Abstract
Turbidity, caused by suspended particles in the water column, induces light scattering and shifts in the wavelengths of light. These changes may impair the ability of fish to use physical cues and hence may modify social interactions. We experimentally investigated the social interactions of guppies, Poecilia reticulata, in clear and turbid water. Fish were significantly less active, formed smaller shoals and were found to be more often alone in turbid than in clear water. A Markov chain analysis revealed significant differences in the social dynamics when comparing clear and turbid water conditions. The probability of leaving a particular nearest neighbour and the probability of choosing some neighbour after swimming around alone differed between the treatments. Our results indicate that turbidity has a number of different effects on the social interactions of the guppy, and we discuss their potential costs and benefits and wider implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Turbidity in the water body has become a worldwide phenomenon and is steadily increasing. It is caused by suspended particles that generate light scattering. This is mainly a result of anthropogenic influences (Scheffer et al. 2001). Intense human land use like deforestation, mining and agriculture lead soils releasing clay and silt particles and increased amounts of fertilizers, which are washed into rivers and lakes. While fertilizers raise productivity of aquatic ecosystems, often reflected in substantially increased algae growth (Carpenter et al. 2001; Conley et al. 2009), soils, clay and silt may be suspended in the water column and hence may cloud the water (Tockner et al. 2010). Quarrying is one of the human activities that affects land and has lasting effects on the environment (Maya et al. 2012). With increasing demand for construction material in the centres of dense population and development all over the world, the consequence of quarrying moves into focus. Closely linked but not mandatory to the activity of quarrying is deforestation. Both can lead to higher nutrient concentrations, pH values, temperatures and stronger currents in streams (Likens et al. 1970; Galas 2003). With flash floods, clay from the quarries and soil can be washed into the rivers leaving a thick coating of mud and high turbidity levels (Tockner et al. 2010; Maya et al. 2012) which might influence the physiology of organisms (Ardjosoediro and Ramnarine 2002). In addition, turbidity scatters and reduces the amount of light and may lead to a change of the light spectrum, in the water column (Utne-Palm 2002). Many fish species rely on vision as their main source of information, for example, with respect to foraging and anti-predator behaviour (Guthrie and Muntz 1993). Therefore, changes in the natural environment that modify the optical properties of water or the visibility per se may have important consequences for the biology of fishes (Endler 1992; Utne-Palm 2002; Chapman et al. 2009, 2010; Sluijs et al. 2010).
In fish, visual communication plays a role in kin recognition and familiarity (Griffiths and Magurran 1999; Hain and Neff 2007), identification of predators and social learning (Laland and Williams 1997; Day et al. 2001) as well as foraging and shoaling behaviour (Hoare et al. 2000). In highly turbid water, visibility may be reduced and a decrease of information exchange may occur. Fish may be able to counteract such constraint by increasing their activity. Such a response has been found in juvenile cod (Gadus morhua) where fish under turbid conditions kept their foraging rate constant by increasing their activity levels (Meager and Batty 2007). In the presence of predator cues, pike larvae (Esox lucius) hid within the vegetation less and exploited the open water more when under turbid conditions than when under clear water conditions (Lehtiniemi et al. 2005). However, African cichlid showed a marginally significant decrease in activity in turbid water when fish were reared in turbidity (Gray et al. 2012).
To investigate the influence of turbidity on social behaviour, we selected the Trinidadian guppy, Poecilia reticulata, which has become a classic study system in behavioural ecology and evolutionary biology over the last 50 years (Magurran 2005). The guppy is subject to high levels of turbidity in large parts of the Northern Mountain Range of Trinidad as a result of quarrying activities. After rainfall, runoff from quarries enters the rivers causing dramatic short-term increases in turbidity (KKB and RJGC pers. obs.). In our study, we examined changes of activity and social behaviours in guppies exposed to turbidity. To investigate the changes of social structure, we carried out generalized linear mixed model. The social dynamics of the fission-fusion process was analysed with a Markov chain approach. We hypothesize that increased turbidity has an effect on the activity of fish as well as on the association behaviour with conspecifics and social dynamics. We predict that activity of the fish will differ under clear and turbid water conditions. Further, we predict that group size will decrease and the frequency of fish being alone will increase in turbid water because encounter probabilities between individuals should be reduced in turbidity.
Methods
Study species
Guppies caught from the Northern Mountain Range of Trinidad, Arima River, were bred for about 20 generations in the laboratory. They were fed twice a day with TetraMin dry food. Water had a constant temperature of 24 °C, and diel illumination rhythm was at 12/12 h.
Experimental setup
One hundred fish (50 males, 2.2–2.8 cm; 50 females: 2.8–3.8 cm) were individually marked with fluorescent elastomer which are known to have no impact on fish behaviour (Croft and Krause 2004). Fish were divided into 10 groups of 10 fish (five males and five females) each and were kept for 12 days in 40 × 30 cm tanks to become familiar with each other (Griffiths and Magurran 1997). Subsequently, the groups were transferred to test tanks (100 × 100 × 20 cm with a grid of 10 × 10 cm squares placed below the tank to facilitate the recording of two-dimensional positions of individual fish) and left overnight for acclimation either in clear or in turbid water. The next day, each group of 10 fish was observed for 2 × 30 min. After observation, groups were transferred to test tanks with the opposite water quality treatment and left overnight for acclimation. Observations were conducted the next day. Half of the groups experienced turbidity first, and the other half experienced clear water first to control for treatment order. To facilitate individual recognition of fish during experiments from above even under turbid conditions, water depth was limited to 2 cm. Guppies often enter very shallow waters in the field (KKB and JK pers. obs.); therefore, such shallow water does not provide unusual conditions for them. Furthermore, pilot experiments showed that the fission-fusion dynamics of fish in tanks was consistent with that shown in the wild (see Wilson et al. 2014). Turbidity was created using 5 g of Kaolin (Amberger Kaolinwerke, Germany) to achieve a level of about 1000 nephelometric turbidity unit (NTU). We used Kaolin to turbid the water as it consists of little clay particles with a big surface that keeps the particles suspended for a long time (Meager et al. 2005). Turbidity levels dropped to 700 NTU during experiments but the optical range of the fish was consistently lower than 5 cm (pre-test showed that fish reacted towards other fish only within a 5-cm range). For comparison, turbidity levels in the clear water treatment were about 5–10 NTU. Similar NTU ranges, namely about 900–1000 NTU for turbid and about 7 NTU for clear water, were measured in natural guppy habitats at the Turure River, Trinidad. Turbidity was measured with Horiba Water quality checker U-53, Kyoto, Japan. To avoid sedimentation of Kaolin during the experiments, we gently stirred up the bottom of the tank in the evening, in the morning and before each observation period. We did so in both treatments to control for potential disturbance of fish.
Data collection
Each fish (in a group of ten) was individually tracked by direct observation for 2 × 3 min per treatment. The position of the focal fish on the grid and the identities of its neighbours were recorded every 10 s giving 2 × 18 data points per focal fish. We defined a focal fish’s neighbours as follows: first, all fish which were within four body lengths of the focal fish (which is a commonly used criterion for social associations, Krause and Ruxton 2002) and second, all fish which were within four body lengths of the above mentioned neighbours (if present). The 10-s duration and the observation time of 3 min were chosen because previous work indicated that this approach captures their social dynamics (Wilson et al. 2014).
The following response variables were calculated:
-
1.
Mean shoal size. We calculated the arithmetic mean of shoal size for each focal fish out of the number of its neighbours from 2 × 18 data points.
-
2.
Mean time alone. Out of the 2 × 18 data points for each focal fish, we recorded how many times a fish was observed alone, i.e. no other fish was located within four body lengths of the focal fish. From these counts, we calculated the arithmetic mean.
-
3.
Mean activity. If a fish changed its grid position (as measured by the squares under the tank) from one 10-s recording to the next, we defined this as a square crossing (regardless of the number of squares moved). We calculated the mean activity as the mean number of square crossings of each fish.
Statistical analysis
From the original 10 groups, one group had to be excluded because of incomplete data. Furthermore, two single male fish from separate groups had missing data or died before the first observation. Therefore, the subsequent analysis was carried out with 88 fish in nine groups. To evaluate whether social behaviour of guppies differed between the treatments and fish sexes, we calculated generalized linear mixed models (GLMM). Models were constructed with treatment and sex as fixed factors, and individual fish nested within groups as a random factor. All response variables were approximated as count data; therefore, we applied the Poisson family distribution with its canonical link function (Kachman 2000). Correlations between activity and solitary fish and activity and mean shoal size were calculated by Pearson’s r. All statistical calculations were performed by the function glmmPQL from MASS package (Venables and Ripley 2002) in R version 2.14.1 (R Core Team 2011) and Sigma Plot 12.0.
Markov chains
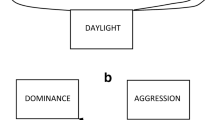
In order to better understand the underlying dynamics of the patterns of social behaviour and to investigate differences between the two treatments, we used a fission-fusion model based on Markov chains. Following Wilson et al. (2014), we constructed two variants (with different levels of detail) that describe the social behaviour common to all focal individuals as sequences of ‘behavioural states’. In the simple model (Fig. 1a), a focal fish can either be social (with a conspecific) denoted by i or alone (no conspecific within four body lengths) denoted by x. By regarding i and x as states of a first-order Markov chain, the transition probabilities between these states can be estimated from the data points in our observations (see supplementary material and Wilson et al. 2014 for more details). Our simple model then predicts the distributions of the lengths of phases of social contact and of being alone. In the more detailed model (Fig. 1b), the identities of the neighbours of the focal fish are also taken into account, i.e. the state i of the simple model is split up into k states i 1 , …, i k , where k is the number of potential neighbours and the state i j indicates that the focal fish is currently associated with individual j. The more detailed model has k + 1 states, one for each potential neighbour plus the state x. In addition to the simple model, the more detailed model predicts the lengths of contact phases with the same neighbour.
Markov chain models of social behaviour in the guppy. All probabilities are estimates for the clear water treatment. a The simple model. A fish can either be social (with a conspecific) denoted by i or alone (no conspecific within four body lengths) denoted by x. p 2 and p 3 indicate the respective probabilities for changing states and q 2 = 1 − p 2 and q 3 = 1 − p 3 those for retaining current states. b The more detailed model. In this model, state i (dotted circle) is split up into as many states as there are potential neighbours, and the focal fish is in state i g, if individual g is its nearest neighbour. In our model, the dynamics do not depend on the neighbour’s identity and is the same for each state i g. Therefore, for the sake of clarity, the figure only shows the state i 1. A focal fish stays with its current nearest neighbour with probability q 1. When the contact with this neighbour ends, it decides to be alone with probability p 2 or switches to a different neighbour with probability p s . If there are k potential neighbours other than i 1, the probability of choosing a particular one is p s /k
In a first-order Markov chain, the next state only depends on the current state. In our case, this means, for example, that the decision of a focal fish to leave its current neighbour and to swim around alone (only) depends on its current state. Wilson et al. (2014) showed that this is a valid assumption for female guppies observed in the wild.
In the supplementary material (Figs S1 and S2), it is demonstrated that the simple and the more detailed Markov models capture the dynamics of the state changes in our observations and that the observed lengths of social contact, of contact with a particular nearest neighbour and of being alone are well approximated by the model predictions.
The model probabilities are estimated as simple proportions (see supplementary material for more details). This allows us to investigate differences between the treatments regarding the lengths of social contact phases, of contact phases with the same neighbour and of being alone, where the length is measured as multiples of time intervals of 10 s. We used the two-sample test for equality of proportions with continuity correction in R for our tests.
Results
The mean shoal size was significantly greater in clear than in turbid water (Table 1; Fig. 2a), and fish in turbid water were significantly more often alone than fish in clear water (Table 1; Fig. 2b). Furthermore, fish in clear water were significantly more active than fish in turbid water (Table 1; Fig. 3). Males and females differed significantly in mean activity across both treatments with males showing higher levels of activity (Table 1). In the following, we investigated the potential influence of activity on social behaviour. We found no significant correlation between activity and mean shoal size (Pearson correlation, r = −0.364, p = 0.335) and activity and mean time alone in turbid water (r = 0.293, p = 0.445). Furthermore, no significant correlation was found between activity and mean shoal size (r = −0.488, p = 0.182) and activity and time spent alone in clear water (r = 0.513, p = 0.158).
For each treatment (clear and turbid water), the Markov model probabilities were computed across all groups (Table 2). The probability p s of switching the nearest neighbour (0.52 in clear water and 0.43 in turbid water) and the probability p 3 of choosing some neighbour after swimming around alone (0.53 in clear water and 0.38 in turbid water) differed between the treatments. Both probabilities were significantly greater in clear water than in turbid water (two-sample test for equality of proportions with continuity correction, both p values <0.001). The probability of ending social time (i.e., leaving any neighbour) did not differ significantly between the treatments (Table 2). This means that both the contact phases with a particular nearest neighbour and the phases of being alone were longer in turbid water than in clear water, while the lengths of general social contact did not differ.
The reciprocal values of the model probabilities specify the mean number of data points over which a focal fish will retain a state. Multiplying this number by 10 s (the time between two data points) yields the mean time spent in each state. The mean length of social contact in clear water was about 47 s and in turbid water about 50 s. Social contacts included 3.4 different nearest neighbours in clear water and 3.2 in turbid water on average. The mean length of contact with a particular nearest neighbour was 14 s in clear water and 16 s in turbid water.
Discussion
Our results show that turbidity leads to a strong behavioural response, which has potentially important implications for information transfer and predator recognition. Furthermore, females and males differed in activity levels.
Altered social behaviour in turbid water may be a result of a sensory constraint in that fish are less able to detect conspecifics visually. Therefore, individuals cling longer to a particular neighbour or remain longer alone after leaving their neighbour, leading to smaller group sizes. Smaller group sizes and less frequent switching of the nearest neighbours suggest that guppies under turbid conditions might have less access to public information and fewer opportunities for social learning about food locations (Laland and Williams 1998; Bates and Chappell 2002) and identities of predators (Magurran and Higham 1988). Lower activity rates suggest that fish conduct less general sampling to keep up to date with their surroundings (Webster and Laland 2011).
Smaller group size and longer time alone could also be interpreted as an adaptive response to changed environmental conditions. Several studies suggest that turbidity might reduce the perceived risk of predation in fish from visually hunting predators (Gregory and Northcote 1993; Maes et al. 1998). In studies of Pacific salmon (Oncorhynchus tshawytscha), it was shown that fish perceived less predation risk in turbidity and foraged more often in open water in turbidity than in clear water (Gregory 1993; Gregory and Levings 1998). Furthermore, Fischer and Frommen (2013) showed that in turbidity, the three-spined stickleback (Gasterosteus aculeatus) lost its preference for bigger shoals. Smaller group size and more time alone in turbidity could be therefore an adjustment to reduced perceived predation risk (Abrahams and Kattenfeld 1997). In addition, competition for food is reduced in smaller groups (Hoare et al. 2004).With the Markov chain approach, we were able to shed more light on the dynamics behind the observation that fish are more often alone in turbid water than in clear water. Obviously, the fish did not increase the number of phases of being alone but rather their lengths, supporting our first hypothesis that turbidity might be experienced as a constraint.
It has been proposed that under turbid conditions, predator-prey interactions are primarily characterized by the encounter rate of predator and prey (Abrahams and Kattenfeld 1997). In clear water, prey can watch for predators and use anti-predator behaviour for protection whereas the only chance under high turbidity and short recognition distances is to avoid encounter. Therefore, decreased activity interpreted as an active response to turbidity would allow fish to reduce actively the encounter probability with predators. A supporting result was found in sticklebacks where individuals switched less often between shoals in turbid water (Fischer and Frommen 2013). Generally, males were found to be more active than females. Since females are receptive approximately every 4 weeks, a reasonable interpretation might be that males are more active to find new mates (Magurran 2005).
Fish showed a strong behavioural response to turbidity in our study. Such behavioural flexibility might be used to minimize negative effects in order to cope with environmental change (Wright et al. 2010; Sih et al. 2011; Tuomainen and Candolin 2011). In fact, a complex behavioural and cognitive repertoire is known to influence the probability of colonization (Huizinga et al. 2009; Bisazza 2011) and the guppy is probably the world’s most widely distributed tropical fish (Magurran 2005). However, regular occurrence of turbidity implies regular disturbance of the social dynamics of fish and they might learn to better cope with it and develop different strategies (Sih 2013). For example, guppy males are known to be selected for colourful pigments by females (Houde 1997; Magurran 2005). Under regular occurrence of turbidity, females may switch to another sense for mate choice, for example olfaction, reducing visual selection pressure as it has been shown in sticklebacks (Heuschele et al. 2009). Cichlid flocks in the great lakes of Africa have also been strongly affected by turbidity within the last decades. Cichlids showed a strong radiation, where species were sexually isolated by visual mate choice. With increasing and enduring turbidity caused by algae blooms due to eutrophication, species radiation collapsed. Cichlid females were not able to differentiate between different colour morphs of males anymore (Seehausen et al. 1997). Here, females apparently, were not able to switch to another sense to maintain speciation.
However, not only the visual sense of fishes is impacted by anthropogenic influences. Road traffic, for example, masked females perception of male signals in the grey tree frog, Hyla chrysoscelis, (Bee and Swanson 2007) or provoked altered singing frequencies in the grey shrike-thrush, Colluricincla harmonica (Parris and Schneider 2009). In bottlenose dolphins, Tursiops sp., communication range was found to be reduced by vessel noise (Jensen et al. 2009).
With our study, it is not possible to disentangle whether we observed an active behavioural change to turbidity or whether it acted as a constraint. However, Trinidad is increasingly affected by quarries and deforestation (Agard et al. 2004) and changes in social behaviour of affected fish should be closely monitored. Even more when we take into account that turbidity induced by quarry runoffs has many other effects like periodically higher water levels and stronger currents associated with rainfall. Further systematic studies are urgently needed to understand how regular occurring environmental changes affect the behaviour, ecology and evolution of guppy populations, which have been a focus point of scientific attention for more than 70 years (Houde 1997). This will also require novel methods to assess changes in behaviour as a result of disturbance. In our study, we used a first-order Markov chain (MC) as an individual-based model to approximate the social dynamics of guppies. A MC represents the simplest possible individual-based model and provided a good fit to our data. There is a twofold benefit of modelling fish social behaviour in this way. Firstly, the MC captured the dynamics of the social behaviour unlike most network approaches which are based on accumulating observations on such a coarse temporal scale that the dynamics is lost (Sundaresan et al. 2007; Henzi et al. 2009). Second, we can circumvent some of the problems associated with conventional social network comparisons because MCs avoid the confounding influence of differences in network density (Croft et al. 2008). The fact that the MC modelling approach worked for guppy social behaviour in both the lab (this study) and the field (Wilson et al. 2014) makes it a promising candidate for future studies that extend to other contexts (than shoaling behaviour) and other species.
References
Abrahams MV, Kattenfeld MG (1997) The role of turbidity as a constraint on predator-prey interactions in aquatic environments. Behav Ecol Sociobiol 40:169–174
Agard J, Alkins-Koo M, Cropper A et al (2004) Report of an assessment of the Northern Range of Trinidad and Tobago
Ardjosoediro I, Ramnarine IW (2002) The influence of turbidity on growth, feed conversion and survivorship of the Jamaica red tilapia strain. Aquaculture 212:159–165
Bates L, Chappell J (2002) Inhibition of optimal behavior by social transmission in the guppy depends on shoaling. Behav Ecol 13:827–831
Bee MA, Swanson EM (2007) Auditory masking of anuran advertisement calls by road traffic noise. Anim Behav 74:1765–1776
Bisazza A (2011) Cognition. In: Evans JP, Pilastro A, Schlupp I (eds) Ecology and evolution of livebearing fishes. University of Chicago Press Chicago, IL, pp 165–173
Carpenter SR, Cole JJ, Hodgson JR, Kitchell JF, Pace ML, Bade D, Cottingham KL, Essington TE, Houser JN, Schindler DE (2001) Trophic cascades, nutrients, and lake productivity: whole-lake experiments. Ecol Monogr 71:163–186
Chapman BB, Morrell LJ, Krause J (2009) Plasticity in male courtship behaviour as a function of light intensity in guppies. Behav Ecol Sociobiol 63:1757–1763
Chapman BB, Morrell LJ, Tosh CR, Krause J (2010) Behavioural consequences of sensory plasticity in guppies. Proc R Soc Lond B 277:1395–1401
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE (2009) Controlling eutrophication: nitrogen and phosphorus. Science 323:1014–1015
Croft D, Krause M (2004) Is sexual segregation in the guppy, Poecilia reticulata, consistent with the predation risk hypothesis? Environ Biol Fish 71:127–133
Croft DP, James R, Krause J (2008) Exploring animal social networks. Princeton Univ Press, Princeton
Day RL, MacDonald T, Brown C, Laland KN, Reader SM (2001) Interactions between shoal size and conformity in guppy social foraging. Anim Behav 62:917–925
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Am Nat S125–S153
Fischer S, Frommen JG (2013) Eutrophication alters social preferences in three-spined sticklebacks (Gasterosteus aculeatus). Behav Ecol Sociobiol 67:293–299
Galas J (2003) Limnological study on a lake formed in a limestone quarry (Kraków, Poland). I. Water chemistry. Pol J Environ Stud 12:297–300
Gray SM, McDonnell LH, Cinouemani FG, Chapman LJ (2012) As clear as mud: turbidity induces behavioral changes in the African cichlid Pseudocrenilabrus multicolor. Curr Zool 58:143–154
Gregory RS (1993) Effect of turbidity on the predator avoidance behaviour of juvenile Chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 50:241–246
Gregory RS, Levings CD (1998) Turbidity reduces predation on migrating juvenile Pacific salmon. Trans Am Fish Soc 127:275–285
Gregory RS, Northcote TG (1993) Surface, planktonic, and benthic foraging by juvenile Chinook salmon (Oncorhynchus tshawytscha) in turbid laboratory conditions. Can J Fish Aquat Sci 50:233–240
Griffiths SW, Magurran AE (1997) Familiarity in schooling fish: how long does it take to acquire? Anim Behav 53:945–949
Griffiths SW, Magurran AE (1999) Schooling decisions in guppies (Poecilia reticulata) are based on familiarity rather than kin recognition by phenotype matching. Behav Ecol Sociobiol 45:437–443
Guthrie DM, Muntz WRA (1993) Role of vision in fish behaviour. In: Pitcher TJ (ed) Behaviour of teleost fishes, 2nd edn. Chapman & Hall, London, pp 89–128
Hain TJA, Neff BD (2007) Multiple paternity and kin recognition mechanisms in a guppy population. Mol Ecol 16:3938–3946
Henzi SP, Lusseau D, Weingrill T, Schaik CP, Barrett L (2009) Cyclicity in the structure of female baboon social networks. Behav Ecol Sociobiol 63:1015–1021
Heuschele J, Mannerla M, Gienapp P, Candolin U (2009) Environment-dependent use of mate choice cues in sticklebacks. Behav Ecol 20:1223–1227
Hoare D, Krause J, Peuhkuri N, Godin J-GJ (2000) Body size and shoaling in fish. J Fish Biol 57:1351–1366
Hoare D, Couzin I, Godin J-GJ, Krause J (2004) Context-dependent group size choice in fish. Anim Behav 67:155–164
Houde AE (1997) Sex, color, and mate choice in guppies. Princeton University Press, Princeton
Huizinga M, Ghalambor CK, Reznick DN (2009) The genetic and environmental basis of adaptive differences in shoaling behaviour among populations of Trinidadian guppies, Poecilia reticulata. J Evol Biol 22:1860–1866
Jensen F, Bejder L, Wahlberg M, Aguilar Soto N, Johnson M, Madsen PT (2009) Vessel noise effects on delphinid communication. Mar Ecol-Prog Ser 395:161–175
Kachman SD (2000) An introduction to generalized linear mixed models. In: Proc. of a Symposium at the Organizational Meeting for a NCR Coordinating Committee on “Implementation Strategies for National Beef Cattle Evaluation”, Athens, Greece, pp 59-73
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, New York
Laland KN, Williams K (1997) Shoaling generates social learning of foraging information in guppies. Anim Behav 53:1161–1169
Laland KN, Williams K (1998) Social transmission of maladaptive information in the guppy. Behav Ecol 9:493–499
Lehtiniemi M, Engström-Öst J, Viitasalo M (2005) Turbidity decreases anti-predator behaviour in pike larvae, Esox lucius. Environ Biol Fish 73:1–8
Likens GE, Bormann FH, Johnson NM, Fisher DW, Pierce RS (1970) Effects of forest cutting and herbicide treatment on nutrient budgets in the Hubbard Brook watershed-ecosystem. Ecol Monogr 40:23–47
Maes J, Taillieu A, Van Damme PA, Cottenie K, Ollevier F (1998) Seasonal patterns in the fish and crustacean community of a turbid temperate estuary (Zeeschelde Estuary, Belgium). Estuar Coast Shelf Sci 47:143–151
Magurran AE (2005) Evolutionary ecology: the Trinidadian guppy. Oxford University Press, Oxford
Magurran AE, Higham A (1988) Information transfer across fish shoals under predator threat. Ethology 78:153–158
Maya K, Santhosh V, Padmalal D, Kumar SRA (2012) Impact of mining and quarrying in Muvattupuzha river basin, Kerala - An overview on its environmental effects. Bonfring Int J Ind Eng Manag Sci 2:36–40
Meager JJ, Batty RS (2007) Effects of turbidity on the spontaneous and prey-searching activity of juvenile Atlantic cod (Gadus morhua). Philos T Roy Soc B 362:2123–2130
Meager JJ, Solbakken T, Utne-Palm AC, Oen T (2005) Effects of turbidity on the reactive distance, search time, and foraging success of juvenile Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 62:1978–1984
Parris KM, Schneider A (2009) Impacts of traffic noise and traffic volume on birds of roadside habitats. Ecol Soc 14:29
R Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org/
Scheffer M, Carpenter S, Foley JA, Folke C, Walkerk B (2001) Catastrophic shifts in ecosystems. Nature 413:591–596
Seehausen O, van Alphen JJ, Witte F (1997) Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277:1808–1811
Sih A (2013) Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim Behav 85:1077–1088
Sih A, Ferrari MCO, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387
Sluijs I, Gray SM, Amorim MCP et al (2010) Communication in troubled waters: responses of fish communication systems to changing environments. Evol Ecol 25:623–640
Sundaresan SR, Fischhoff IR, Dushoff J, Rubenstein DI (2007) Network metrics reveal differences in social organization between two fission–fusion species, Grevy’s zebra and onager. Oecologia 151:140–149
Tockner K, Pusch M, Borchardt D, Lorang MS (2010) Multiple stressors in coupled river–floodplain ecosystems. Freshw Biol 55:135–151
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657
Utne-Palm A (2002) Visual feeding of fish in a turbid environment: physical and behavioural aspects. Mar Freshw Behav Physiol 35:111–128
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, Berlin
Webster MM, Laland KN (2011) Innovation and social learning. In: Evans JP, Pilastro A, Schlupp I (eds) Ecology and evolution of livebearing fishes. University of Chicago Press, Chicago, IL, pp 155–164
Wilson ADM, Krause S, James R, Croft DP, Ramnarine IW, Borner KK, Clement RJG, Krause J (2014) Dynamic social networks in guppies (Poecilia reticulata). Sociobiol, Behav Ecol
Wright TF, Eberhard JR, Hobson EA, Avery ML, Russello MA (2010) Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethol Ecol Evol 22:393–404
Acknowledgments
KKB would like to thank Romain JG Clément for the help with the turbidity measurements in the field, Ralf HJM Kurvers for statistical discussion and the participants of the workshop “Scientific writing” at the Leibniz-Institute of Freshwater Ecology and Inland Fisheries for helpful discussions at an early stage of manuscript. Further, we thank three anonymous referees for the helpful comments. This study was enabled by a scholarship provided by Elsa-Neumann-Stiftung.
Conflict of interest
The authors declare that they have no conflict of interest
Compliance with ethical standards
This research was performed in accordance with the laws, guidelines and ethical standards of the country in which they were performed (Trinidad).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Lindström
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 53.2 kb)
Rights and permissions
About this article
Cite this article
Borner, K.K., Krause, S., Mehner, T. et al. Turbidity affects social dynamics in Trinidadian guppies. Behav Ecol Sociobiol 69, 645–651 (2015). https://doi.org/10.1007/s00265-015-1875-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1875-3