Abstract
Alien predators may impose a great threat to naïve prey. Ibiza wall lizards (Podarcis pityusensis) live in Ibiza, a snake-free island until 2003. We studied the lizards’ discrimination of scents of two invader snakes: one that depredates lizards, the horseshoe whip snake (Hemorrhois hippocrepis), and another that does not, the ladder snake (Rhinechis scalaris). We compared two populations of Ibiza wall lizards: one from the main island of Ibiza, which coexists with both snakes, and another from the nearby snake-free islet of Sal Rossa. Lizards from Ibiza recognized the scent of the horseshoe whip snake and responded with clear antipredatory behaviours. However, they reacted to the scent of the ladder snake similarly to that of the controls (odourless control and pungent scent). Lizards from Sal Rossa did not respond to any of the snakes or the controls. Our results show that lizards can rapidly acquire the ability to react to a novel predator. As only about ten generations of lizards have coexisted with snakes, the most plausible explanation to our results is that lizards have learned to associate the scent of the predatory snake with a threat. This is the first study reporting the rapid acquisition of lizards’ antipredatory responses to the chemical cues of novel predators. However, more research is needed in order to identify the mechanisms implicated in the response.
Significance statement
How naïve prey acquire antipredator behaviour is both important for basic scientific research and useful for the conservation of native species subjected to biological invasions. The island of Ibiza (Spain) has been free from snakes until their introduction by humans in 2003. We compared the reaction of lizards from Ibiza (where presently three species of snakes cohabitate) and lizards from Sal Rossa (a nearby snake-free islet) to the scent of two snakes (one that feeds on lizards and other that does not). The results were clear: lizards from Ibiza react to the scent of the predatory snake with antipredatory behaviours, while ignoring the scent of the non-predatory snake. Lizards from the snake-free island of Sal Rossa did not react to any of the snakes. Our study shows that lizards can rapidly acquire the ability to react to a completely new type of predator, most likely by learning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation is one of the main evolutionary forces in animals (Endler 1986; Lima and Dill 1990). The hunting efficiency of predators usually coevolves with the antipredatory behaviours of the prey (Greene 1988; Vermeij 1994). Nonetheless, the invasion of alien predators imposes new predator-prey interactions among animals that do not share a common evolutionary history (Strauss et al. 2006; Sih et al. 2010; Simberloff et al. 2013). In this scenario, naïve native prey may be unable to recognize predators or respond to them appropriately (Banks and Dickman 2007; Kovacs et al. 2012; Cooper et al. 2014). In addition, sometimes, the alien predator has coevolved with similar prey in its original habitat, hence becoming a highly effective hunter of the naïve prey (Sih et al. 2010), which may result in the extinction of the naïve prey (e.g. Blackburn et al. 2004). However, there are many cases in which naïve prey may recognize and avoid novel predators despite not having a shared evolutionary history (e.g. Strauss et al. 2006; Anson and Dickman 2013; Nunes et al. 2014). Such an ability may be innate and hence fixed by evolution (e.g. Losos et al. 2006; Nunes et al. 2014), or it may be a trait that is caused by predator exposure and subsequent learning to avoid novel predators by the naïve prey (Kelley and Magurran 2003; Griffin 2004; Ferrari et al. 2012). Learning is well documented in fish and has been reported to occur rapidly, only some days after the first visual encounter with the novel predator, and even faster antipredator responses have been recorded with using predator chemical cues (usually by means of chemical alarm signals of conspecifics or heterospecifics; Chivers et al. 1995; Brown et al. 1997).
Many animals are able to recognize predators by chemoreception, which allows potential prey to avoid risky encounters and, often, the costs of fleeing (Kats and Dill 1998; Apfelbach et al. 2005; Cooper et al. 2009). Reptiles have highly developed mechanisms of chemoreception (Schwenk 1995), and several species of lizards have been reported to use them to identify and react to native predators (Thoen et al. 1986; Dial et al. 1989; Webb et al. 2009; Mencía et al. 2016). However, long-term isolations from predators may result in that prey may have lost the ability to recognize predators (Blumstein 2002; Blumstein and Daniel 2005; Cox and Lima 2006).
The Mediterranean island of Ibiza has never been inhabited by snakes (Kotsackis 1981; Alcover 2010) until their recent introduction via olive trees that were used for ornamental gardening in 2003 (Álvarez et al. 2010; Silva-Rocha et al. 2015). Hence, the Ibiza wall lizard, Podarcis pityusensis, first encountered snakes around one decade ago. Three invasive snake species (the horseshoe whip snake, Hemorrhois hippocrepis, the ladder snake, Rhinechis scalaris, and the Montpellier snake, Malpolon mosnpessulanus) are now present on the main island of Ibiza, while coastal islets are still free from snakes (Silva-Rocha et al. 2015). Thus, Ibiza wall lizards from coastal islets have never encountered snakes. We took advantage of this unique situation in order to test if recognition and response to alien predators can be acquired in a naïve prey that lacks a common evolutionary history with their potential predators. To do this, we conducted experiments on chemical recognition of alien snakes by Ibiza wall lizards. We exposed lizards from Ibiza (which have been in contact with snake predators for about one decade) and lizards from the snake-free islet of Sal Rossa to the scents of two of the alien snakes: the horseshoe whip snake and the ladder snake.
In order to study the reaction of insular lizards to the chemical cues of the recent invader species of snakes, we conducted laboratory experiments with different odour treatments. If lizards recognize the chemical cues of the snakes, we would expect that they show significant differences in their behaviours to harmless odours vs. odours from potential predators. Thus, one would expect lizards to react with antipredatory behaviours if they recognize the odour as a threat, whereas no such behaviours would be displayed when encountering harmless odours.
If the ability to recognize the scents of predatory snakes is not innate, we would expect that lizards from Sal Rossa, which have never encountered snake scents before, would not display antipredatory behaviours. These lizards would act normally and similarly to controls and to the scent of snakes. If lizards from Ibiza, which coexist with both types of snake during the last decade, are able to recognize the snake’s scent as a threat, we would expect that they react to the scent with antipredatory behaviour but react normally to the controls. Finally, it is also possible that lizards from Ibiza discriminate and react only to the scent of the predatory snake, the horseshoe whip snake, and react normally to controls and to the scent of the ladder snake.
Materials and methods
Study system
The Ibiza wall lizard is a medium-sized lacertid lizard that inhabits the main islands of Ibiza and Formentera and 42 surrounding islets (Salvador 1984, 2014; Salvador and Pérez-Mellado 1984). On Ibiza, the wall lizards are exposed to several predators: mammals, such as genets (Genetta genetta) and feral cats (Felis silvestris), as well as birds such as kestrels (Falco tinnunculus) and barn owls (Tyto alba; Alcover 1984; Salvador 2014). As mentioned previously, a decade ago, three species of snakes have invaded Ibiza and hence constitute three additional potential predators. Sal Rossa is an islet (<0.5 Ha) that is close to the Southeastern coast of Ibiza, where the only potential predators are birds.
The horseshoe whip snake inhabits the South of the Iberian Peninsula and the North of Tunisia, Algeria and Morocco (Carranza et al. 2005; Silva-Rocha et al. 2015). Adult horseshoe whip snakes are active foraging snakes and feed mainly on mammals and reptiles such as lizards, but their diet also includes birds and invertebrates (Pleguezuelos and Moreno 1990; Pleguezuelos and Fahd 2004; Pleguezuelos and Feriche 2014). The ladder snake inhabits the Iberian Peninsula, the Southeast of France and the Northeast of Italy (Pleguezuelos 1998). Adult ladder snakes are active foragers which feed on mammals and birds and do not depredate lizards (Pleguezuelos 1998; Pleguezuelos et al. 2007). Both species have been recently introduced to Ibiza through ornamental gardening, and they have stable reproductive populations in Ibiza (Pinya and Carretero 2011; Silva-Rocha et al. 2015).
Experimental design
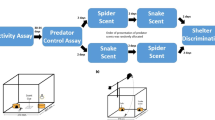
During June of 2014, we conducted three experiments: (1) ‘Ibiza horseshoe whip snake’, to test if lizards from Ibiza respond to the scent of adult horseshoe whip snakes, (2) ‘Ibiza ladder snake’, to test if lizards from Ibiza respond to the scent of adult ladder snakes and (3) ‘Sal Rossa’, to test if lizards from Sal Rossa recognize and respond to the scents of adult horseshoe whip snakes and adult ladder snakes. We used three different sets of 15 adult male lizards, one set for each experiment: 15 adult males from Ibiza for the experiment Ibiza horseshoe whip snake, another 15 males from Ibiza for the experiment Ibiza ladder snake and 15 males from the islet of Sal Rossa for the experiment Sal Rossa. All lizards were captured by noosing and immediately placed into individual cloth bags and transported to the laboratory. The body length of studied lizards is 65.95 ± 5.36 mm (mean snout-vent length (SVL) ± SD), and the mean weight is 7.68 ± 1.64 g. On Ibiza, we captured the lizards in the municipality of Santa Eulària des Riu, within the area of the highest density of the invader snakes (Álvarez et al. 2010). There, we also captured one adult male horseshoe whip snake (SVL = 872 mm) and one adult male ladder snake (SVL = 750 mm). Due to the difficulty in finding snakes, only a single snake was used. Snakes were transported in a different vehicle than lizards in order to avoid any odour mixture.
The experiments were conducted in the research facilities of Sa Casilla (Sant Josep de Sa Talaia, Ibiza). Lizards were kept in individual terraria (40 × 25 × 30 cm) with a substrate of artificial grass and fed daily with crickets and Tenebrio molitor larvae and provided with water ad libitum. Snakes were housed in a different room and kept in individual terraria (50 × 30 × 30 cm) with a substrate of artificial grass and water ad libitum.
Our experimental design was based on previous studies (e.g. Thoen et al. 1986; Van Damme and Quick 2001) and was similar to the one used in Mencía et al. (2016). The experiments consisted of registering the behaviour of lizards in terraria subjected to different chemical cues (odours), i.e. odours from an adult horseshoe whip snake, an adult ladder snake and two controls: an odourless control and a pungent control (cologne) without biological significance for lizards (Cooper et al. 2003). The type of treatments varied in the three experiments. For the experiment Ibiza horseshoe whip snake, we used three different treatments: ‘odourless control’, ‘pungent’ and ‘horseshoe whip snake’. For the experiment Ibiza ladder snake, we used three different experimental terraria: odourless control, pungent and ‘ladder snake’. Finally, for the experiment Sal Rossa, we used four different experimental terraria: odourless control, pungent, horseshoe whip snake and ladder snake.
We placed an absorbent paper on the floor in each of four different experimental terraria (60 × 40 × 40 cm), except for the odourless control. We then allowed three different odours; i.e. horseshoe whip snake, ladder snake and cologne impregnate the absorbent paper. The snakes were placed into the terrarium 24 h before the beginning of the experiment and were placed inside them once again during 40 min between trials. The occlusive plastic cover of the terrarium was closed to avoid odour loss. The snakes were removed from the terrarium 2 min before each experiment trial. We used the absorbent paper in multiple trials with different lizards, cleaning the terrarium and replacing it with new paper each night. Thus, it is possible that lizard alarm odours could be left in the paper, confounding the effect of the chemical cues of the snakes. However, in order to minimize such an effect, we conducted the trials in a random order (i.e. lizards were introduced randomly into the different treatments) and the data was analyzed with repeated measure tests (values of each behavioural variable were tested within individuals).
Each lizard was subjected once to each treatment, resulting in 45 trials (15 lizards × 3 treatments) for each experiment of lizards from Ibiza and 60 trials for the experiment of the lizards from Sal Rossa (15 lizards × 4 treatments). Each lizard was tested once a day within their normal activity period (0800 to 1700 GMT). The experimental room was dark, and only the terrarium was illuminated by a 75-W bulb 50 cm above it, providing homogeneous lighting. We maintained a homogeneous constant temperature of 30 °C in the experimental room in order to avoid possible variations in the behaviour of lizards due to temperature. We drew six equal sectors on the transparent surface of each terrarium in order to count the number of times that lizards moved among sectors. Each trial began by introducing the lizard into the experimental terrarium, closing the terrarium with the occlusive transparent cover in order to avoid scent loss and registering lizard behaviour with a digital recorder for 10 min. Two observers were placed in front of the terrarium, opposite each other: one observer recorded the behavioural variables with binoculars and the other recorded the number of movements and changes among the sectors of the terrarium. Snakes were not fed for the entire duration of all three experiments in order to avoid the potential influence in the lizard’s response to the snakes’ chemical cues due to their diet. It was not possible to record data blindly because terraria were clearly labelled to avoid mistakes. Additionally, the snakes were re-introduced into their terraria after trials. In any case, bias should be low since lizards’ behaviours registered by the two observers. All animals remained healthy throughout the study period. Once we finished each of the three experiments, we released all lizards to their capture sites.
Behavioural variables
Antipredatory vs. normal behavioural patterns are well defined in the lacertid lizard Iberolacerta galani (Mencía et al. 2016). Antipredatory responses include slow movements, jerky movements as tail waving and foot shakes (Thoen et al. 1986; Van Damme et al. 1995; Mencía et al. 2016). By moving less than normal and/or moving in a slow motion, an individual is less likely to be visually detected by the predator (Labra and Niemeyer 2004). Tail waving is another clear antipredatory behaviour related to caudal autotomy of lacertid lizards (Arnold 1984). On the other hand, normal exploratory behaviours of lizards, expected under neutral odours, include walking normally, rubbing the snout of the walls of the terrarium, scratching the walls of the terrarium and/or raising the head (Mencía et al. 2016).
Accordingly, we recorded 16 behavioural variables: (1) ‘walk latency’: time until the first ‘walk’ movement; (2) walk: the lizard walks normally, as it would move in the wild; (3) ‘change among sectors’: the lizard moves between the six predefined sectors of the experimental terrarium; (4) ‘slow’: the lizard walks slowly and with stalking or scattered movements (Thoen et al. 1986; Mencía et al. 2016); (5) tongue flick (TF) latency, i.e. time until the first TF; (6) ‘TF’: number of TFs; (7) ‘snout’: the lizard taps the wall of the terrarium with the snout; (8) ‘rubbing’: the lizard rubs its head against the walls of the terrarium; (9) ‘stand and scratching’: the lizard stands up against the wall of the terrarium and scratches it with its forelegs, as if it were trying to escape; (10) ‘head bob’: the lizard shakes its head up and down; (11) ‘head raise’: the lizard raises the head with its forelimbs straightened; (12) ‘tail waving’: the lizard waves its tail about in a horizontal plane; (13) ‘foot shake’: the lizard moves its forelimbs rapidly up and down; (14) ‘walk time’: total amount of time that the lizard moves normally; (15) ‘slow time’: total amount of time that the lizard moves in a slow motion; and (16) ‘no move’: total amount of time that the lizard stays immobile. The variables were quantified as frequencies, except for walk latency, ‘TF latency’, walk time, slow time and no move, which were quantified in seconds. We started to record the behaviour of each lizard 5 s after placing it in the centre of the experimental terrarium.
Data analysis
We conducted all analyses on R, version 3.1.3 (R Core Team 2015). Because neither the original nor the log-transformed data met the requirements of parametric statistics, we analyzed the data with non-parametric tests. For each experiment, we used the repeated measures of the Friedman test to assess possible differences in the behavioural variables among treatments. When the result of the Friedman test was significant, we performed post hoc multiple comparisons for the Friedman test in order to locate the differences between treatments (Giraudoux 2012).
Results
Experiment Ibiza horseshoe whip snake
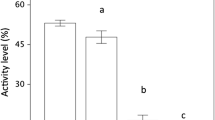
All the 15 lizards from Ibiza detected and reacted to the scent of the adult horseshoe whip snake. The values of 11 of the 16 studied behavioural variables were significantly different in the Friedman test of horseshoe whip snake compared to the odourless control and ‘pungent control’ (Table 1). The post hoc tests of Friedman identified the significant differences between the horseshoe whip snake treatment and the odourless control treatment and between the horseshoe whip snake treatment and the pungent treatment (Table 2). For example, the frequency of normal lizard movements was significantly lower containing the scent of the horseshoe whip snake compared to the two controls (Fig. 1). Other behavioural variables, such as the frequency of movements in a slow motion and the frequency of tail waving, only occurred when lizards where exposed the scent of the horseshoe whip snake.
Boxplots (the line in the middle of the box depicts the median, the box extends from the 25th to the 75th percentiles, and the whiskers depict the 10th and 90th percentiles) of the number of normal movements (variable ‘walk’) of Podarcis pityusensis lizards for each experimental condition recorded during the three experiments
Experiment Ibiza ladder snake
None of the 15 lizards from Ibiza reacted to the chemical cues of the adult ladder snake. All behavioural variables, except for TF latency, showed similar values in the Friedman test for the three treatments: ladder snake, odourless control and pungent (Table 3).
Experiment Sal Rossa
None of the 15 lizards from Sal Rossa reacted to the scent of the adult horseshoe whip snake or the adult ladder snake. All behavioural variables showed similar values in the Friedman test for the four treatments: horseshoe whip snake, ladder snake, pungent and odourless control (Table 4).
Discussion
The results from the present study show that Ibiza wall lizards from the main island of Ibiza, after approximately one decade of coexistence, recognized and reacted to the scent of the adult horseshoe whip snake by displaying antipredatory behaviours, while ignoring the scent of the adult ladder snake. Importantly, Ibiza wall lizards from Sal Rossa did not recognize or responded to any of the snakes.
Prior to the arrival of humans, birds were the only predator of the Ibiza wall lizard (Alcover 2010). About 5000 years ago, humans arrived and introduced mammalian predators such as genets and feral cats (Brown et al. 2008). However, until 2003, Ibiza has been as snake-free island (Alcover 2010; Silva-Rocha et al. 2015). The present study shows that after less than 15 years of coexistence, Ibiza wall lizards are able of recognizing and displaying antipredator behaviours, such as tail waving and slow motion movements, to the chemical cues of the adult horseshoe whip snake (which frequently feed on lizards), while they ignore the chemical cues of the adult ladder snake. These antipredatory responses are similar to those exhibited by other European lacertid lizards, which have coexisted with predatory snakes for thousands of years (Thoen et al. 1986; Van Damme and Quick 2001; Mencía et al. 2016). However, lizards from the snake-free islet of Sal Rossa ignored the scent of both snakes, behaving similarly to the controls (odourless and pungent odours). Our results show that Ibiza wall lizards have acquired the ability to recognize and respond to the scent of the horseshoe whip snake during this brief period of coexistence. Neophobia, i.e. a general avoidance of novel stimuli, has been shown to be associated with high predation risk (Brown et al. 2013). However, if neophobia was underpinning the lizards’ antipredatory behaviours, they should have responded similarly to the odours of both the snakes and not only to the scent of the horseshoe whip snake, hence making neophobia an unlikely mechanism to strong antipredator response to the adult horseshoe whip snake observed in the lizards from the island of Ibiza. Thus, there are two possible explanations to the rapid adaptation of these lizards to the novel predator: (1) rapid evolution of the observed antipredatory behaviour and/or (2) learning to respond to the scent of the horseshoe whip snake.
Rapid evolution requires a strong predation pressure and background genetic variability on which to act (Nunes et al. 2014). Both requirements seem to apply here. Horseshoe whip snakes prey on similar lacertid lizards in their native habitats of the Iberian Peninsula (Pleguezuelos and Moreno 1990), so they are most likely highly effective at capturing Ibiza wall lizards. As the generation time of Ibiza wall lizards is 1 year, such an evolutionary response would have evolved during the last ten generations of coexistence with horseshoe whip snakes. Losos et al. (2006) reported strong and directional selective pressures elicited by predators within one generation of lizards. Additionally, Nunes et al. (2014) reported that the innate antipredator recognition of tadpoles of the Iberian green frog (Pelophylax perezi) had evolved in only 20 years of predator coexistence. Furthermore, Freeman and Byers (2006) demonstrated that mussels changed in morphology after one decade after the introduction of a novel predator. It is therefore possible that the Ibiza wall lizards have evolved a fixed antipredatory behaviour in the presence of cues from the horseshoe whip snake. However, only a subsequent experiment using captive hatched neonate lizards will be able to elucidate whether the antipredatory behaviours recorded are an innate response to this novel predator.
On the other hand, several species have been shown to rely on learning to recognize and respond to novel predators (Ferrari et al. 2015). For example, introduction of northern pike (Esox lucius) into a pond previously only inhabited by fathead minnows (Pimephales promelas) resulted in that the minnows displayed antipredatory behaviours to pike odours only 3 days after the introduction (Brown et al. 1997). Learning only requires one successful encounter with the predator, enabling the surviving naïve prey to rapidly recognize chemical and/or visual cues of the predator (Griffin 2004; Ferrari et al. 2010; Brown et al. 2011; Crane and Ferrari 2013). Moreover, wall lizards (Podarcis muralis) have been shown to combine both chemical and visual cues to detect snake predators (Amo et al. 2004); it is therefore possible that visual cues may have reinforced the Ibiza wall lizards’ antipredatory learning response to horseshoe whip snakes.
Ibiza wall lizards have been subjected to a continuous high predation pressure from several predatory birds and human-introduced mammals over many thousands of years (Pérez-Mellado et al. 1997; Cooper and Pérez-Mellado 2012) as revealed by their high autotomic capacity (Pérez-Mellado et al. 1997) and long flight initiation distance (Cooper and Pérez-Mellado 2012). We therefore propose that previous high-level predation has further enabled the lizards to maintain the ability to learn how to rapidly recognize and respond to new predators. This ability could potentially save Ibiza wall lizards from possible significant population decline or extinction caused by the alien snake invaders. However, as mentioned previously, further experiments have to be conducted in order to elucidate which of the two antipredatory mechanisms, i.e. innate or learned, being the major underpinning of the strong antipredator responses displayed by the wall lizards from the island of Ibiza to a novel snake predator, the horseshoe whip snake.
References
Alcover JA (1984) Uber die Nahrung der Ginsterkatze Genetta genetta (Linnaeus, 1758) auf der Inseln Mallorca, Ibiza und Cabrera. Saügetierkd Mitt 31:189–195
Alcover JA (2010) A century of insular vertebrate paleontology research on the Balearic Islands. In: Pérez-Mellado V, Ramon C (eds) Islands and evolution. Institut Menorqui d’Estudis, Mao, pp. 59–83
Álvarez C, Mateo JA, Oliver J, Mayol J (2010) Los ofidios ibéricos de introducción reciente en las Islas Baleares. Bol Asoc Herpetol Esp 21:126–131
Amo L, López P, Martín J (2004) Wall lizards combine chemical and visual cues of ambush snake predators to avoid overestimating risk inside refuges. Anim Behav 670:647–653
Anson JR, Dickman CR (2013) Behavioural responses of native prey to disparate predators: naiveté and predator recognition. Oecologia 171:367–377
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144
Arnold EN (1984) Evolutionary aspects of tail shedding in lizards and their relatives. J Nat Hist 18:127–169
Banks PB, Dickman CR (2007) Alien predation and the effects of multiple levels of prey naiveté. Trends Ecol Evol 22:229–230
Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ (2004) Avian extinction and mammalian introductions on oceanic islands. Science 305:1955–1958
Blumstein DT (2002) Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J Biogeogr 29:685–692
Blumstein DT, Daniel JC (2005) The loss of anti-predator behaviour following isolation on islands. Proc R Soc Lond B 272:1663–1668
Brown GE, Chivers DP, Smith RJF (1997) Differential learning rates of chemical versus visual cues of a northern pike by fathead minnows in a natural habitat. Environ Biol Fish 49:89–96
Brown GE, Ferrari MC, Chivers DP (2011) Learning about danger: chemical alarm cues and threat-sensitive assessment of predation risk by fishes. In: Brown C, Laland K, Krause J (eds) Fish cognition and behavior. Wiley-Blackwell, Oxford, pp. 59–80
Brown GE, Ferrari MC, Elvidge CK, Ramnarine I, Chivers DP (2013) Phenotypically plastic neophobia: a response to variable predation risk. Proc R Soc B 280:20122712
Brown RP, Terrasa B, Pérez-Mellado V, Castro JA, Hoskisson PA, Picornell A, Ramon MM (2008) Bayesian estimation of post-Messinian divergence times in Balearic Island lizards. Mol Phylogenet Evol 48:350–358
Carranza S, Arnold EN, Pleguezuelos JM (2005) Phylogeny, biogeography and evolution of two Mediterranean snakes Malpolon monspessulanus and Hemorrhois hippocrepis (Squamata, Colubridae), using mtDNA sequence. Mol Phylogenet Evol 40:532–546
Chivers DP, Brown GE, Smith RJF (1995) Acquired recognition of chemical stimuli from pike, Esox lucius, by brook sticklebacks, Culaea inconstans (Osteichthyes, Gasterosteidae). Ethology 99:234–242
Cooper WE, Pérez-Mellado V (2012) Historical influence of predation pressure on escape by Podarcis lizards in the Balearic Islands. Biol J Linn Soc 107:254–268
Cooper WE, Hawlena D, Pérez-Mellado V (2009) Interactive effect of starting distance and approach speed on escape behavior challenges theory. Behav Ecol 20:542–546
Cooper WE, Pérez-Mellado V, Vitt LJ, Budzynski B (2003) Cologne as a pungency control in tests of lizard chemical discriminations: effects of concentration, brand, and simultaneous and sequential presentation. J Ethol 21:101–106
Cooper WE, Pyron RA, Garland T (2014) Island tameness: living on islands reduces flight initiation distance. P Roy Soc Lond B Bio 281:20133019. doi:10.1098/rspb.2013.3019
Cox JG, Lima SL (2006) Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680
Crane AL, Ferrari MCO (2013) Social learning of predation risk: a review and prospectus. In: Clark K (ed) Social learning theory: phylogenetic considerations across animal, plant, and microbial taxa. Nova Science Publisher, New York, pp. 53–82
Dial BE, Weldon PJ, Curtis B (1989) Chemosensory identification of snake predators (Phyllorhynchus decurtatus) by banded geckos (Coleonyx variegatus). J Herpetol 3:224–229
Endler JA (1986) Defense against predators. In: Feder ME, Lauder GV (eds) Predator-prey relationships. Perspectives and approaches from the study of lower vertebrates. The University of Chicago Press, Chicago, pp. 109–134
Ferrari MCO, McCormick MI, Allan BJ, Choi R, Ramasamy RA, Johansen JL, Mitchell MD, Chivers DP (2015) Living in a risky world: the onset and ontogeny of an integrated antipredator phenotype in a coral reef fish. Sci Rep 5:15537
Ferrari MCO, Vrtělová J, Brown GE, Chivers DP (2012) Understanding the role of uncertainty on learning and retention of predator information. Anim Cogn 15:807–813
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Freeman AS, Byers JE (2006) Divergent induced responses to an invasive predator in marine mussel populations. Science 313:831–833
Giraudoux P (2012) pgirmess: data analysis in ecology. R package version 1.5.6., http://CRAN.R-project.org/package=pgirmess
Greene HW (1988) Antipredator mechanisms in reptiles. In: Gans C, Huey RB (eds) Biology of Reptilia, Vol. 16. Ecology B: defense and life history. Alan R. Liss, New York, pp. 1–152
Griffin AS (2004) Social learning about predators: a review and prospectus. Anim Learn Behav 32:131–140
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kelley JL, Magurran AE (2003) Learned predator recognition and antipredator responses in fishes. Fish Fish 4:216–226
Kotsackis T (1981) Le lucertole (Lacertidae, Squamata) del Pliocene, Pleistocene e Olocene delle Baleari. Bol Soc Hist Nat Balears 25:135–150
Kovacs EK, Crowther MS, Webb JK, Dickman CR (2012) Population and behavioural responses of native prey to alien predation. Oecologia 168:947–957
Labra A, Niemeyer HM (2004) Variability in the assessment of snake predation risk by Liolaemus lizards. Ethology 110:649–662
Lima SL, Dill LM (1990) Behavioural decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Losos JB, Schoener TW, Langerhans RB, Spiller DA (2006) Rapid temporal reversal in predator-driven natural selection. Science 314:1111
Mencía A, Ortega Z, Pérez-Mellado V (2016) Chemical discrimination of sympatric snakes by the mountain lizard Iberolacerta galani (Squamata: Lacertidae). Herpetol J 26:151–157
Nunes AL, Orizaola G, Laurila A, Rebelo R (2014) Rapid evolution of constitutive and inducible defenses against an invasive predator. Ecology 95:1520–1530
Pérez-Mellado V, Corti C, Lo Cascio P (1997) Tail autotomy and extinction in Mediterranean lizards. A preliminary study of continental and insular populations. J Zool 243:533–541
Pinya S, Carretero MA (2011) The Balearic herpetofauna: a species update and a review on the evidence. Acta Herpetol 6:59–80
Pleguezuelos JM (1998) Elaphe scalaris (Schinz, 1822). In: Ramos MA, Alba J, Bellés X, Golsálbez J, Guerra A, Macpherson E, Martín F, Serrano J, Templado J (eds) Reptiles, 1st edn, Fauna Ibérica, vol 10. Museo Nacional de Ciencias Naturales, CSIC, Madrid, pp. 390–407
Pleguezuelos JM, Fahd S (2004) Body size, diet and reproductive ecology of Coluber hippocrepis in the Rif (Northern Morocco). Amphibia-Reptilia 25:287–302
Pleguezuelos JM, Feriche M (2014) Hemorrhois hippocrepis (Linnaeus, 1758). In: Salvador A (ed) Reptiles, 2nd edn, Fauna Ibérica, vol 10. Museo Nacional de Ciencias Naturales, CSIC, Madrid, pp. 723–739
Pleguezuelos JM, Moreno M (1990) Alimentación de Coluber hippocrepis en el SE de la Península Ibérica. Amphibia-Reptilia 11:325–337
Pleguezuelos JM, Fernandez-Cardenete JR, Honrubia S, Feriche M (2007) Correlates between morphology, diet and foraging mode in the Ladder Snake Rhinechis scalaris (Schinz, 1822). Contrib Zool 76:179–186
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/
Salvador A (1984) A taxonomic study of the Eivissa wall lizard, Podarcis pityusensis Boscá 1883. In: Kuhbier H, Alcover JA, Guerau d’Arellano Tur C (eds) Biogeography and ecology of the Pityusic Islands. Springer Netherlands, The Hague, pp. 393–427
Salvador A (2014) Podarcis pityusensis (Boscá, 1883). In: Salvador A (ed) Reptiles, 2nd edn, Fauna Ibérica, vol 10. Museo Nacional de Ciencias Naturales, CSIC, Madrid, pp. 589–601
Salvador A, Pérez-Mellado V (1984) The amphibians and reptiles of the Pityusic Islands. In: Kuhbier H, Alcover JA, Guerau d’Arellano Tur C (eds) Biogeography and ecology of the Pityusic Islands. Springer Netherlands, The Hague, pp. 429–439
Schwenk K (1995) Of tongues and noses: chemoreception in lizards and snakes. Trends Ecol Evol 10:7–12
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Silva-Rocha I, Salvi D, Sillero N, Mateo JA, Carretero MA (2015) Snakes on the Balearic Islands: an invasion tale with implications for native biodiversity conservation. PLoS One 10:e0121026
Simberloff D, Martin JL, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:357–374
Thoen C, Bauwens D, Verheyen RF (1986) Chemoreceptive and behavioural responses of the common lizard Lacerta vivipara to snake chemical deposits. Anim Behav 34:1805–1813
Van Damme R, Quick K (2001) Use of predator chemical cues by three species of lacertid lizards (Lacerta bedriagae, Podarcis tiliguerta, and Podarcis sicula). J Herpetol 35:27–36
Van Damme R, Bauwens D, Thoen C, Vanderstighelen D, Verheyen RF (1995) Responses of naive lizards to predator chemical cues. J Herpetol 29:38–43
Vermeij GJ (1994) The evolutionary interaction among species: selection, escalation, and coevolution. Annu Rev Ecol Evol S 25:219–236
Webb JK, Du WG, Pike DA, Shine R (2009) Chemical cues from both dangerous and nondangerous snakes elicit antipredator behaviours from a nocturnal lizard. Anim Behav 77:1471–1478
Acknowledgments
This work was supported by the Spanish Ministry of Science and Innovation (project CGL2012-39850-CO2-02) and the University of Salamanca (predoctoral grants to AM and ZO). We thank the city council of Sant Josep de Sa Talaia (Ibiza) for providing us accommodations and research facilities at Sa Casilla. We thank A. Pérez-Cembranos for her assistance with the capturing of lizards and Mario Garrido, Gonzalo Rodríguez and Alicia León for their support with the writing and M.T. Mencía and Joseph McIntyre for language revision. We also thank Axios Review, Thomas Madsen and two anonymous reviewers that helped to improve the first version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Spanish Ministry of Science and Innovation (project CGL2012-39850-CO2-02) and the University of Salamanca (predoctoral grants to AM and ZO).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of animals were followed. Lizards were sampled under permits issued by the Balear Government. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was not required.
Additional information
Communicated by T. Madsen
Zaida Ortega and Abraham Mencía are cofirst authors.
Rights and permissions
About this article
Cite this article
Ortega, Z., Mencía, A. & Pérez-Mellado, V. Rapid acquisition of antipredatory responses to new predators by an insular lizard. Behav Ecol Sociobiol 71, 1 (2017). https://doi.org/10.1007/s00265-016-2246-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-016-2246-4