Abstract
The immune system is an important defence against pathogens but requires resources that hosts may also use otherwise. Thus, trade-offs between investment in immunity versus other life-history traits may exist, especially during resource-demanding periods such as reproduction. Here, we investigated the potential trade-off between an activated immune system and parental care in free-living great tits. We also studied whether variation in baseline immune indices prior to immunization contributes to individual differences in the responses to an immune challenge. To this end, we injected free-living great tit females with either phosphate-buffered saline (PBS) or with bacterial lipopolysaccharides (LPS) when nestlings were 9 days old and subsequently recorded parental feeding rates. We quantified potential fitness consequences via the growth and survival of their nestlings. Exposure to LPS tended to decrease female feeding rates. However, nestling body mass was not affected by the maternal immune challenge, probably because males compensated for the change in feeding rate of their partner. We found a negative relationship between haptoglobin levels and female feeding rates pre-treatment, but not with any of the other innate immune traits. Although there was substantial variation in female innate immune indices, we found no evidence that baseline immunity affected how females reacted to an immune challenge in terms of changes in parental behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals are faced with a wide range of parasite taxa, which exploit the host for resources they need for their own replication. In response to this, hosts have evolved a wide range of adaptations to prevent an initial parasite infection and to eradicate established infections (Clayton and Wolfe 1993; Christe et al. 1994; Sheldon and Verhulst 1996; Murphy et al. 2012). One of the most important adaptations is the immune system and its different components. However, even though an immune response confers a fitness benefit against a parasite, as it increases the survival probability, it also requires resources (Sheldon and Verhulst 1996). Mounting an immune response and maintaining an efficient immune system is thought to be a demanding process due to the damaging effects of reactive oxygen species (ROS) and the energetic and nutritional costs associated with it (Sheldon and Verhulst 1996; Lochmiller and Deerenberg 2000; Bonneaud et al. 2003; Costantini and Møller 2009; Hasselquist and Nilsson 2012). Based on the costs associated with mounting an immune response and maintaining the immune system, there may be a trade-off between investment in central life-history components such as reproduction and an investment in the immune system (Sheldon and Verhulst 1996; Lochmiller and Deerenberg 2000; Norris and Evans 2000; Demas and Nelson 2012). Indeed, several studies have shown that an increased reproductive effort impairs a bird’s immune responsiveness, parasite resistance and a bird’s ability to control (chronic) infections (Siikamäki et al. 1997; Nordling et al. 1998; Knowles et al. 2009). Vice versa, induction of an immune response via immunization with a diphtheria-tetanus vaccine or the endotoxin lipopolysaccharide (LPS) led to reduced feeding rates (Ilmonen et al. 2000; Raberg et al. 2000; Bonneaud et al. 2003). Thus, an activated immune system can lower breeding success, and this could be caused by an energetic or nutritional trade-off between immune function and workload when feeding young. Reducing the workload could also be an adaptive response to infection to avoid oxidative stress, as workload and immune system activation are both ROS producing activities (Hasselquist and Nilsson 2012).
Costs of immunity have, as detailed above, mainly been studied in terms of deployment costs, which arise when an immune response is mounted. These deployment costs are due to the use of energy and resources or are a consequence of collateral damage caused by the immune system when mounting the response (McKean et al. 2008). However, the immune system is also costly because of maintenance costs that are related to the energy and resources an individual invests into the infrastructure, continuous surveillance and maintenance in the absence of an infection (=baseline immunity). Individuals differ in their baseline immune characteristics, yet the costs and benefits of high levels of baseline immunity are less clear. Variation in baseline immunity may be linked to how individuals will respond to an infection, eventually preventing additional responses of the acquired immune system or the acceleration of the course of the immune reaction.
Baseline immunity consists in particular of components of innate immunity such as natural antibodies (NAbs) and complement activity. NAbs broadly recognize and bind to antigens, a process which can result in activation of the complement cascade and ends with the lysis of foreign cells (Boes 2000; Ochsenbein and Zinkernagel 2000; Matson et al. 2005; Murphy et al. 2012). Acute phase proteins (APPs) such as haptoglobin (Hp) play an important role too. APPs have several antimicrobial functions, such as opsonizing bacteria and activating the complement cascade (Murphy et al. 2012). APP concentrations can rise significantly in response to an acute infection, trauma or inflammation (Murata et al. 2004; Quaye 2008; Cray et al. 2009; Matson et al. 2012).
Here, we investigated the trade-off between an activation of the immune system and parental care in free-living female great tits (Parus major) while also linking the observed responses to their baseline innate immunity. We investigated potential consequences of an immune challenge on reproductive effort. Females were immunized with the non-replicating antigen LPS when nestlings were 9 days old, and we compared their responses with those of females injected with PBS (control treatment). We recorded parental feeding rates prior to and after the immune challenge via nest box cameras. Furthermore, we quantified the consequences of the injected endotoxin on offspring growth and survival by repeatedly measuring nestling body weight and determining fledging success. We collected a blood sample prior to injecting the female with either LPS or PBS in order to link the maintenance of several aspects of the innate immune function, which is generally non-specific and serves as an initial line of defence against invading pathogens, with potential deployment costs. We expected a negative effect of the LPS challenge on reproductive performance, and we hypothesized that the individual immune characteristics of each female, that is variation in baseline immune indices, are responsible for individual differences in the (fitness) consequences of our immune challenge.
Materials and methods
Study sites and data sampling
This study was performed during the breeding season of 2013 at three established great tit populations around Antwerp, Belgium (Hoboken: N 51 10 10, E 4 20 44.4 and N 51 9 48.3, E 4 20 48.9; Wilrijk: N 51 9 56.3, E 4 22 36.7). All study sites are deciduous park areas located in urbanized areas, which differ in their levels of pollution (Janssens et al. 2001). Experimental groups were therefore equally distributed among sites, and potential side effects were additionally statistically tested. Yet, we found no differences in female baseline immune indices among populations (HA: P = 0.21; HL: P = 0.32; Hp: P = 0.06) and no significant differences in the response to the immune challenge among locations (=three-way interactions between time (pre or post), field site and immune indices (for more details please see below): HA: χ² = 4.03, df = 2, P = 0.13; HL: χ² = 2.12, df = 2, P = 0.35; Hp: χ² = 2.96, df = 2, P = 0.23). Great tits living within these different populations breed in nest boxes with approximately 30 to 60 nest boxes (12.5 × 15 × 25 cm, entrance hole diameter: 3 cm, opens at the top) per study site. Nest boxes were checked every other day to determine laying date, clutch size, start of incubation, and exact hatch day. Breeding females (N = 53) were captured on their nest, when sleeping, between 21:00 hours and midnight when chicks were 9 days old (hatch day = day 1, average brood size = 7 nestlings). We collected a blood sample (∼150 μL) from the brachial vein using a Microvette CB 300 lithium-heparin tube (Sarstedt) from each female. Subsequently, females were injected in the pectoral muscle with either 0.05 mL of a 500 μg/kg body mass lipopolysaccharide solution (LPS) or with 0.05 mL of a phosphate-buffered saline solution (PBS). The stock solution of 500 μg/kg was based on an average weight of 17 g, based on the body mass of previously captured females; thus, every bird received 8.5 μg of LPS. An injection with LPS induces an inflammatory response as it non-specifically activates a wide range of cells such as heterophils and B and T lymphocytes within a few hours after injection. The initial inflammatory response starts an immune cascade and results in the production of specific antibodies for LPS (Poxton 1995; Bonneaud et al. 2003; Fang et al. 2004; Abbas and Lichtman 2010). We injected females in the evening to be sure that the first acute effects of the immune response took place when the female was on the nest at night. Consequently, by the next morning, the females’ immune responses will be in some sort of transition phase between the first inflammatory response and the start-up of the acquired immune response (carry over effect of the acute response). By using this approach, any induced acute sickness behaviour (e.g. fever (Gray et al. 2013)) will be terminated by the time females start feeding their nestlings the next morning, while there will still be an ongoing start-up of other immune responses. After manipulation, we marked females on the head using white typing correction fluid (Tipp-ex) and returned them to their nest box. Nest entrances were blocked for a few minutes to prevent females from escaping directly after they were returned. Collected blood was stored under cool conditions and centrifuged at 7000 rpm for 10 min after returning to the lab that same evening. Resulting plasma samples were stored at −80 °C until use in further immunological assays. Generally, all blood was sampled immediately after taking the females out of the nest to minimize the potential effects of stress on baseline immune functions (Matson et al. 2006; Millet et al. 2007; Buehler et al. 2008).
We recorded parental feeding rates of both females and males using infrared cameras (Pakatak PAK-MIR5, Essex, UK) when nestlings were 9 days old (morning before injection = pre-injection feeding rates) and when nestlings were 10 days old (morning after injection = post-injection feeding rates, Fig. 1). Cameras were installed on the day prior to the morning of recording to make sure that birds had some time to adapt to the altered appearance of their nest box. So, the pre-camera was placed on the nest box at day 8 and programmed to start recording the morning of day 9. The same method was used for the post-recording where the camera was placed on the nest box at day 9 to start recording the morning of day 10. For every nest, we analysed 3 h of parental feeding rates pre-injection (from 07:00 to 10:00 hours) and 3-h post-injection (from 07:00 to 10:00 hours) by scoring the number of visits to the nest for both females and males using The Observer XT 10 software (Noldus). This software provides, based on the entered feeding scores, a feeding rate for every individual expressed as the feeding rate per minute. To minimize observer bias, blinded methods were used for the analysis of feeding behaviour.
Nestling body mass was regularly evaluated by weighing the chicks (with an accuracy of 0.1 g) at day 9 (pre-injection), day 10, day 12 and day 14 (Figs. 1 and 2). On day 12, chicks (N = 316) were also ringed with a uniquely coded metal ring. Due to our alternating injection scheme, there is an equal distribution of PBS and LPS-injected females per field site. In total, we manipulated females belonging to 53 different nests of which 26 were assigned to an LPS treatment and 27 which were assigned to a PBS treatment. Four nests were excluded from analysis due to technical problems with nest box cameras (2 LPS, 2 PBS), while one extra nest (PBS) was excluded since we were unable to collect enough blood from the female. One female (LPS) died during the experiment, and data belonging to this nest were therefore excluded. For this reason, further analyses were based on 23 LPS-injected females and 24 PBS-injected females. In both groups, we had one female feeding the nestlings without the help of her partner. We experienced no nest abandonment after capture and immune challenges.
Mean body mass ± SE of nestlings of females injected with phosphate-buffered saline (PBS) or lipopolysaccharide (LPS) on day 9, day 10, day 12 and day 14. Body mass on day 9 was determined before the treatment of their mothers, while body mass for day 10, 12 and 14 was determined after the treatment of their mothers
Immunological assays
Haemolysis-haemagglutination assay
To assess the levels of natural antibodies and complement activity, we used the haemolysis-haemagglutination assay as developed by Matson et al. (2005) with some minor alterations (Vermeulen et al. 2015). This assay is based on the interaction of great tit plasma and rabbit red blood cells which results in agglutination and natural antibody-mediated complement activation. Agglutination scores (HA) represent the interaction between natural antibodies in the plasma and antigens present in the rabbit blood, whereas lysis scores (HL) represent the activity of the complement system. Titers for agglutination and lysis were blindly scored from digitized images as the negative log2 of the last plasma dilution at which agglutination or lysis occurred. Half scores were assigned to wells which showed intermediate agglutination or lysis. All samples were scored twice on different days. We used Spearman’s rank correlations to quantify repeatability between the two agglutination scores (ρ = 0.88, P < 0.0001) and between the two lysis scores (ρ = 0.99, P < 0.0001).
Haptoglobin assay
Plasma haptoglobin concentrations (mg/mL) were quantified using the manufacturer’s instructions provided with the commercially available colorimetric assay (PHASE Haptoglobin assay, Tridelta Development Ltd). We performed this analysis last, therefore, due to shortage of plasma, the results for haptoglobin are based on 14 LPS-injected females and 10 PBS-injected females.
Statistical analysis
To assess which parameters influenced nestling body mass, we constructed a linear mixed model containing body mass as dependent variable, field site, age (d9, d10, d12 and d14) and treatment (LPS or PBS) as fixed factors, individual and nest as random factors (with individual nested in nest and nest nested in field site) and brood size was included as a covariate. The model also contained the interaction terms field site by treatment, treatment by age and brood size by age.
To establish which parent feeds on average the most before the treatment (natural conditions), we conducted a paired t test. To investigate whether the effects of our treatment differed between the sexes, we used a linear mixed model containing feeding rate as a dependent variable, field site, treatment (LPS or PBS), time (pre or post) and sex (male or female) as fixed factors (with all possible interactions between time, sex and treatment), treatment day was included as a covariate, nest as a random factor and individual as a random factor (with individual nested in nest and in field site). Since we found that there was a significant three-way interaction (time by treatment by sex), we wanted to examine in more detail whether there were significant differences in pre- and post-treatment feeding rates for females and males. Therefore, we used linear mixed models for both sexes separately. We used (female or male) feeding rate as a dependent variable, field site, treatment (LPS or PBS) and time (pre or post) as fixed factors (with the interaction time by treatment), treatment day was included as a covariate and individual as a random factor (with individual nested in field site). Relationships between female immunity and female feeding rates (for the complete dataset and for the dataset excluding females feeding without males) were investigated using Spearman’s rank correlations following normality checks.
To establish whether the change in feeding rate following a LPS treatment was correlated with baseline immunity of the females, we used Pearson correlations (HA and HL) or Spearman rank correlations (Hp) depending on model assumptions. To see if we could use our data on female immunity and feeding rates to make a prediction on how the female would react to a challenge, we constructed linear mixed models to investigate the time by immunity interaction with feeding rate as a dependent variable, field site and time (pre or post) as fixed factors, brood size and immunity (HA, HL or Hp) as covariates and individual as a random factor (with individual nested in field site).
Mixed models were constructed using the lmer function imbedded in the package lme4 in R (Bates et al. 2013) starting from a model containing all independent variables of interest and their interactions. Subsequently, we used the backward elimination procedure for model reduction. We used the maximum likelihood method since we tested fixed effects, their interactions and covariables. Decisions to keep parameters in the model were based on a significance level of 5 %. Model assumptions were checked using Q-Q plots and Shapiro-Wilk normality tests. All statistical analyses were performed in R 2.15.3 (R development core team 2013-03-01 release; www.r-project.org).
Results
Influence of female immunization on nestling body mass
There was no significant field site by treatment interaction (χ² = 3.01, df = 2, P = 0.22) or brood size by age interaction (χ² = 1.61, df = 3, P = 0.66). We found no evidence that the treatment of the mother had an effect on nestling body mass (treatment by age interaction χ² = 3.09, df = 3, P = 0.38; Fig. 2). Further, we found no effect of brood size (χ² = 1.66, df = 1, P = 0.20) or treatment (χ² = 2.51, df = 1, P = 0.12) on nestling body mass. Field site had an effect on nestling body mass (χ² = 17.68, df = 2, P < 0.001), and as expected, age also influenced the body mass of nestlings (χ² = 1679.9, df = 3, P < 0.0001). Female immunization did not affect fledging success as we did not find any dead chicks in the nests during a nest box check at day 25, suggesting that all nestlings had fledged.
Effects of the immune challenge on parental feeding rates
Prior to treatment, male (0.38 ± 0.02 average feeding rate per minute ± SE) great tits had higher feeding rates compared to female (0.25 ± 0.02 average feeding rate per minute ± SE) great tits (t = 5.5, df = 44, P < 0.0001). In our first model, we found a significant three-way interaction (time by treatment by sex χ² = 8.13, df = 1, P = 0.004). We repeated the analysis for each sex separately, which showed that the change in female feeding rates between pre- and post-treatment tended to differ for immunized females and sham-treated (PBS) females (time by treatment interaction χ² = 3.00, df = 1, P = 0.08; Fig. 3a). LPS-injected females decreased their feeding rate by 3.1 % after the injection, whereas PBS-injected females increased their feeding rate by 14.9 %. The change between pre- and post-treatment feeding rates also differed for males according to the treatment of their female partner (time by treatment interaction χ² = 5.39, df = 1, P = 0.02; Fig. 3b). Males of PBS-injected females decreased their feeding rate by 6.3 %, whereas males of LPS-injected females increased their feeding rate by 11.0 %.
Relationship between immunity and provisioning in females
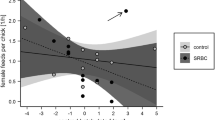
All correlation coefficients for the relationships between baseline immune indices and female pre-treatment feeding rates were negative, but only the negative correlation between haptoglobin and pre-treatment feeding rate was statistically significant (Spearman rank correlation coefficients: HA: r s = −0.15, P = 0.33; HL: r s = −0.17, P = 0.25; Hp: r s = −0.42, P = 0.04; Fig. 4a). We repeated the analyses while excluding the two females who were feeding their nestlings without the help of a male (Spearman rank correlation coefficients: HA: r s = −0.06, P = 0.70; HL: r s = −0.15, P = 0.32; Hp: r s = −0.48, P = 0.02; Fig. 4b).
Relationships between haptoglobin concentrations Hp (mg/mL) and the feeding rate of females pre-treatment (a) on the complete dataset where black circles represent females feeding with the help of their male and open circles represent nests where only the female was feeding the nestlings, while b shows the relationships for the dataset excluding the females that were feeding without the help of their males
Variation in baseline immunity as a possible predictor
The change in feeding rate following LPS injection was not correlated with females baseline innate immunity prior to injection (HA: r p = 0.18, P = 0.42; HL: r p = 0.15, P = 0.50; Hp: r s = 0.42, P = 0.13). Similarly, when taking a different statistical approach, baseline immunity appeared not to be suitable to make predictions on how females react to a challenge in terms of a change in feeding rate, since we found no significant time by immunity interaction (time by HA interaction χ² = 0, df = 1, P = 1.00; time by HL interaction χ² = 0.01, df = 1, P = 0.91; time by Hp interaction χ² = 0.21, df = 1, P = 0.65).
Discussion
In this study, we explored whether variation in baseline innate immune indices contributes to individual differences in the consequences of an immune challenge. We found a negative relationship between innate immunity and female feeding rates pre-treatment only for one out of three parameters studied. There was no evidence that baseline immunity affected the way females reacted to an immune challenge in terms of changes in parental behaviour, although there was substantial variation in female innate immunity.
Effects of the immune challenge on parental feeding rates
Once immunized, treated females tended to feed their young less frequently compared to control females. The observed reduction in feeding rate of females injected with LPS has thus to be interpreted cautiously but supports the idea that mounting an immune response carries a cost in the currency of parental effort as shown in previous studies (Ilmonen et al. 2000; Raberg et al. 2000; Bonneaud et al. 2003). However, species vary in their responses. For example, injected female blue tits (Cyanistes caeruleus) and pied flycatchers (Ficedula hypoleuca) decreased their feeding effort and had lower reproductive output than control females (Ilmonen et al. 2000; Raberg et al. 2000), whereas a study on female European starlings (Sturnus vulgaris) found that an experimentally enhanced immune function did not depress reproductive output (Williams et al. 1999). However, it is possible that a trade-off becomes more visible when an individual has to simultaneously enhance its investment in both reproduction and immunity (Siikamäki et al. 1997; Bonneaud et al. 2003). In the case of our study, females reared their own un-manipulated broods, so their workload remained unaltered (equal to normal conditions).
We do not have a clear explanation as to why control females increased their parental care on a daily basis. It may relate to the stress of handling and sampling, and this effect may have been masked by the immune challenge in treated females. However, this remains speculative, and we will therefore not discuss this in more detail. But the fact that the sex-specific feeding patterns were complementary renders it unlikely that the overall levels of demand increased.
Males of which the female reduced her feeding rate after being injected with an endotoxin responded to this change in their partner’s behaviour by compensating. This corresponds with a comparable study showing that male great tits indeed increase their feeding investment to ensure the success of their offspring (Christe et al. 1996). Our results are also in line with a study in house sparrows (Passer domesticus), showing that males of LPS-injected females tended to increase their feeding rates to compensate for the reduction in female feeding effort (Bonneaud et al. 2003). Yet, in the case of blue tits and pied flycatchers, males of diphtheria-tetanus vaccinated females did not compensate for the reduced feeding rates of their mates (Ilmonen et al. 2000; Raberg et al. 2000). However, these latter studies are in contrast to the general pattern in birds, which is to partially compensate for reduced partner effort (reviewed in Harrison et al. 2009). Yet, it should be noted that also the type of manipulation used can mediate the response of parents (Harrison et al. 2009). For example in our case, males with LPS-injected females were capable to compensate for a decreased partner feeding rate. Whereas great tit males of which the female was handicapped (by clipping a number of feathers) did not compensate but even tended to decrease their feeding rates, while females with a handicapped partner fully compensated (Sanz et al. 2000). In both cases, males are able to recognize and respond (either by reducing their feeding rates or by compensating) to a change in their females’ state. The fact that males compensated for the reduced feeding effort of their females probably explains why we did not find any effect of treatment on nestling body mass, in contrast to comparable studies on pied flycatchers and blue tits (Ilmonen et al. 2000; Raberg et al. 2000).
Relationship between immunity and provisioning in females
The correlations between female provisioning and female baseline immune parameters were negative for all innate immune parameters measured here, but only the relationship with the acute phase protein haptoglobin was statistically significant. This significant relationship existed for both females feeding with their partner as for females feeding without the help of their male. However, it remains elusive why such relationship was restricted to Hp, and the results have thus to be interpreted cautiously. Unfortunately, thus far, no other study has focused on natural variation in immunity in relation to parental provisioning, which limits the comparison with earlier studies and other species. Most studies to date have examined the link between life-history decisions and immune defences in birds for example via brood size manipulations or injections activating the immune system (Deerenberg et al. 1997; Nordling et al. 1998; Ilmonen et al. 2000; Raberg et al. 2000; Bonneaud et al. 2003; Verhulst et al. 2005). But, only very few have studied natural variation in immunity to examine the trade-off between reproduction and other important fitness components (Merilä and Andersson 1999) or the capacity of baseline immunity to predict subsequent immune responses (Matson et al. 2012). To our knowledge, this study is the first to investigate correlations between baseline innate immunity and provisioning in un-manipulated free-living birds. However, care must be taken when interpreting these results since the relationships found in this study show only weak support for the existence of a trade-off. Further, we would like to highlight that the birds used in this study were presumably infested with their natural parasites (e.g. hen fleas, Ixodes ricinus, mites, bacteria) since we performed our research under natural conditions. The presence of these natural parasites might have partially contributed to the variation in baseline immune levels we observed. Thus, the possibility of natural parasites causing variation should be kept in mind when interpreting the results of this study.
Variation in baseline immunity as a possible predictor
Baseline immune indices have previously been used as a predictor of local recruitment, establishment success, dispersal ability and long-term survival (e.g. Saino et al. 1997; Møller and Cassey 2004; Møller et al. 2004; Møller and Saino 2004; Cichon and Dubiec 2005; Moreno et al. 2005). However, the capacity of baseline immune parameters to predict subsequent immune responses is as yet unknown (Matson et al. 2012). In this study, we explored if several innate immune measures (NAbs, complement activity and haptoglobin concentrations) were able to predict the consequences of an immune challenge, here measured in terms of changes in parental effort. However, we found no evidence that baseline immunity altered the way how females reacted to our immune challenge. As pointed out above, there is variation in the response of birds to an immune challenge (Williams et al. 1999; Ilmonen et al. 2000; Raberg et al. 2000; Bonneaud et al. 2003), and we hypothesized that this variation might at least partly be due to variation in baseline immunity (Cichon and Dubiec 2005). However, our results show that having a high standing level of immunity does not necessarily mean that an individual will pay different fitness costs in response to an immune challenge. Innate immunity might be a potential mechanism to compensate for a suboptimal major histocompatibility complex diversity (Kurtz et al. 2004), indicating the relevance of investigating different immune traits simultaneously. Yet, here, the adaptive value of elevated baseline immunity, as well as the causes and consequences of variation in baseline immunity, remains unclear.
Conclusions
Our results provide only weak support for the existence of a trade-off between investment in reproduction and investment in immunity in female birds during the period of parental care. Interestingly, males compensated for the change in female feeding behaviour, and we consequently did not observe any changes in offspring development or survival. Further, we showed that variation neither in NAbs nor in complement activity nor in haptoglobin concentrations predicted variation in the consequences of an immune challenge, even though there was substantial variation in female innate immune indices.
References
Abbas AK, Lichtman AH (2010) Basic immunology: functions and disorders of the immune system. Saunders/Elsevier, Philadelphia
Bates D, Maechler M, Bolker B, Walker S (2013) lme4: linear mixed-effects models using Eigen and S4. R package version 1.0-4, http://CRAN.R-project.org/package=lme4.
Boes M (2000) Role of natural and immune IgM antibodies in immune responses. Mol Immunol 37:1141–1149
Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G (2003) Assessing the cost of mounting an immune response. Am Nat 161:367–379
Buehler DM, Bhola N, Barjaktarov D, Goymann W, Schwabl I, Tieleman BI, Piersma T (2008) Constitutive immune function responds more slowly to handling stress than corticosterone in a shorebird. Physiol Biochem Zool 81:673–681
Christe P, Oppliger A, Richner H (1994) Ectoparasite affects choice and use of roost sites in great tit, Parus major. Anim Behav 47:895–898
Christe P, Richner H, Oppliger A (1996) Begging, food provisioning, and nestling competition in great tit broods infested with ectoparasites. Behav Ecol 7:127–131
Cichon M, Dubiec A (2005) Cell-mediated immunity predicts the probability of local recruitment in nestling blue tits. J Evol Biol 18:962–966
Clayton DH, Wolfe ND (1993) The adaptive significance of self-medication. Trends Ecol Evol 8:60–63
Costantini D, Møller AP (2009) Does immune response cause oxidative stress in birds? A meta-analysis. Comp Biochem Physiol A 153:339–344
Cray C, Zaias J, Altman NH (2009) Acute phase response in animals: a review. Comparative Med 59:517–526
Deerenberg C, Arpanius V, Daan S, Bos N (1997) Reproductive effort decreases antibody responsiveness. Proc R Soc Lond B 264:1021–1029
Demas GE, Nelson RJ (2012) Ecoimmunology. Oxford University Press, Oxford
Fang HQ, Pengal RA, Cao XH, Ganesan LP, Wewers MD, Marsh CB, Tridandapani S (2004) Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J Immunol 173:360–366
Gray DA, Marais M, Maloney SK (2013) A review of the physiology of fever in birds. J Comp Physiol B 183:297–312
Harrison F, Barta Z, Cuthill I, Szekely T (2009) How is sexual conflict over parental care resolved? A meta-analysis. J Evol Biol 22:1800–1812
Hasselquist D, Nilsson JA (2012) Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Anim Behav 83:1303–1312
Ilmonen P, Taarna T, Hasselquist D (2000) Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc R Soc Lond B 267:665–670
Janssens E, Dauwe T, Bervoets L, Eens M (2001) Heavy metals and selenium in feathers of great tits (Parus major) along a pollution gradient. Environ Toxicol Chem 20:2815–2820
Knowles SCL, Nakagawa S, Sheldon BC (2009) Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: a meta-regression approach. Funct Ecol 23:405–415
Kurtz J, Kalbe M, Aeschlimann PB, Häberli MA, Wegner KM, Reusch TBH, Milinski M (2004) Major histocompatibility complex diversity influences parasite resistance and innate immunity in sticklebacks. Proc R Soc Lond B 271:197–204
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98
Matson KD, Ricklefs RE, Klasing KC (2005) A hemolysis-hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev Comp Immunol 29:275–286
Matson KD, Tieleman BI, Klasing KC (2006) Capture stress and the bactericidal competence of blood and plasma in five species of tropical birds. Physiol Biochem Zool 79:556–564
Matson KD, Horrocks NPC, Versteegh MA, Tieleman BI (2012) Baseline haptoglobin concentrations are repeatable and predictive of certain aspects of a subsequent experimentally-induced inflammatory response. Comp Biochem Physiol A 162:7–15
McKean KA, Yourth CP, Lazzaro BP, Clark AG (2008) The evolutionary costs of immunological maintenance and deployment. BMC Evol Biol 8:76
Merilä J, Andersson M (1999) Reproductive effort and success are related to haematozoan infections in blue tits. Ecoscience 6:421–428
Millet S, Bennett J, Lee KA, Hau M, Klasing KC (2007) Quantifying and comparing constitutive immunity across avian species. Dev Comp Immunol 31:188–201
Møller AP, Cassey P (2004) On the relationship between T-cell mediated immunity in bird species and the establishment success of introduced populations. J Anim Ecol 73:1035–1042
Møller AP, Saino N (2004) Immune response and survival. Oikos 104:299–304
Møller AP, Martίn-Vivaldi M, Soler JJ (2004) Parasitism, host immune defence and dispersal. J Evol Biol 17:603–612
Moreno J, Merino S, Sanz JJ, Arriero E, Morales J, Tomás G (2005) Nestling cell-mediated immune response, body mass and hatching date as predictors of local recruitment in the pied flycatcher Ficedula hypoleuca. J Avian Biol 36:251–260
Murata H, Shimada N, Yoshioka M (2004) Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J 168:28–40
Murphy K, Travers P, Walport M, Janeway C (2012) Janeway’s immunobiology. Garland Science, New York
Nordling D, Andersson M, Zohari S, Gustafsson L (1998) Reproductive effort reduces specific immune response and parasite resistance. Proc R Soc Lond B 265:1291–1298
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11:19–26
Ochsenbein AF, Zinkernagel RM (2000) Natural antibodies and complement link innate and acquired immunity. Immunol Today 21:624–630
Poxton IR (1995) Antibodies to lipopolysaccharide. J Immunol Methods 186:1–15
Quaye IK (2008) Haptoglobin, inflammation and disease. Trans Roy Soc Trop Med Hyg 102:735–742
Raberg L, Nilsson JA, Ilmonen P, Stjernman M, Hasselquist D (2000) The cost of an immune response: vaccination reduces parental effort. Ecol Lett 3:382–386
Saino N, Bolzern AM, Møller AP (1997) Immunocompetence, ornamentation, and viability of male barn swallows (Hirundo rustica). P Natl Acad Sci USA 94:549–552
Sanz JJ, Kranenbarg S, Tinbergen JM (2000) Differential response by males and females to manipulation of partner contribution in the great tit (Parus major). J Anim Ecol 69:74–84
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Siikamäki P, Ratti O, Hovi M, Bennett GF (1997) Association between haematozoan infections and reproduction in the Pied Flycatcher. Funct Ecol 11:176–183
Verhulst S, Riedstra B, Wiersma P (2005) Brood size and immunity costs in zebra finches Taeniopygia guttata. J Avian Biol 36:22–30
Vermeulen A, Müller W, Matson KD, Tieleman BI, Bervoets L, Eens M (2015) Sources of variation in innate immunity in great tit nestlings living along a metal pollution gradient: an individual-based approach. Sci Total Environ 508:297–306
Williams TD, Christians JK, Aiken JJ, Evanson M (1999) Enhanced immune function does not depress reproductive output. Proc R Soc Lond B 266:753–757
Acknowledgments
We greatly thank Jel D’Hollander for assisting in the field, Josie Meaney-Ward for improving the English and Thomas Raap for his valuable comments on the manuscript. Further, we would like to thank Kevin D. Matson for teaching us the immune techniques. We would also like to thank the University of Antwerp for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethical committee of the University of Antwerp (ID number 201131), and it was performed in accordance with Belgian and Flemish laws.
Funding
This study was made possible by a PhD studentship to AV from the University of Antwerp.
Informed consent
This article does not contain experiments with human participants.
Additional information
Communicated by I. R. Hartley
Significance Statement
Innate immunity is a central component of vertebrate immunity, representing the first line of defence. However, there is a large amount of among individual variation in baseline innate immunity, potentially indicating differences in individual defence strategies, as baseline levels may be costly to maintain while being central for the effectiveness of a first immune response. To study the adaptive significance of baseline innate immunity levels, we investigated whether the trade-off between raising an immune response and parental provisioning depended on (previously measured) baseline innate immunity levels. We found only weak support for the existence of a trade-off, which was also unaffected by the levels of baseline innate immunity. Thus, the functional consequences of variation in innate immunity levels remain unclear, urging for further studies to explore the relatively unknown field of innate immunity.
Rights and permissions
About this article
Cite this article
Vermeulen, A., Eens, M., Zaid, E. et al. Baseline innate immunity does not affect the response to an immune challenge in female great tits (Parus major). Behav Ecol Sociobiol 70, 585–592 (2016). https://doi.org/10.1007/s00265-016-2077-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2077-3