Abstract
Division of labour is the hallmark of the success of many social animals. It may be especially important with regard to waste management because waste often contains pathogens or hazardous toxins and worker specialisation can reduce the number of group members exposed to it. Here we examine waste management in a fungus-farming, leaf-cutting ant, Acromyrmex echinatior, in which waste management is necessary to protect their vulnerable fungal crop. By marking ants with task-specific paint colours, we found clear division of labour between workers that engage in waste management and those that forage, at least during the fine timescale of the 3-day marking period. This division of labour was influenced by both age and size, with waste management workers tending to be smaller and younger than foragers. The role of preventing contaminated ants from entering the colony was fulfilled mainly by medium-sized workers. When the level of waste was experimentally increased, most of the ants that responded to remove the waste were workers previously engaged in tasks inside the nest rather than external waste workers or foragers. These responding workers tended to be young and medium-sized. Surprisingly, the responding ants were subsequently able to revert back to working within the fungus garden, but the probability of them doing so depended on their age and the length of time they were exposed to waste. The results demonstrate the importance of division of labour with regard to waste management in A. echinatior and show that this is adaptable to changing needs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The efficient organisation of work enhances the success of social organisms. In many social insects, this involves division of labour, with individuals carrying out different tasks and thereby potentially improving overall colony efficiency (Oster and Wilson 1978; but see Dornhaus 2008). The probability of an individual engaging in a particular task may be affected by a variety of factors that can include age, location, morphological caste, genotype and experience (Hölldobler and Wilson 1990; Winston 1995; Ravary et al. 2007; Smith et al. 2008). The strength of stimuli for tasks will vary both spatially and temporally, and will thus be experienced differently by different individuals. A number of models have been proposed to explain the emergence of division of labour in social insect colonies (Gordon 1996; Beshers and Fewell 2001; Seid and Traniello 2006), many of which are based on individuals having different stimulus-response thresholds (Robinson 1992; Bonabeau et al. 1996; Theraulaz et al. 1998; Beshers et al. 1999; Bonabeau and Theraulaz 1999). Increases in stimuli then result in more individuals engaging in the relevant task, with those with a lower response threshold for the stimulus engaging at a lower level of stimulus. There is now good evidence from a range of social insects that workers do in fact differ in their response thresholds and thus their likelihood of carrying out particular tasks, depending on their morphological caste, age, development, experience or genotype (Detrain and Pasteels 1991; Pankiw and Page 1999; Beshers and Fewell 2001; Weidenmuller 2004; Ravary et al. 2007; Jeanson et al. 2008; Smith et al. 2008; Uribe-Rubio et al. 2008). The result is that the profile of workers engaged in a task depends on the stimulus, with an increase in stimulus resulting in workers that might not normally be involved in the relevant task switching to engage in it (Robinson 1992; Fewell and Bertram 1999; Beshers and Fewell 2001; Breed et al. 2002; Chapman et al. 2007; Hughes and Boomsma 2007).
Division of labour is likely to be particularly important with regard to waste management. All societies generate waste, including excreta, food refuse and deceased group members, and its effective management is essential for the society to be successful (Jackson and Hart 2009). Waste can often be hazardous, containing pathogens or toxic compounds (Schmid-Hempel 1998; Bot et al. 2001; Hughes et al. 2004; Brown et al. 2006); division of labour can reduce the proportion of the group exposed to infection by minimising the number of individuals that contact hazardous material. Such division of labour between waste management and other tasks has accordingly been recorded in a diversity of social insects, from those with complex social systems such as honeybees and leaf-cutting ants to those with more simple societies (Michener 1974; Hölldobler and Wilson 1990; Benton and Foster 1992; Winston 1995; Gordon and Mehdiabadi 1999; Breed et al. 2002).
For most social insects, therefore, waste management is necessary to protect the vulnerable brood and queen, as well as adult workers. However, leaf-cutting ants (Atta and Acromyrmex) have a further incentive to practice waste management. They are part of a complex symbiosis involving at least five parties (Currie et al. 1999b; Little and Currie 2008; Mueller et al. 2008), and are reliant upon a mutualistic fungus which they cultivate for food on the vegetation they harvest. Due to its long coevolutionary history with the ants (Currie et al. 2003; Mikheyev et al. 2008), this cultivated fungus is vulnerable to competitors and parasites, particularly the specialised fungal parasite Escovopsis which can destroy the fungal crop and result in colony death (Currie et al. 1999a; Currie 2001). Waste material frequently contains Escovopsis as well as other harmful microorganisms (Bot et al. 2001; Currie and Stuart 2001; Hughes et al. 2004; Brown et al. 2006), so to reduce the risk of contamination, leaf-cutting ants need to deal efficiently with waste. Accordingly, leaf-cutting ants deposit waste either in deep underground chambers or in external piles downhill and downwind of the nest (Bot et al. 2001; Hart and Ratnieks 2002; Ballari et al. 2007). The task of waste removal may be partitioned, with workers from the fungus gardens depositing waste in a cache from which it is transported to the waste piles by dedicated waste management workers (Hart and Ratnieks 2001). There can also be division of labour: waste management workers are distinct from foragers in Atta colombica and Atta cephalotes (Hart and Ratnieks 2001, 2002), are older in Acromyrmex subterraneus (Camargo et al. 2007) and smaller than foragers in Atta sexdens and Acromyrmex lobicornis (Wilson 1980b; Ballari et al. 2007). It appears that this division of labour is reinforced by treating aggressively waste management workers that try to enter the fungus gardens (Hart and Ratnieks 2001; Ballari et al. 2007). Older workers tend to be more aggressive in many social insects and aggression at nest entrances in Atta leaf-cutting ants is mediated by small workers (Hughes and Goulson 2001), but whether size or age affect aggression towards individuals contaminated with waste is not known.
Here we examine waste management of the leaf-cutting ant, Acromyrmex echinatior. In experiment 1, we first confirmed that division of labour was present between waste management and foraging, as well as determining the size and age of ants normally engaged in working with waste. In experiment 2, we then examined whether ants contaminated by waste are prevented by nestmate aggression from entering the fungus garden, and which size and age of ants were responsible for this. Finally, in experiment 3 we tested the stimulus-response threshold model by artificially increasing the stimulus for waste management to establish whether the number of waste workers within the colony was fixed, or whether, and which, other workers would aid in waste removal. We followed this up by determining whether ants that responded to work with the added waste are then able to revert back to their original roles.
Materials and methods
Five colonies of A. echinatior were used (Ae213, Ae216, Ae310, Ae312 and Ae321) which had been collected from Gamboa, Panama, between 2003 and 2006. Colonies were maintained at 80 ± 5% relative humidity, 26 ± 2°C and 12:12 light/dark cycle, fed twice weekly on privet leaves (Ligustrum spp.) and provided with water ad libitum. Colonies were kept in plastic boxes (height 17 cm, width 36 cm, depth 54 cm) and provided with a pot in one corner in which they deposited waste. Fungus gardens were contained within inverted plastic beakers. At the time of the experiments, colonies Ae310 and Ae321 had c. 0.5 l of fungus garden, colonies Ae216 and Ae312 had c. 1 l, and colony Ae213 had c. 1.5 l.
Experiment 1: division of labour between waste management and foraging
For four colonies (Ae213, Ae216, Ae310 and Ae312), the ants engaged in waste management and foraging were marked over the course of 3 days with task-specific coloured paint. Ants were removed from colonies, cooled on ice and marked with paint. The ants were then kept in individual pots for 5 min after marking to confirm that they behaved normally before being replaced in their colony. Previous studies (Julian and Cahan 1999; Walker and Hughes 2009), as well as preliminary trials, confirmed that such paint marking does not affect behaviour. This procedure was then repeated for 8 h per day for 3 days (see Table 1 for the total numbers marked). The number, size (small, medium or large) and age (young or old) of workers marked for each task were recorded. ‘Young’ ants were those whose cuticles were medium brown in colour, while ‘old’ ants were dark brown. No light brown or callow individuals were included in the experiment. The size of ants was estimated by eye (Wilson 1980b; Hughes et al. 2003), and ants were classed as small, medium or large if their head widths were ≤1.3, 1.4–1.8 and ≥1.9 mm, respectively. In addition, the head widths were measured for 50 waste workers and 50 foragers, randomly selected from colony Ae312.
Experiment 2: behavioural response to ants contaminated with waste
Young, medium-sized workers ants were collected from the fungus gardens of four colonies (Ae213, Ae216, Ae310 and Ae321). Test ants were exposed to waste particles (not including pieces of leaf or ant cadavers) from their colony in 1.5-ml Eppendorf tubes for 5 s or for 10, 20 or 30 min (ten ants per exposure time per colony). Control ants were treated similarly, but in empty Eppendorf tubes (again ten ants per exposure time per colony). In total, 40 test and 40 control ants were observed for each of the four colonies. After the exposure periods, the ants were cooled at −20°C for 20 s and placed back into their colony close to the entrance of the fungus garden. Ants were taken from their colonies in batches of 20 individuals, of which ten were exposed to waste and ten used as controls. The ants were reintroduced individually, with colonies being left for 1 h in between batches. We recorded for each ant whether it entered the fungus garden without interacting with any nestmates, or in its first interaction with a nestmate was treated aggressively (nestmate bites or chases focal ant, or opens mandibles in alarm; Hughes and Goulson 2001; Hughes et al. 2001), or non-aggressively (nestmate antennates focal ant). For the ants exposed to waste for 5 s or 10 min, we also examined if ant size influences the behavioural response by recording the number of ants of each size class (small, medium or large) within 5 cm of the location where the focal ant was placed and the proportions of these ants showing aggression.
Experiment 3: response to increased stimulus for waste management
After all waste workers and foragers were marked as outlined above, a 5-ml volume of waste particles from the colony’s waste pile was placed immediately next to the entrance of the fungus garden. The position was such that the added waste was encountered by foragers and waste workers as well as the other ants present near the nest entrance. During the following 10 min, all individuals seen transporting this waste to the waste pile were collected. Immediately after this 10-min period, a snapshot count was made of the ants working with the added waste. All collected ants were then marked with paint, using a different colour to before, and their size, age and original task [waste management, forager or internal (unmarked)] recorded. After marking, the ants were placed on ice for 20 s and then replaced in their colony. The ant marking and data collection for a colony was completed within a 20-min period. This procedure was then repeated for 6 h. The colonies were then left for 24 h to allow individuals to revert back to their previous tasks if they were able to do so. After this time had elapsed, the colony’s waste area was observed to determine whether any of the newly marked ants were still dealing with waste. Such ants were removed from the colony over the course of 30 min. The numbers, original task, size and age of newly marked ants engaged in waste management were then recorded to determine what effect previous task, age or size had on the probability of reverting.
Statistical analysis
G tests for heterogeneity were used to test whether size categories or ages differed in their representation in the waste management workers or foragers, and whether size, age or task of origin affected the likelihood of a worker responding to deal with the experimentally added waste or their likelihood of remaining a waste management worker 24 h later (Sokal and Rohlf 1995). General linear models were used to investigate the results of the behavioural response experiment. Separate models examined the effects of exposure time and treatment on: whether an ant entered a colony immediately, was aggressed or treated non-aggressively; the log(x + 1) transformed numbers of ants of different sizes which interacted with the focal ant; the arcsin transformed proportions of interactions by ants of different sizes which were aggressive or non-aggressive.
Results
Experiment 1: division of labour between waste management and foraging
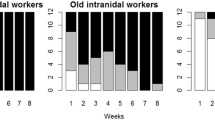
All colonies showed clear division of labour between waste management and foraging, at least over short time spans. During the 3-day marking period, workers seen (and marked) engaged in waste management were afterwards observed almost exclusively working with waste while workers seen (and marked) foraging were almost never seen working with waste. Out of 1,522 waste workers and 1,325 foragers that were marked, only three were observed to switch from foraging to waste management or vice versa. This further supports the observation that the paint-marking procedure or paint has no effect on behaviour. In colony Ae312 where individuals were measured, the workers engaged in waste management were significantly smaller than those engaged in foraging (1.49 ± 0.05 vs 2 ± 0.04 mm head width respectively; Mann–Whitney U test—U = 277, N = 101, P < 0.0001). In all four colonies, the waste management workers differed significantly from foragers in the representation of the small, medium and large worker categories (Ae213—G Het = 41.9, df = 2, P < 0.0001; Ae216—G Het = 149, df = 2, P < 0.0001; Ae312—G Het = 326, df = 2, P < 0.0001; Ae321—G Het = 34, df = 2, P < 0.0001). The majority of foragers were large, with most of the remainder being medium-sized and virtually none small (Fig. 1a). In contrast, most waste management workers were medium-sized, with many being small and only few large (Fig. 1a). In colonies Ae213 (G adj = 168, df = 1, P < 0.0001) and Ae312 (G adj = 93.4, df = 1, P < 0.0001), waste management workers also differed significantly from foragers in that a greater proportion of them were young (Fig. 1b). However, there was no difference in age between waste management workers and foragers in colony Ae216 (G adj = 3.9, df = 1, P = 0.142; Fig. 1b).
Division of labour in the four colonies (Ae213, Ae216, Ae312 and Ae321) during experiment 1. The proportion of ants engaged in waste management or foraging which were: a small (black), medium (grey) or large (white) in size, and b ‘young’ (white) or ‘old’ (black). The numbers of ants recorded are given above the columns. Waste management workers differed significantly from foragers at P < 0.05 in the frequencies of the three sizes of worker in all four colonies, and in the frequencies of the two ages in colonies Ae213 and Ae312 but not Ae216. The ages of ants were not recorded for colony Ae321
Experiment 2: behavioural response to ants contaminated with waste
Very few of the ants exposed to waste entered the fungus garden immediately without interacting first with a nestmate (Fig. 2a). Significantly more of the control ants did so, with the number increasing with the length of time the control ant had been kept isolated from its colony (treatment × exposure time interaction—F 3,24 = 9.59, P = 0.0002). There was also a significant interaction between treatment and exposure/isolation time on the number of introduced ants which were treated aggressively (F 7,24 = 13.6, P < 0.0001). Very few of the control ants were aggressed, regardless of the length of time they were kept isolated (Fig. 2c). Substantially more of the ants exposed to waste were aggressed, with most ants exposed to waste for 20 or 30 min being treated aggressively compared to only about half of the ants exposed to waste for 5 s or 10 min (Fig. 2c). Ants which were exposed for 10 min, and which were not treated aggressively, tended to be intensively allogroomed by their nestmates.
The three sizes of worker differed significantly in their representation in the general area (F 2,69 = 112, P < 0.0001), with 67 ± 2% of workers being medium-sized while large (14 ± 1%) and small (19 ± 2%) workers were less frequent. Medium-sized workers were also the main group to interact with the focal ant (50 ± 4% of ants, compared with 27 ± 4% for large and 23 ± 5% for small workers; F 2,54 = 76.6, P < 0.0001), regardless of treatment or exposure time (both main effects, and all interactions P > 0.05). The likelihood of an ant being aggressive towards the focal ant depended significantly upon the size of the ant responding (F 2,40 = 3.73, P = 0.033), with small workers being more, and large workers less aggressive towards the focal ant (Fig. 3).
Experiment 3: response to increased stimulus for waste management
Following the placement of the additional waste in the colonies, a variety of workers engaged in moving it to the waste pile. These ants did not show alarm behaviour [gaping mandibles, rapid movement, looping runs (Hughes and Goulson 2001; Hughes et al. 2001)]. In all four colonies, there was a significant effect of an ant’s original task on its likelihood of dealing with the additional waste (Ae213—G Het = 82.8, df = 2, P < 0.0001; Ae216—G Het = 67.8, df = 2, P < 0.0001; Ae312—G Het = 15.9, df = 2, P = 0.0004; Ae321—G Het = 34, df = 2, P < 0.0001). Many internal workers, but vey few foragers, dealt with the added waste (Fig. 4a). Medium-sized ants were the most common size to deal with the waste in all four colonies (Ae213—G Het = 81.6, df = 2, P < 0.0001; Ae216—G Het = 55.1, df = 2, P < 0.0001; Ae312—G Het = 78.3, df = 2, P < 0.0001; Ae321—G Het = 19.3, df = 2, P < 0.0001; Fig. 4b). The effect of age was also consistent across colonies, with more young ants responding than old ants (Ae213—G Het = 14.5, df = 1, P = 0.0007; Ae216—G Het = 7.6, df = 1, P = 0.022; Ae312—G Het = 5.24, df = 1, P = 0.073; Ae321—G Het = 11.9, df = 1, P = 0.003; Fig. 4c). The number responding decreased over time and ants that responded continued working with the waste until it had been completely removed.
Proportions of workers dealing with the additional waste in experiment 3 from the four experimental colonies which were: a previously engaged in waste management (black), internal work (grey) or foraging (clear); b small (black), medium-sized (grey) or large (clear); c ‘young’ (black) or ‘old’ (grey). The numbers of ants counted are given above the columns
After 24 h, most ants that had dealt with the additional waste had reverted back to their original task, with only a few still engaged in waste management (Fig. 5). The size and age of ants influenced whether they reverted or remained as waste management workers in colonies Ae213 (G Het = 41, df = 4, P < 0.0001) and Ae216 (G Het = 12.5, df = 4, P = 0.014), but not colony Ae312 (G Het = 6.21, df = 4, P = 0.184). In colony Ae213, young, medium-sized workers were more likely to revert whereas old, large workers were more likely to remain as waste management workers (Fig. 5). In colony Ae216, though, it was young, medium-sized workers that were less likely to revert while old, medium-sized workers were less likely to stay as waste management workers (Fig. 5). The effect of size and age on likelihood of reversion was therefore somewhat variable across colonies, with the only general pattern in the data being that old, large workers were most likely to remain as waste management workers (Fig. 5).
Proportions of the workers in colonies Ae213, Ae216 and Ae312 in experiment 3 that switched to deal with the added waste and which then either reverted back to their original task (white) or stayed as waste management workers (black) 24 h after the additional waste had been dealt with. Data are separated into workers that were a ‘young’ or b ‘old’, and which were small, medium or large in size. The numbers of ants counted are given above the columns and circled when they were significant at P < 0.05 in individual G tests within that colony. No data on reversion was collected for colony Ae321
Discussion
In experiment 1, we found that under normal conditions, and at least for the 3-day duration of the marking period, certain workers specialised in waste management and did not engage in other tasks, while foragers similarly specialised in their task and did not engage in waste management. Both size and age affected this division of labour. Our results therefore show division of labour between waste management and foraging in A. echinatior colonies. This is in accord with previous studies which have similarly shown division of labour between waste management and foraging in leaf-cutting ants and other social insects (Hölldobler and Wilson 1990; Gordon and Mehdiabadi 1999; Hart and Ratnieks 2001, 2002; Ballari et al. 2007). As found previously in Atta and Acromyrmex (Wilson 1980b; Wetterer 1999; Hughes et al. 2003), the largest workers engaged in foraging which is due to their greater efficiency at cutting vegetation (Wilson 1980a). Waste management workers were significantly smaller. This was also found to be the case in Atta sexdens and Acromyrmex lobicornis (Wilson 1980b; Ballari et al. 2007), although not in Atta colombica (Hart and Ratnieks 2002), and may be because waste material is relatively light to transport. Waste management workers also differed from foragers in two of the three colonies analysed in that many of them were of medium brown cuticular colouration. This indicates that they were on average younger than foragers, in contrast to the results of Camargo et al. (2007). Our study only very crudely estimated age so was a conservative estimate of the effect of age and greater age polyethism is likely to have been present. Taken together, these results suggest that foraging and waste management are not equally alternative end-of-life tasks for leaf-cutting ant workers. Instead smaller individuals appear to become waste management workers while larger individuals tend to become foragers, with the former doing so at a younger age than the latter.
The results from experiment 2 show that the division of labour in waste management in A. echinatior is reinforced by aggression. Workers that had come into contact with waste were treated aggressively by their nestmates, which is consistent with the results of other studies of leaf-cutting ants (Hart and Ratnieks 2001; Ballari et al. 2007). However, the level of aggression depended on the duration of exposure to waste. Aggression was high towards ants that had been exposed to waste for 20 or 30 min, but was substantially lower towards ants exposed to waste for 10 min or only a few seconds. In addition, ants exposed for 10 min and not aggressed tended to receive intensive allogrooming, a behaviour which is effective at removing parasites from the cuticle (Hughes et al. 2002; Yanagawa et al. 2008). This pattern suggests A. echinatior workers are sensitive to the level of hazard presented by a contaminated ant, attempting to clean it if contamination is low and treating it aggressively when contamination is high.
The aggressive response was also related to worker size. Medium-sized ants were the most numerous size in the area just outside the fungus garden where the introduced ants were placed, and thus the most likely to interact with the introduced ant. Medium-sized workers make up a relatively small proportion of the overall population of an Acromyrmex colony (Wetterer 1999; Hughes et al. 2003), but may be more abundant in this area due to their generalist role (Forti et al. 2004). Small workers though, were most likely to be aggressive. Previous studies have found that Atta small workers both defend foraging workers against parasitoids (Feener and Moss 1990; Orr 1992; Linksvayer et al. 2002; Yackulic and Lewis 2007) and more generally act as sentries on foraging trails, playing the key role in the alarm reaction to threats (Hughes and Goulson 2001). Our results suggest that Acromyrmex small workers may also play a key role in detecting and responding to threats.
When presented with an increase in waste in experiment 3, individuals that had not previously been marked as engaged in waste management dealt with the added waste. Some of these may have been waste management workers that had simply not been marked. However, 81% of the 441 workers that responded ceased waste management once the added waste had been dealt with, indicating that most had responded specifically to it. This is in keeping with the stimulus-response threshold model of division of labour (Robinson 1992). The probability of an ant responding was influenced by the previous task, age and size of ants. Foragers were unlikely to switch task, possibly because the behavioural complexity of foraging either makes it inefficient or neurologically difficult for them to switch task. In accord with this, switching between foraging and waste management under normal circumstances in A. echinatior, and other leaf-cutting ants (Hart and Ratnieks 2002; Ballari et al. 2007), appears to almost never occur. Most of the ants that responded were medium-sized, young, internal workers. The size of ants that switched was thus similar to that of the workers that normally dealt with waste. This may in part be because this is the optimum size for dealing with waste (Hart and Ratnieks 2002), but it is also likely to be due to their relative abundance in the area immediately outside the fungus garden and their generalist role (Forti et al. 2004). Possibly the internal workers that responded could have been inactive waste management workers, but this seems unlikely as the rarity of such a sudden increase in waste stimulus would make this very inefficient. More probably, these ants represent a ‘reserve’ force of inactive workers that were not committed to any task, and which were then able to respond rapidly to the sudden increase in stimulus for waste management.
Previous studies have found that waste management workers are unable to re-enter the main nest (Hart and Ratnieks 2001; Ballari et al. 2007), and in experiment 2 we also found that A. echinatior workers contaminated with waste are treated aggressively. It is therefore surprising that most of the ants which dealt with the added waste in experiment 3 reverted back to working within the fungus garden once the waste had been removed. Presumably this was because their contamination with waste was relatively low and did not pose a threat to the colony or could be cleaned. Reverting to previous tasks after task switching has been observed in the honeybee, in which the probability of reversion depends upon the length of time engaged in the task (Page et al. 1992). Our results therefore also suggest that the behavioural demands of dealing with the waste were insufficient for ants to become fixed in the task.
This investigation has demonstrated how A. echinatior colonies organise waste management and respond to increasing need. Both ant age and size influence the division of labour between waste management and foraging, as well as the probability of individuals responding to an increase in waste. Waste management is an important task for leaf-cutting ants because waste is hazardous to both the ants themselves and their mutualistic fungal crop (Bot et al. 2001; Currie and Stuart 2001; Hart and Ratnieks, 2002; Hughes et al. 2004; Brown et al. 2006). Our results suggest that the flexibility of task allocation in A. echinatior colonies allows them to respond rapidly and effectively deal with an increase in the hazard while minimising the risk of further contamination of the colony.
References
Ballari S, Farji-Brener A, Tadey M (2007) Waste management in the leaf-cutting ant Acromyrmex lobicornis: division of labour, aggressive behaviour, and location of external refuse dumps. J Ins Behav 20:87–98
Benton TG, Foster WA (1992) Altruistic housekeeping in a social aphid. Proc R Soc Lond B 247:199–202
Beshers SN, Fewell JH (2001) Models of division of labor in social insects. Annu Rev Entomol 46:413–440
Beshers SN, Robinson GE, Mittenthal J (1999) Response thresholds and division of labor in insect colonies. In: Detrain C, Deneubourg JL, Pasteels JM (eds) Information processing in social insects. Birkhauser, Basel, pp 115–140
Bonabeau E, Theraulaz G (1999) Role and variability of response thresholds in the regulation of division of labor in insect societies. In: Detrain C, Deneubourg JL, Pasteels JM (eds) Information processing in social insects. Birkhauser, Basel, pp 141–164
Bonabeau E, Theraulaz G, Deneubourg J-L (1996) Quantitative study of the fixed threshold model for the regulation of division of labour in insect societies. Proc R Soc Lond B 263:1565–1569
Bot ANM, Currie CR, Hart AG, Boomsma JJ (2001) Waste management in leaf-cutting ants. Ethol Ecol Evol 13:225–237
Breed MD, Williams DB, Queral A (2002) Demand for task performance and workforce replacement: undertakers in honeybee, Apis mellifera, colonies. J Ins Behav 15:319–329
Brown MJF, Bot ANM, Hart AG (2006) Mortality rates and division of labor in the leaf-cutting ant Atta colombica. J Ins Sci 6:18
Camargo RS, Forti LC, Lopes JFS, Andrade APP, Ottati ALT (2007) Age polyethism in the leaf-cutting ant Acromyrmex subterraneus brunneus Forel, 1911 (Hym., Formicidae). J Appl Entomol 131:139–145
Chapman NC, Oldroyd BP, Hughes WOH (2007) Differential responses of honeybee (Apis mellifera) patrilines to changes in stimuli for the generalist tasks of nursing and foraging. Behav Ecol Sociobiol 61:1185–1194
Currie CR (2001) Prevalence and impact of a virulent parasite on a tripartite mutualism. Oecologia 128:99–106
Currie CR, Stuart AE (2001) Weeding and grooming of pathogens in agriculture by ants. Proc R Soc Lond B 268:1033–1039
Currie CR, Mueller UG, Malloch D (1999a) The agricultural pathology of ant fungus gardens. Proc Natl Acad Sci U S A 96:7998–8002
Currie CR, Scott JA, Summerbell RC, Malloch D (1999b) Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398:701–704
Currie CR, Wong B, Stuart AE, Schultz TR, Rehner SA, Mueller UG, Sung GH, Spatafora JW, Straus NA (2003) Ancient tripartite coevolution in the attine ant–microbe symbiosis. Science 299:386–388
Detrain C, Pasteels JM (1991) Caste differences in behavioral thresholds as a basis for polyethism during food recruitment in the ant Pheidole pallidula (Nyl.) (Hymenoptera: Myrmicinae). J Insect Physiol 4:157–176
Dornhaus A (2008) Specialization does not predict individual efficiency in an ant. PLoS Biol 6:e285
Feener DH, Moss KAG (1990) Defense against parasites by hitchhikers in leaf-cutting ants—a quantitative assessment. Behav Ecol Sociobiol 26:17–29
Fewell JH, Bertram SM (1999) Division of labor in a dynamic environment: response by honeybees (Apis mellifera) to graded changes in colony pollen stores. Behav Ecol Sociobiol 46:171–179
Forti LC, Camargo RS, de Matos CAO, de Andrade APP, Lopes JFS (2004) Aloetismo em Acromyrmex subterraneus brunneus Forel (Hymenoptera, Formicidae), durante o forrageamento, cultivo do jardim de fungo e devolução dos materials forrageados. Rev Bras Ent 48:59–63
Gordon DM (1996) The organization of work in social insect colonies. Nature 380:121–124
Gordon DM, Mehdiabadi NJ (1999) Encounter rate and task allocation in harvester ants. Behav Ecol Sociobiol 45:370–377
Hart AG, Ratnieks FLW (2001) Task partitioning, division of labour and nest compartmentalisation collectively isolate hazardous waste in the leafcutting ant Atta cephalotes. Behav Ecol Sociobiol 49:387–392
Hart AG, Ratnieks FLW (2002) Waste management in the leaf-cutting ant Atta colombica. Behav Ecol 13:224–231
Hölldobler B, Wilson EO (1990) The Ants. Belknap, Cambridge
Hughes WOH, Boomsma JJ (2007) Genetic polymorphism in leaf-cutting ants is phenotypically plastic. Proc R Soc Lond B 274:1625–1630
Hughes WOH, Eilenberg J, Boomsma JJ (2002) Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc R Soc Lond B 269:1811–1819
Hughes WOH, Goulson D (2001) Polyethism and the importance of context in the alarm reaction of the grass-cutting ant, Atta capiguara. Behav Ecol Sociobiol 49:503–508
Hughes WOH, Howse PE, Vilela EF, Goulson D (2001) The response of grass-cutting ants to natural and synthetic versions of their alarm pheromone. Physiol Entomol 26:165–172
Hughes WOH, Sumner S, Van Borm S, Boomsma JJ (2003) Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc Natl Acad Sci U S A 100:9394–9397
Hughes WOH, Thomsen L, Eilenberg J, Boomsma JJ (2004) Diversity of entomopathogenic fungi near leaf-cutting ant nests in a Neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. J Invertebr Pathol 85:46–53
Jackson DE, Hart AG (2009) Does sanitation facilitate sociality? Anim Behav 77:e1–e5
Jeanson R, Clark RM, Holbrook CT, Bertram SM, Fewell JH, Kukuk PF (2008) Division of labour and socially induced changes in response thresholds in associations of solitary halictine bees. Anim Behav 76:593–602
Julian GE, Cahan S (1999) Undertaking specialization in the desert leaf-cutter ant Acromyrmex versicolor. Anim Behav 58:437–442
Linksvayer TA, McCall AC, Jensen RM, Marshall CM, Miner JW, McKone MJ (2002) The function of hitchhiking behavior in the leaf-cutting ant Atta cephalotes. Biotropica 34:93–100
Little AEF, Currie CR (2008) Black yeast symbionts compromise the efficiency of antibiotic defenses in fungus-growing ants. Ecology 89:1216–1222
Michener CD (1974) The social behavior of bees. Harvard University Press, Cambridge
Mikheyev AS, Vo T, Mueller UG (2008) Phylogeography of post-Pleistocene population expansion in a fungus-gardening ant and its microbial mutualists. Mol Ecol 17:4480–4488
Mueller UG, Dash D, Rabeling C, Rodrigues A (2008) Coevolution between attine ants and actinomycete bacteria: a reevaluation. Evolution 62:2894–2912
Orr MR (1992) Parasitic flies (Diptera, Phoridae) influence foraging rhythms and caste division of labor in the leaf-cutter ant, Atta cephalotes (Hymenoptera, Formicidae). Behav Ecol Sociobiol 30:395–402
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton
Page RE, Robinson GE, Britton DS, Fondrk MK (1992) Genotypic variability for rates of behavioral development in worker honeybees (Apis mellifera L). Behav Ecol 3:173–180
Pankiw T, Page RE (1999) The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 185:207–213
Ravary F, Lecoutey E, Kaminski G, Chaline N, Jaisson P (2007) Individual experience alone can generate lasting division of labor in ants. Curr Biol 17:1308–1312
Robinson GE (1992) Regulation of division of labor in insect societies. Annu Rev Entomol 37:637–665
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton
Seid M, Traniello J (2006) Age-related repertoire expansion and division of labor in Pheidole dentata (Hymenoptera: Formicidae): a new perspective on temporal polyethism and behavioral plasticity in ants. Behav Ecol Sociobiol 60:631–644
Smith CR, Toth AL, Suarez AV, Robinson GE (2008) Genetic and genomic analyses of the division of labour in insect societies. Nature Rev Gen 9:735–748
Sokal RR, Rohlf FJ (1995) Biometry. Freeman, New York
Theraulaz G, Bonabeau E, Deneubourg JL (1998) Response threshold reinforcement and division of labour in insect societies. Proc R Soc Lond B 265:327–332
Uribe-Rubio J, Guzmán-Novoa E, Vázquez-Peláez C, Hunt G (2008) Genotype, task specialization, and nest environment influence the stinging response thresholds of individual Africanized and European honeybees to electrical stimulation. Behav Genet 38:93–100
Walker TN, Hughes WOH (2009) Adaptive social immunity in leaf-cutting ants. Biol Lett 5:446–448
Weidenmuller A (2004) The control of nest climate in bumblebee (Bombus terrestris) colonies: interindividual variability and self reinforcement in fanning response. Behav Ecol 15:120–128
Wetterer JK (1999) The ecology and evolution of worker size-distribution in leaf-cutting ants (Hymenoptera: Formicidae). Sociobiology 34:119–144
Wilson EO (1980a) Caste and division of labor in leaf-cutter ants (Hymenoptera, Formicidae, Atta). 2. The ergonomic optimization of leaf cutting. Behav Ecol Sociobiol 7:157–165
Wilson EO (1980b) Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). I. The overall pattern in A. sexdens. Behav Ecol Sociobiol 7:143–156
Winston ML (1995) The biology of the honey bee. Harvard University Press, London
Yackulic CB, Lewis OT (2007) Temporal variation in foraging activity and efficiency and the role of hitchhiking behaviour in the leaf-cutting ant, Atta cephalotes. Ent Exp Appl 125:125–134
Yanagawa A, Yokohari F, Shimizu S (2008) Defense mechanism of the termite, Coptotermes formosanus Shiraki, to entomopathogenic fungi. J Invertebr Pathol 97:165–170
Acknowledgements
We thank the Smithsonian Tropical Research Institute for facilities in Gamboa, the Autoridad Nacional del Ambiente (ANAM) for permission to collect and export the ants, J.J. Boomsma for providing the ant colonies, L. A. Santorelli, C. L. Frost, F. R. Ryan and A. Reynolds for technical assistance, the referees for their constructive comments and the Leverhulme Foundation for funding. The work described in this article complies with the current laws of the countries in which it was performed. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: J.Traniello
Rights and permissions
About this article
Cite this article
Waddington, S.J., Hughes, W.O.H. Waste management in the leaf-cutting ant Acromyrmex echinatior: the role of worker size, age and plasticity. Behav Ecol Sociobiol 64, 1219–1228 (2010). https://doi.org/10.1007/s00265-010-0936-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0936-x