have experienced drastically different turbidity levels in their recent past.
Methods
Study system
During August 2016, guppies were collected from turbid/non-turbid stream pairs in two southern drainages (the Caroni and Oropouche drainages) in the Northern Range mountains of Trinidad. Within the Caroni drainage, fish were collected from the chronically turbid Arima River (with quarry operations for the last seven decades; lat 10° 41′ 10.1646″, lon − 61° 17′ 23.3556″) and also from the typically clear Aripo River (lat 10° 39′ 24.8934″, lon − 61° 13′ 35.004″). In the Oropouche drainage, fish were collected from the chronically turbid Turure River (with quarry operations occurring for the last decade; lat 10° 39′ 24.5232″, lon − 61° 10′ 5.1384″) and the typically clear Quare River (lat 10° 39′ 50.4576″, lon − 61° 11′ 34.4148″). The two turbid populations, Arima and Turure, are both located downstream from nearby rock quarries which routinely wash sediment into streams, resulting in chronic turbidity. The clear streams have no upstream quarries and are only exposed to elevated turbidity during periods of heavy rain. All populations sampled were from typical “high-predation” reaches of river characterized by low flow velocities, high predation rates, and semi-open canopies (Reznick et al. 1996; Magurran 2005). Guppies were collected using handheld nets during the day from 900 to 1300 h. Collected guppies were housed at the William Beebe Tropical Research Station in the Arima Valley, Trinidad, at a density of approximately five fish per liter in 5-l tanks with clear water, and fed once daily ad libitum with brine shrimp. Fish were housed in separate tanks by population, with each tank containing an approximately equal mix of males and females. Guppies were held for at least 2 days, but not more than 1 week, after collection, then assayed for mating behavior.
Mating behavior
Mating behavior assays were performed using the four populations described above. A total of 144 males were initially used in the study, but due to missing data or mortality, only 129 of these yielded data that could be analyzed (32 males from each population, except Turure, which had 33). Assays consisted of a male and two size-matched females from the same population being placed in a tank of either turbid or clear water. Two females were used to reduce possible female stress (Chapman et al. 2009). Sessions were then recorded for a total of 30 min (consisting of a 15-min acclimation period and a 15-min assay period) using an HD video recorder placed approximately 15 cm directly in front of the tank.
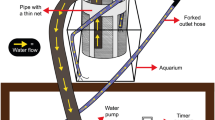
Assays took place between 7 and 15 September 2016, with four to six rounds of assays each day. All assays were performed in the same location between the hours of 9:00 and 17:00 with ambient light provided by overhead fluorescent lights. A total of eight males—one per assay tank—were assayed in each round, with two males from each of the four populations assayed in each round in both conditions (turbid or clear). Assay tanks were separated by pieces of laminated printer paper to eliminate any bystander effects from adjacent guppies. Between each round, tanks were systematically rotated to eliminate any tank position effect. Before each round, tanks were emptied, cleaned, and refilled with turbid or clear water. Tank treatments were also randomized between days. Turbid water was created by mixing 2.25 g of fine powder bentonite clay, a commonly used additive to produce turbidity (Gardner 1981; Heubel and Schlupp 2006; Ranåker et al. 2012; Ehlman et al. 2015), into approximately 20 l of water using an immersion blender. This concentration of bentonite clay yields a turbidity level of ~ 20 NTU (nephelometric turbidity units), which is below the average NTU range of 40–60 NTU found in turbid Trinidadian streams (Luyten and Liley 1985; SME unpublished data), but well above the mean NTU of streams unaffected by quarrying. This treatment was chosen so that human observers could accurately view guppy behavior, while still producing similar turbidity levels to those found in the wild. Air was pumped into tanks through an airstone, which helped to keep bentonite suspended and maintain oxygen levels in the water.
Each male was assayed twice—once in clear water, once in turbid water—on the same day, with water condition experienced on the first round being randomly selected. The first and second assays for each male were separated by approximately 2 to 4 h. Assaying each male twice allowed for the calculation of behavioral repeatability across trials. Females were used in trials only once. For each round, females were randomly selected from home tanks (females came from the same tank as each other but not as the focal male) and introduced into the assay tanks first. Only gravid females (determined visually by the presence of a gravid spot) were selected for the study, and each female was measured to the nearest 0.1 mm. Males were then randomly selected from home tanks and introduced to assay tanks with the females. After the assay, females were placed in a separate tank for “retired females,” and males were placed into individual marked containers to be used for their second round of assays.
Videos were viewed between 9 January and 12 February 2017 in VLC media player, and behaviors were scored using Jwatcher 2.0 (Blumstein and Daniel 2007). Videos were watched in a randomized order, and the observer was blind of population origin (but not treatment condition, since that was readily apparent). All instances of displays, sneaks, gonopodial thrusts, copulations, and tail bites were recorded, as well as the time that the males spent near at least one female (defined as within two body lengths). A sneak had two components: chase and gonopodial thrust (Endler 1987). Gonopodial thrusts were scored as a particularly escalated chase that progressed to propelling towards the female with gonopodium fully extended. Females typically responded by rapidly darting away (Houde 1997) and in these trials, gonopodial thrusts never successfully contacted a female’s genital pore. Displays were characterized by the males orienting themselves in front of the female and contorting their body into a sigmoid shape, splaying their fins and, during intense displays, quivering their body (Houde 1997). All displays, sneaks, thrusts, and tail bites necessarily occurred, while males were proximate to at least one female. Lastly, in addition to comparing rates of specific mating behaviors between treatments, we also compared overall mating effort, which we defined as a rate composed of the sum of display and sneak rates.
Statistical analyses
Since individuals assayed in turbid water were in sight less frequently during assays than those assayed in clear water, we kept track of when individuals were in versus out of sight. Behaviors were then analyzed as rates: \( \frac{time\ behavior\ observed}{total\ time\ male\ in\ sight} \). We used an arbitrary threshold of 2 min (out of the total 15) for the amount of time that a male fish needed to be in sight for us to include that assay in the analysis. Results were not qualitatively different when we chose a threshold that varied from 1 to 5 min. To evaluate mating behavior, we constructed linear mixed models using the lme4 package (Bates et al. 2015) in R (R Core Team 2017) with stream turbidity type (“population type”), assay turbidity condition, time of day, and combined mass of females as fixed effects and origin stream (i.e., population), fish ID, assay tank, date, and round (i.e., first or second assay) as random effects. P values were calculated using the lmerTest package (Kuznetsova et al. 2016). Models originally tested for an interaction between stream condition and assay condition, but no significant interactions were revealed. Thus, results are reported using models without interaction terms. When variance attributed to random effects was determined to be zero, we reran the model without that factor to ensure model convergence (Bolker 2015). Though doing so did not change fixed effect estimates and inferences drawn, all results derive from models that retain all random effects aforementioned. Due to few tail bites and no copulations observed, we were only able to analyze display, sneak, and thrust rates.
Male consistency in mating behaviors across contexts (turbid vs. clear) was calculated using the rptR package (Stoffel et al. 2017), and 95% confidence intervals around repeatability estimates were calculated via parametric bootstrapping. We then compared individual consistencies of mating behavior for fish from different population types (clear vs turbid streams) using bootstrapped confidence intervals.
Results
Model formulation and statistical results for all mating behaviors are shown in Table 1.
Overall mating effort in turbid and clear assays
When analyzed over the entire 15-min assay period, overall mating effort did not vary between population type (i.e., turbid or clear streams) or treatment condition (Fig. 1a). However, when assayed in turbid conditions, males spent less time near a female than when they were assayed in clear water (Fig. 1b). When comparing mating effort only while fish were within two body lengths, males assayed in turbid water increased their mating effort during bouts of proximity relative to those assayed in clear water (Fig. 1c).
a Overall mating effort (number of displays and sneaks per second) in assays of clear and turbid water. b The proportion of time that males from clear and turbid populations spent within two body lengths of a female in either clear or turbid conditions. Left of dashed line represents fish from clear water streams; right represents fish from turbid streams. c Mating effort per second spent near a female in clear and turbid assay conditions. Lines represent standard error (SE) around mean values
Male mating tactics in turbid and clear treatments
The increase in male mating effort while near a female in turbid conditions was evident across all mating behaviors scored: display rate, sneak rate, and thrust rate (Fig. 2). We did not find an effect of assay condition on the proportion of sneak attempts that ended in gonopodial thrusting (estimate ± SE = 0.071 ± 0.080, t value = 0.884, p = 0.381), nor did we find an effect of turbidity on the relative display vs sneak rate (estimate ± SE = − 0.009 ± 0.005, t value = − 1.587, p = 0.118).
Males from clear streams showed highly consistent among-individual differences in display rate (repeatability point estimate 0.602, 95% CI 0.336–0.787), but not in sneaking rate (point estimate 0.097, 95% CI 0–0.406) or thrusting rate (point estimate 0.233, 95% CI 0–0.441) behaviors. Males from turbid streams, however, showed no detectable consistency in behaviors across clear and turbid assays: display rate (point estimate 0.012, 95% CI 0–0.287), sneaking rate (point estimate 0.220, 95% CI 0–0.472), and thrusting rate (point estimate 0.233, 95% CI 0–0.466). Comparing individual consistency of display behavior of males from clear vs turbid populations, males from clear populations were significantly more repeatable (i.e., individually consistent) in their display behavior than those from turbid populations across assays, as evidenced by a lack of overlap in their 95% confidence intervals (Fig. 3).
Repeatability of display, sneaking, and thrusting rates (per time within two body lengths of female) across turbid and clear conditions for males from turbid clear streams. Males from turbid conditions are those represented in brown. Those from clear conditions are represented in blue. Males were assayed once in each condition, with assays separated by 2 to 4 h. Lines represent 95% bootstrapped confidence intervals
Discussion
Our results indicate that, in response to visually obscuring turbidity, guppies plastically adjust mating behaviors. Specifically, we document turbidity-induced increases in mating effort while males were near a female, which allowed guppies to compensate for decreased time spent near a female in turbid conditions. While other studies have shown that guppies spend less time with schooling conspecifics or form looser social bonds in turbid water (Borner et al. 2015; Kimbell and Morrell 2015), ours is the first to test how anthropogenic increases in turbidity interact with evolutionary or developmental histories to affect mating behavior in this system.
A number of previous studies have found effects of turbidity on mating systems. While for some species, turbidity seems to cause breakdown or weakening of sexual selection in visually based mating systems (Seehausen et al. 1997; Järvenpää and Lindström 2004; Wong et al. 2007; Maan et al. 2010; Sundin et al. 2010, 2016), other species seem to be able to cope somewhat via behavioral plasticity (Engström-Öst and Candolin 2007; Heuschele et al. 2009; current study). In some systems, including that presented here, the question remains, however, whether behavioral coping mechanisms involved in mate choice are enough to fully compensate for changes to the visual environment (i.e., such that the rate of reproduction and the strength of sexual selection remains stable across turbid and clear environments).
It is evident that significant variation exists in the propensity of species or populations to behaviorally adjust mating behaviors in response to turbidity. But why do some species fail to adjust while others succeed? In other systems, variation in response to environmental change may be attributable to variation in developmental or evolutionary histories (Sih 2013). Here, contrary to expectations, strong differences among populations in previous exposure (evolutionary and developmental) to turbidity did not significantly influence average mating behaviors. All populations, regardless of previous exposure to turbidity, showed similar patterns of plastic adjustment to experimentally altered turbidity.
Interestingly, past exposure to turbidity influenced behavioral repeatability (Bell et al. 2009), a key metric for measuring individual differences in animal personality (Sih et al. 2004). Guppies from clear streams exhibited consistent individual differences in their display rates across turbid and clear water treatments while those from chronically turbid streams exhibited no detectable consistent differences among individuals. A previous study found quite high consistency in display and sneak behaviors among male guppies measured in differing sex-ratio scenarios (Magellan and Magurran 2007), suggesting that even for the same behaviors, the context in which consistency is measured matters. Our finding of a relative lack of individual consistency for fish from turbid streams suggests that fish from turbid streams exhibit greater individual differences in plasticity (i.e., larger individual × environment interaction; Dingemanse et al. 2010) in mating behavior between turbid and clear conditions than those from clear streams. The underlying significance of increased variation in mating behavior plasticity for populations in turbid streams remains to be determined. Whatever the case, these consistent individual differences in mating behaviors between turbid and clear populations may be induced developmentally; guppies exhibit remarkable developmental plasticity in a whole host of traits in response to varying environments (e.g., Kodric-Brown 1989; Chapman et al. 2010; Ruell et al. 2013), including differing turbidity regimes (Ehlman et al. 2015). Alternatively, such differences may be due to rapid evolution of guppies in turbid streams, as guppies are fully capable of rapid evolution in the wild within the timescale of anthropogenic turbidity increases in Trinidad (Reznick et al. 1997). Further research is needed to measure potential differences between populations in clear and turbid streams and assess the underlying mechanisms driving differences. One potentially useful experiment would rear F2 offspring of guppies from streams of varying turbidities in a common garden design to tease apart genetic and developmental differences between populations.
Understanding how environmental changes affect sexual selection is critical (Candolin and Heuschele 2008): sexual selection can have large consequences for evolutionary trajectories and macroevolutionary patterns (West-Eberhard 1983; Maan and Seehausen 2011) and species persistence (Martínez Ruiz and Knell 2017). Thus, shifts in mating systems driven by HIREC, such as those associated with increasing turbidity, can have large consequences for populations and their evolution. While we found that guppies compensate for lost time spent near females by increasing their mating effort during the reduced time that they are around females, it is unclear whether or not the strength of guppy sexual selection is altered in turbid water. For instance, there are a few reasons why this compensation may not be complete. First of all, we did not assess male mating success. If male mating success during sneaking was proportionally greater (in relation to displays) in turbid water, sexual selection on color is likely weakened. This could occur if females were less prepared to take evasive action against males’ sneak attempts, perhaps due to females being less able to see approaching males in turbid water. We also did not measure aspects of female mate choice. Even if male behavior were not altered in turbid water, if females are less able to discriminate between males of varying quality during displays in turbid water, the degree of reproductive skew in the population could be affected, with males of lower quality increasing their contributions to the gene pool. Over time, this could lead to a decrease in male coloration through decreased selection for bright and ornate males, since coloration is costly (Godin and McDonough 2003). A loss of mating signal reliability would also result in a weakening of preference for the sexually selected trait and could shift preference to more reliable signals (Shenoy and Crowley 2011); in turbid water, for example, the properties of certain color patches may shift depending on the visual environment (Castillo Cajas et al. 2012). Furthermore, while we have not investigated the effects of turbidity on male-male interactions, it is possible that turbidity could hamper the abilities of males to assess other potential suitors; since other types of visual obstructions alter male mating behavior (Hibler and Houde 2006), this is a plausible route through which turbidity may further alter male mating behaviors.
In conclusion, due to the global nature of HIREC-associated increases in turbidity, understanding the role of behavioral plasticity as a mechanism to cope with such changes should be a priority. Here, we offer one of the first studies to investigate the role of past history or provenance of wild animals in creating variation in mating behavior in turbid waters. Thus, one promising research direction in the study of behavioral responses to HIREC is one that takes a similar comparative approach across populations or species in order to understand variation in responses.
References
Abrahams M, Kattenfeld M (1997) The role of turbidity as a constraint on predator-prey interactions in aquatic environments. Behav Ecol Sociobiol 40:169–174. https://doi.org/10.2307/4601315?ref=search-gateway:48e013fd369e1ef06f43b1b5b0eaf554
Archard GA, Cuthill IC, Partridge JC (2009) Light environment and mating behavior in Trinidadian guppies (Poecilia reticulata). Behav Ecol Sociobiol 64:169–182. https://doi.org/10.1007/s00265-009-0834-2
Baerends GP, Brouwer R, Waterbolk HTJ (1955) Ethological studies on Lebistes reticulatus (Peters). Behav 8:249–332. https://doi.org/10.1163/156853955X00238
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783. https://doi.org/10.1016/j.anbehav.2008.12.022
Blumstein DT, Daniel JC (2007) Quantifying behavior the JWatcher way. Sinauer Associates, Inc., Sunderland, MA
Bolker BM (2015) Linear and generalized linear mixed models. In: Fox GA, Negrete-Yankelevich S, Sosa VJ (eds) Ecological statistics: contemporary theory and application. Oxford University Press, Oxford, pp 309–333
Borner KK, Krause S, Mehner T, Uusi-Heikkilä S, Ramnarine IW, Krause J (2015) Turbidity affects social dynamics in Trinidadian guppies. Behav Ecol Sociobiol 69:645–651. https://doi.org/10.1007/s00265-015-1875-3
Candolin U, Heuschele J (2008) Is sexual selection beneficial during adaptation to environmental change? Trends Ecol Evol 23:446–452. https://doi.org/10.1016/j.tree.2008.04.008
Castillo Cajas RF, Selz OM, Ripmeester EAP, Seehausen O, Maan ME (2012) Species-specific relationships between water transparency and male coloration within and between two closely related Lake Victoria cichlid species. Int J Evol Biol 2012:161306–161312. https://doi.org/10.1155/2012/161306
Chapman BB, Morrell LJ, Krause J (2009) Plasticity in male courtship behaviour as a function of light intensity in guppies. Behav Ecol Sociobiol 63:1757–1763. https://doi.org/10.1007/s00265-009-0796-4
Chapman BB, Morrell LJ, Tosh CR, Krause J (2010) Behavioural consequences of sensory plasticity in guppies. Proc R Soc Lond B 277:1395–1401. https://doi.org/10.1098/rspb.2009.2055
Clark E, Aronson LR (1951) Sexual behavior in the guppy, Lebistes reticulatus. Zoologica 36:49–66
Davies-Colley RJ, Smith DG (2001) Turbidity, suspended sediment, and water clarity: a review. J Am Water Resour As 37:1085–1101. https://doi.org/10.1111/j.1752-1688.2001.tb03624.x
Dearing JA, Jones RT (2003) Coupling temporal and spatial dimensions of global sediment flux through lake and marine sediment records. Glob Planet Change 39:147–168. https://doi.org/10.1016/S0921-8181(03)00022-5
Dingemanse NJ, Kazem AJN, Réale D, Wright J (2010) Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol 25:81–89. https://doi.org/10.1016/j.tree.2009.07.013
Ehlman SM, Sandkam BA, Breden F, Sih A (2015) Developmental plasticity in vision and behavior may help guppies overcome increased turbidity. J Comp Physiol A 201:1125–1135. https://doi.org/10.1007/s00359-015-1041-4
Endler JA (1980) Natural selection on color patterns in Poecilia reticulata. Evolution 34:1–20
Endler JA (1987) Predation, light intensity and courtship behaviour in Poecilia reticulata (Pisces: Poeciliidae). Anim Behav 35:1376–1385. https://doi.org/10.1016/S0003-3472(87)80010-6
Engström-Öst J, Candolin U (2007) Human-induced water turbidity alters selection on sexual displays in sticklebacks. Behav Ecol 18:393–398. https://doi.org/10.1093/beheco/arl097
Evans JP, Kelley JL, Ramnarine IW, Pilastro A (2002) Female behaviour mediates male courtship under predation risk in the guppy (Poecilia reticulata). Behav Ecol Sociobiol 52:496–502. https://doi.org/10.1007/s00265-002-0535-6
Gardner MB (1981) Effects of turbidity on feeding rates and selectivity of bluegills. Trans Am Fish Soc 110:446–450. https://doi.org/10.1577/1548-8659(1981)110<446:EOTOFR>2.0.CO;2
Godin J-GJ, McDonough HE (2003) Predator preference for brightly colored males in the guppy: a viability cost for a sexually selected trait. Behav Ecol 14:194–200. https://doi.org/10.1093/beheco/14.2.194
Godin J-GJ (1995) Predation risk and alternative mating tactics in male Trinidadian guppies (Poecilia reticulata). Oecologia 103:224–229. https://doi.org/10.1007/BF00329084
Gregory RS, Levings CD (1998) Turbidity reduces predation on migrating juvenile pacific salmon. Trans Am Fish Soc 127:275–285. https://doi.org/10.1577/1548-8659(1998)127<0275:TRPOMJ>2.0.CO;2
Grether GF (2005) Environmental change, phenotypic plasticity, and genetic compensation. Am Nat 166:E115–E123. https://doi.org/10.1086/432023
Heubel KU, Schlupp I (2006) Turbidity affects association behaviour in male Poecilia latipinna. J Fish Biol 68:555–568. https://doi.org/10.1111/j.0022-1112.2006.00941.x
Heuschele J, Mannerla M, Gienapp P, Candolin U (2009) Environment-dependent use of mate choice cues in sticklebacks. Behav Ecol 20:1223–1227. https://doi.org/10.1093/beheco/arp123
Hibler TL, Houde AE (2006) The effect of visual obstructions on the sexual behaviour of guppies: the importance of privacy. Anim Behav 72:959–964 https://doi.org/10.1016/j.anbehav.2006.03.007
Horkel JD, Pearson WD (1976) Effects of turbidity on ventilation rates and oxygen consumption of green sunfish, Lepomis cyanellus. Trans Am Fish Soc 105:107–113. https://doi.org/10.1577/1548-8659(1976)105<107:EOTOVR>2.0.CO;2
Houde AE (1997) Sex, color, and mate choice in guppies. Princeton University Press, Princeton
Houde AE (1987) Mate choice based upon naturally occurring color-pattern variation in a guppy population. Evolution 41:1–10. https://doi.org/10.2307/2408968
Järvenpää M, Lindström K (2004) Water turbidity by algal blooms causes mating system breakdown in a shallow-water fish, the sand goby Pomatoschistus minutus. Proc R Soc Lond B 271:2361–2365. https://doi.org/10.1098/rspb.2004.2870
Kimbell HS, Morrell LJ (2015) Turbidity influences individual and group level responses to predation in guppies, Poecilia reticulata. Anim Behav 103:179–185. https://doi.org/10.1016/j.anbehav.2015.02.027
Kodric-Brown A (1989) Dietary carotenoids and male mating success in the guppy: an environmental component to female choice. Behav Ecol Sociobiol 25:393–401. https://doi.org/10.1007/BF00300185
Kolluru GR, Grether GF (2005) The effects of resource availability on alternative mating tactics in guppies (Poecilia reticulata). Behav Ecol 16:294–300. https://doi.org/10.1093/beheco/arh161
Kolluru GR, Grether GF, Contreras H (2006) Environmental and genetic influences on mating strategies along a replicated food availability gradient in guppies (Poecilia reticulata). Behav Ecol Sociobiol 61:689–701. https://doi.org/10.1007/s00265-006-0299-5
Kuznetsova A, Brockhoff PB, Christensen RHB (2016) lmerTest: tests in linear mixed effects models. R package version 2.0–33, https://CRAN.R-project.org/package=lmerTest
Leahy SM, McCormick MI, Mitchell MD, Ferrari MCO (2011) To fear or to feed: the effects of turbidity on perception of risk by a marine fish. Biol Lett 7:811–813. https://doi.org/10.1098/rsbl.2011.0645
Lehtiniemi M, Engström-Öst J, Viitasalo M (2005) Turbidity decreases anti-predator behaviour in pike larvae, Esox lucius. Environ Biol Fish 73:1–8. https://doi.org/10.1007/s10641-004-5568-4
Lowe-McConnell RH (1987) Ecological studies in tropical fish communities. Cambridge University Press, Cambridge
Luyten PH, Liley NR (1985) Geographic variation in the sexual behaviour of the guppy, Poecilia reticulata (Peters). Behav 95:164–179. https://doi.org/10.1163/156853985X00109
Luyten PH, Liley NR (1991) Sexual selection and competitive mating success of male guppies (Poecilia reticulata) from four Trinidad populations. Behav Ecol Sociobiol 28:329–336. https://doi.org/10.1007/BF00164382
Maan ME, Seehausen O (2011) Ecology, sexual selection and speciation. Ecol Lett 14:591–602. https://doi.org/10.1111/j.1461-0248.2011.01606.x
Maan ME, Seehausen O, van Alphen JJM (2010) Female mating preferences and male coloration covary with water transparency in a Lake Victoria cichlid fish. Biol J Linn Soc 99:398–406. https://doi.org/10.1111/j.1095-8312.2009.01368.x
Magellan K, Magurran AE (2007) Behavioural profiles: individual consistency in male mating behaviour under varying sex ratios. Anim Behav 74:1545–1550. https://doi.org/10.1016/j.anbehav.2007.03.015
Magurran AE (2005) Evolutionary Ecology: the Trinidadian Guppy. Oxford University Press, Oxford
Martínez Ruiz C, Knell RJ (2017) Sexual selection can both increase and decrease extinction probability: reconciling demographic and evolutionary factors. J Anim Ecol 86:117–127. https://doi.org/10.1111/1365-2656.12601
Milinski M, Bakker T (1990) Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature 344:330–333
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna https://www.R-project.org/
Ranåker L, Jönsson M, Nilsson PA, Brönmark C (2012) Effects of brown and turbid water on piscivore–prey fish interactions along a visibility gradient. Freshw Biol 57:1761–1768. https://doi.org/10.1111/j.1365-2427.2012.02836.x
Reznick DN, Rodd FH, Cardenas M (1996) Life-history evolution of guppies (Poecilia reticulata: Poecilidae). IV. Parallelism in life-history phenotypes. Am Nat 147:319–338. https://doi.org/10.2307/2463211
Reznick DN, Shaw FH, Rodd FH, Shaw RG (1997) Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275:1934–1937. https://doi.org/10.1126/science.275.5308.1934
Ruell EW, Handelsman CA, Hawkins CL, Sofaer HR, Ghalambor CK, Angeloni L (2013) Fear, food and sexual ornamentation: plasticity of colour development in Trinidadian guppies. Proc Roy Soc B 280:20122019. https://doi.org/10.1098/rspb.2012.2019
Seehausen O, van Alphen JJM (1998) The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex). Behav Ecol Sociobiol 42:1–8. https://doi.org/10.1007/s002650050405
Seehausen O, van Alphen JJM, Witte F (1997) Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277:1808–1811. https://doi.org/10.1126/science.277.5333.1808
Shenoy K, Crowley PH (2011) Endocrine disruption of male mating signals: ecological and evolutionary implications. Funct Ecol 25:433–448. https://doi.org/10.1111/j.1365-2435.2010.01787.x
Sih A (2013) Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim Behav 85:1077–1088. https://doi.org/10.1016/j.anbehav.2013.02.017
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. https://doi.org/10.1016/j.tree.2004.04.009
Stoffel MA, Nakagawa S, Schielzeth H, Goslee S (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639-1644. https://doi.org/10.1111/2041-210X.12797
Sundin J, Berglund A, Rosenqvist G (2010) Turbidity hampers mate choice in a pipefish. Ethology 116:713–721
Sundin J, Rosenqvist G, Myhren S, Berglund A (2016) Algal turbidity hampers ornament perception, but not expression, in a sex-role-reversed pipefish. Ethology 122:215–225. https://doi.org/10.1111/eth.12461
West-Eberhard MJ (1983) Sexual selection, social competition, and speciation. Q Rev Biol 58:155–183. https://doi.org/10.1086/413215
Wilkinson CR (1999) Global and local threats to coral reef functioning and existence: review and predictions. Mar Freshw Res 50:867–878. https://doi.org/10.1071/MF99121
Wong BBM, Candolin U, Lindström K (2007) Environmental deterioration compromises socially enforced signals of male quality in three-spined sticklebacks. Am Nat 170:184–189. https://doi.org/10.2307/4541072
Acknowledgments
We would like to thank the Sih lab for offering insights during the analysis phase. The manuscript was improved by helpful edits from A. Pilastro, K. Heubel, and an anonymous reviewer. We also gratefully acknowledge the Asa Wright Nature Centre, with special thanks to Ronnie Hernandez, for facilitating our work at the William Beebe Tropical Research Station. SME was supported by the UC Davis Center for Population Biology and by an NSF Graduate Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Fish were collected under the approval of the Trinidad and Tobago Ministry of Food Production, Aquaculture Division. All procedures herein performed were approved in accordance with national and international ethics standards by the University of California Davis’ Institutional Animal Care and Use Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Data accessibility statement
The dataset collected and analyzed during the current study is available from the corresponding author on reasonable request.
Additional information
Communicated by A. Pilastro
Rights and permissions
About this article
Cite this article
Ehlman, S.M., Martinez, D. & Sih, A. Male guppies compensate for lost time when mating in turbid water. Behav Ecol Sociobiol 72, 46 (2018). https://doi.org/10.1007/s00265-018-2468-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2468-8