Abstract
During the reproductive season, male Hyla versicolor produce advertisement calls to attract females. Females exhibit phonotaxis and approach the individual callers, resulting in amplexus. For frogs that call from dense choruses, the extent to which and the range from which a male’s advertisement call within a chorus can be heard by a receptive female leading to phonotaxis is unclear. We investigated females’ responses to natural choruses in the field and found that they were attracted and showed directed orientation to breeding choruses at distances up to 100 m. To assess the role of acoustic cues in the directed orientation, we conducted acoustic playback experiments in the laboratory using conspecific call and noise as stimuli, as well as chorus sounds (that contained calls from a focal male) recorded at various distances, all played at naturalistic intensities. Using two response metrics (females’ normalized response times and their phonotaxis trajectories) we found that, unlike the field experiments, females oriented and were attracted to chorus sounds from 1 to 32 m only, but not from >32 m, or to band-limited noise. Possible reasons for the observed difference in phonotaxis behavior in the two experimental conditions were discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustic signals are widely used for communication in many taxa and serve numerous functions including sexual advertisement, mate choice, territorial defense, and other interactions. In socially aggregating animals, acoustic recognition, discrimination, and localization are made difficult due to the presence of competing background sound and changes in the signal features due to environmental interactions (Lohr et al. 2003; Feng and Schul 2007; Swanson et al. 2007). Noise from biotic (Gerhardt and Klump 1988a; Wollerman 1999; Wollerman and Wiley 2002) and abiotic (Bee and Swanson 2007) sources compromises signal detection and localization due to masking. Masking by signals of conspecifics is particularly problematic because they share many of the frequency and temporal characteristics with a signal of interest (Jouventin et al. 1999; Aubin and Jouventin 2002). Detection and localization of auditory signals are also hampered by environmental degradation, e.g., atmospheric attenuation, signal absorption, and reflection from vegetation and the substrate (Forrest 1994; Luther and Wiley 2009). Signal-environmental interactions alter both the temporal and spectral characteristics of acoustic signals (Richards and Wiley 1980; Dabelsteen et al. 1993; Penna and Solis 1998).
During the reproductive season, male anurans aggregate and produce advertisement calls to attract mates, while females use these signals as a basis for mate selection (Narins et al. 2007). Most behavioral studies examining female mate choice use clean and well-defined acoustic signals with high signal-to-noise ratios (Gerhardt 1981, 2005; Gerhardt and Doherty 1988; Bush et al. 2002). Such studies have successfully elucidated, for a number of anuran species, the salient features in the male’s call that attract female frogs and give rise to their behavioral preferences (Gerhardt 1981; Gerhardt and Doherty 1988; Gerhardt and Schul 1999; Gerhardt et al. 2000). However, we have limited understanding of the extent to which and the range from which a male’s advertisement call within a chorus, or the chorus itself, is used to guide female’s phonotaxis.
The use of acoustic cues for orientation in complex auditory environments has recently been investigated in a few taxa with mixed results (Slabbekoorn and Bouton 2008). Among anuran amphibians, there is evidence for positive phonotaxis to choruses in several species, including Rana sylvatica (Bee 2007a, b), Hyla gratiosa (Gerhardt and Klump 1988b), and Hyla chrysoscelis (Swanson et al. 2007). These chorus attraction studies have focused on the attractive qualities of the chorus itself, without examining how distance to the chorus affects the spectral and temporal characteristics of individuals’ calls. Male gray treefrogs (Hyla versicolor) also form dense choruses during the mating season from which they emit advertisement calls (Schwartz et al. 2004). The chorus sound can propagate over several hundred meters and remain at levels well above the measured hearing and behavioral thresholds for anurans (Gerhardt and Klump 1988b; Narins and Zelick 1988; Sinsch 1990a). Female H. versicolor may travel hundreds of meters from daytime feeding and resting sites to breeding ponds (Johnson and Semlitsch 2003). The sensory cues that females use to localize and orient towards the ponds remain unclear, however.
In the present study we examined the attraction of female Hyla versicolor to both natural chorus sounds as well as male calls embedded within a chorus. In the first part of the study, we studied females’ responses to natural choruses in the field. In the second part, to assess the role of acoustic cues in directed orientation in the field we examined females’ responses to male calls embedded within a chorus in a laboratory.
Materials and methods
Subjects and collection/study sites
We collected female gray treefrogs (Hyla versicolor) of the northwestern mitochondrial lineage (Ptacek et al. 1994) from breeding choruses in the Thomas Baskett Wildlife Area and Three Creeks State Park, near Ashland, Missouri. Gravid females in amplexus were caught by hand and placed in plastic containers on ice overnight to prevent oviposition. We carried out field and laboratory phonotaxis experiments on the following day. A total of 19 and 34 females were used for the field and laboratory phonotaxis studies, respectively. At the conclusion of each experiment, we released all animals back to their native ponds.
The study site for the chorus recording and field phonotaxis experiments was a large pond, approximately 60 by 30 m, surrounded by woodland. The ground was level around most of the pond, but sloped on the north side, starting about 2–3 m from the pond’s edge, and extending for ~3–4 m away and ~2 m down from the pond. The ground then gently sloped in a north–north-easterly direction. Vegetation consisted of mixed hard and softwoods (with cedar and locust the most prevalent species) as well as woody shrubs <1 m in height. Vegetation was most dense at the pond’s edge, consisting of trees and shrubs. Beyond ~2 m, vegetation decreased in density and was mostly confined to tall (>5 m) trees and short (<1 m) vegetation. The substrate was leaf litter that lying on top of soil.
Field phonotaxis studies
All field phonotaxis studies took place at the Thomas Baskett Wildlife Area, between 22:00 pm and 1:00 am. Only gravid females originally collected from this site were used. Two transects were made from the edge of the main breeding pond out to a distance of 75–100 m. Care was taken to ensure that each transect led directly away from the main pond and not toward any other ponds which could contain choruses and act as potential stimuli. We constructed a series of 4 circular arenas along each transect at the following distances from the pond: 10, 25, 50, and 75 or 100 m (depending on local terrain). A series of 36 numbered marker flags was arraigned in a circle of 1 m radius around the center, in 10° arc intervals. In each arena, the majority of leaf litter, small plants, grasses, and shrubs was removed, leaving a substrate of soil with some leaves. The 10-m arena served as a control trial for the calculation of phonotaxis scores (see “Statistics”), and females were expected to orient directly to the pond at this distance. The other distances served as experimental trials.
Prior to each experimental session, we removed the females from ice and allowed them to warm to ambient temperature for 15–20 min. For each female we chose one of the two transects, and tested the subject in each of the four arenas, in random order. At each distance, we placed the female in the center of the arena in an acoustically transparent plastic release cage (random facing; ~12 cm diameter). Prior to this, the cage floor was dusted with an orange non-toxic UV fluorescent dye powder (DayGlo Color Corp.)—allowing tracking of the female (Windmiller 1996; Eggert 2002). All lights were switched off and the female was given >15–30 s to acclimate. Then the lid of the cage was remotely lifted by an observer standing outside of the arena border, and a timer started. The observer monitored the animal’s locomotion with a night vision monocle (Night Patriot II, ATN Corp.). When the female left the arena boundary, the timer was stopped and the time noted, along with the two flags through which the female passed to exit the arena. If the female did not leave the release cage after 10 min, the female was considered unresponsive, and data were not collected. Exit point determination and animal recovery were aided by visualizing the fluorescent powder the female deposited on the ground using a UV lamp (Blak-Ray ML-49, UVP). After the female was tested at all arenas, any residual powder was washed with pond water and the female was released.
Chorus recordings
Recordings of frog choruses were made from ~22:00 pm to 1:00 am, when the chorus density and amplitude were at their peaks (personal observation). We measured the sound pressure level (SPL) of the chorus before and after recording of the chorus with a digital SPL meter (Bruel and Kjær 2239, fast RMS, C-weighted, re: 20 μPa). C-weighting results in band-pass attenuation of frequencies < ~100 Hz and > ~6 kHz, with low-frequency roll-off less steep than standard A-weighting. The SPL was measured at four different positions (roughly north, south, east, and west of the breeding pond), each approximately 15 m from the edge of the breeding pond. Three SPL values (10 s averaged Leq) were taken at each of the four locations and used as a rough estimate of chorus intensity. The differences in SPL values between each location remained fairly constant throughout the duration of the experiment (data not shown).
We then selected an active calling male H. versicolor at the pond’s edge (perching on a tree at a height of >20 cm from the ground) as a “focal” male. All focal males were at least 1 m distant from the closest neighboring males to ensure that at the closest distances (1–4 m) the calls of the focal male are the most prominent. We recorded his vocal signals using a hypercardioid microphone (Seinnheiser model ME-66 passed through a Sound Devices MP-1 preamplifier) placed within 20 cm of the male. A reel of twine was run in a straight line away from the focal male and chorus, in a direction that avoided nearby ponds with potentially interfering choruses. The twine was used to mark various distances from the focal male: 1, 4, 8, 16, 32, 50, and 100 m. At each distance we set up a single omnidirectional microphone (Audio Technica model AT899 with AT8537 power module) at two elevations (~6 cm and ~1 m above the substrate) to record the chorus sounds. Output from both the hypercardioid and omnidirectional microphones was fed to a digital sound recorder (Nagra ARES-BB; 16-bit mode, 44.1 kHz sampling rate).
Laboratory phonotaxis studies
We conducted laboratory phonotaxis experiments in a temperature-controlled and anechoic chamber at 20 ± 1°C. The chamber measured 15 m long, 3 m wide, and 2.5 m high, with 10 cm acoustic foam (Technifoam, Circle Pines MN) on all surfaces, backed by 10 cm of sound absorbing material. Prior to the start of the experiment, females were removed from the ice and placed in plastic containers in an incubator (kept at 20°C) to acclimate to the temperature of the chamber. Within the chamber was a circular arena 2 m in diameter and 40 cm in height, composed of wire mesh covered in black fabric (to eliminate the potential use of visual cues). The circumference was divided into thirty-six 10° arc segments. Acoustic stimuli were presented via a 3″ loudspeaker (Aura Sound NS3-194-8E in a custom-made casing; ±4 dB frequency response from 200 Hz to 16 kHz) placed on the floor outside the arena wall. For synthetic stimuli and the 1-m chorus stimulus, the loudspeaker was placed directly outside the arena wall, 1 m from the center of the arena. For all remaining chorus stimuli the loudspeaker was placed an additional 0.5 m from the arena wall (1.5 m distant from the center of the arena) to reduce or eliminate possible near-field effects not experienced by frogs exposed to distant chorus sounds. The loudspeaker was placed at different arc segments which were changed at random during the experiments to eliminate possible directional biases of females.
From the chorus recordings we selected exemplars on the basis of the chorus density, as approximated by the SPL measurements (unpublished data). Exemplars were taken from the three nights showing the highest average SPL values (5/19/2006, 5/23/2006, and 5/24/2006). Stimuli were chosen from recordings made from the ground, as females travel along the ground en-route to the chorus (personal observation). From the recordings made on each of the three nights mentioned earlier, we selected a 20-s segment of chorus sound when the focal male called uninterruptedly and not masked by calls of the nearest neighbors. This was looped to create a chorus stimulus with a total duration of 5 min. Chorus stimuli were played back at SPL levels approximating the levels in the field at their respective distances, including fluctuations in SPL between 5 and 12 dB (Table 1), which matched well to values for this species encountered in the field (Gerhardt 1975).
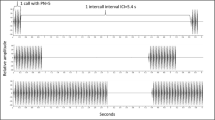
Playback stimuli comprised chorus sounds and three synthetic stimuli: a synthetic conspecific call (‘Hv’) and a band-limited noise (‘Noise’). The synthetic stimuli were created using Adobe Audition, and presented at an inter-stimulus interval of 3.125 s—a period within the range observed from recordings in the field (unpublished data). The synthetic Hv call was a positive control stimulus, as it has been shown to be a highly attractive stimulus in previous phonotactic studies (Gerhardt and Doherty 1988; Bush et al. 2002; Beckers and Schul 2004)—the call consisted of 18 sound pulses of 25 ms duration with an inter-pulse interval of 25 ms, resulting in a total call duration of 875 ms (Fig. 1a). Each sound pulse in the synthetic call consisted of 2 phase-locked sinusoids of 1.1 and 2.2 kHz (the dominant spectral components of natural H. versicolor calls), with the latter 6 dB more intense than the former, and had a rise/fall time of 80 and 20% of the pulse duration, respectively. To control for responses to acoustic energy in general, a band-limited noise stimulus with a flat spectrum over 0.1–22 kHz was also presented (Fig. 1b). All synthetic stimuli were played back at 85 dB SPL, measured at the center of the arena, equivalent to the amplitude of a calling male at ~1 m distance (Gerhardt 1975).
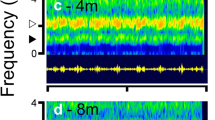
a–h Oscillograms (bottom) and spectrograms (top) of brief (400 ms) segments of control and chorus stimuli used in the laboratory phonotaxis experiments, taken at the middle of the focal male’s call. a Synthetic Hv call, b band-limited noise c 1 m chorus, d 4 m chorus, e 8 m chorus, f 16 m chorus, g 32 m chorus, h 50 m chorus. Insets on c–f show oscillograms of chorus recordings (15 s duration) of chorus recordings during which the focal male called repeatedly. Arrows indicate calls emitted by the focal male
The general experimental protocol followed that of Bush et al. (2002) and Beckers and Schul (2004). For each female, we randomly selected a chorus exemplar; all chorus stimuli presented to the female were from the same recording date. An experimental trial comprised a single chorus exemplar or noise; presentation of synthetic conspecific calls constituted a control trial. At the start of a trial, females were placed inside a wire-mesh release cage (~10 cm diameter) in the center of the arena with a random facing. After 15 s of stimulus playback, the cage was remotely lifted. The animal’s movements were monitored and recorded by a video camera (Fig. 5). We recorded three measures of responsiveness: (1) the time for the female to touch the arena wall regardless of the location on the wall, (2) the time for the female to touch the wall in the immediate vicinity (within 15 cm) of the loudspeaker, (3) the location (arc segment) on the arena wall which the female contacted. A score of ‘No response’ was recorded if the female remained in the release cage for 3 min for synthetic stimuli, or 5 min for chorus stimuli. If a female took longer than 5 min to reach the arena wall for synthetic stimuli, or 10 min with chorus stimuli, a score of ‘No response’ was also recorded.
Each female began an experimental session with three control stimuli, with the first two functioning to acclimate the female with handling, while data were only recorded for the third. If on any subsequent control trial the female took longer to reach the loudspeaker than twice this value, the data for the previous two experimental trials were discarded, and the experimental session was terminated for that female. After each control trial we presented the female with two experimental trials (stimuli chosen at random), followed by another control trial, then two more experimental trials, and so on. In between experimental trials females were returned to the incubator for a minimum of 5 min to maintain constant body temperature.
Statistics
For the field studies, the time it took a female to exit the arena boundary was used to calculate a phonotaxis score, as described by Bush et al. (2002). This measure of signal attractiveness was the ratio of the mean of the response times for the control (10 m) stimuli, to the response of the experimental trial (25, 50 and 75/100 m) itself (t control/t experimental). A score of 1 indicates that the female took the same amount of time to exit in response to an experimental stimulus as to the control stimulus. A score <1 indicates reduced attractiveness (longer time to exit), while a score >1 suggests a greater attractiveness to the stimulus. Data were pooled over transect for each distance in the field, and compared with Kruskal–Wallis tests and Bonferroni comparisons (α = 0.05, Zar 1999). All scores are expressed as the mean ± standard error of the mean (SEM) for each chorus distance.
Orientation data from females exiting the arenas were pooled for each transect and were used to calculate a mean angle (±standard deviation) of orientation for each arena distance, an associated r-statistic (the length of the mean orientation vector), and 95% confidence intervals of the mean. Randomness of the distributions was tested using both Rayleigh and V tests with a specified target angle of 0°, corresponding to a direct line to the chorus (α = 0.05, Batschelet 1981).
For the laboratory phonotaxis experiments, phonotaxis scores were determined in a similar manner to that described above. The phonotaxis score was the ratio of the mean of the response times for the control stimuli before (t Hv pre) and after (t Hv post) an experimental trial, to the response of the experimental trial itself (t control/t experimental, where \( t_{\text{control}} = {\frac{{t_{\text{Hv\,pre}} t_{\text{Hv\,post}} }}{2}} \) . ‘No response’ trials were given a score of 0. All scores are expressed as the mean ± standard error of the mean (SEM) for each acoustic stimulus type.
To justify pooling scores for wall- and speaker-contacts for chorus stimuli over all exemplar dates, we ran multiple Kolmogorov–Smirnov (K–S) tests (α = 0.05) on the cumulative distribution functions of the power spectra of the chorus samples used in stimulus creation. For each chorus distance, the power spectra of each exemplar was calculated with sound analysis software (Audacity; FFT: Hanning window, 512 samples), and the K–S tests was run using Matlab (The Mathworks, Inc.). The null hypothesis of equal distributions could not be falsified, so the data were pooled over the exemplar dates. Wall- and speaker-contact derived phonotaxis scores were compared with Kruskal–Wallis one-way analysis of variance tests with post-hoc Bonferroni comparisons (Zar 1999). In addition, means ±25 and 75% confidence limits were examined to see if they overlapped the value of 1.0, which would suggest that female responsiveness is similar to that of the synthetic control stimuli.
The wall-contact data in the laboratory were pooled by stimulus type over all three exemplar dates. From this we calculated mean angles (±standard deviation), the associated r-statistic, and 95% confidence intervals of the mean for each experimental distance. As in the field studies, we tested randomness using both Rayleigh and V tests with a specified target angle of 0° (α = 0.05, Batschelet 1981). As females were tested multiple times with the control synthetic stimulus, a mean angle (±SD) was calculated for each female to avoid pseudoreplication (Hurlbert 1984). We averaged each female’s responses to the control stimuli. The arena arc segment corresponding to the mean angle was then used as contact data for that female.
Results
Chorus recordings
At 1 m, the calls of the focal male were readily distinguishable from those of neighboring males (Fig. 1c, inset). Away from the focal male, his calls became less intense and the individual pulses became less distinct, while the calls of nearby conspecific males approached and could even exceed the intensity of that of the focal male (Fig. 1d, e, insets). At longer distances (>16 m) the amplitude modulation of individual frog calls merged into the chorus background, the overall intensity dropped, and other biotic noise with energy in higher-frequency bands became more noticeable (Fig. 1f, inset, e–h).
Orientation in the field
Females showed directed orientation to the chorus at all tested distances from the chorus, up to 100 m (Table 1; Fig. 2). Arena exit locations were non-random for all tested distances in the field (V test, p ≤ 0.01). The exit locations, reflected in the angles and lengths of the mean vectors (Fig. 3a–d), were directed toward the pond—the orientation degraded only slightly with increasing distance to the chorus. Their phonotaxis scores at any one distance, as well as the raw arena exit time, were not significantly different from the scores or times at any other distance (Kruskal–Wallis, F 2,50 = 3.18; p ≤ 0.05). The standard error intervals for all three distances overlapped the control value of 1.0, suggesting that the mean phonotaxis scores do not differ from the values for the 10 m control.
Phonotaxis scores (mean ± SEM) from orienting responses to the chorus in the field; data were pooled from all transects. Scores were calculated as the ratio of the time to exit the arena at the 10-m distance to the time to exit the arena at the 25, 50, or 75/100 m distances. The 10-m scores are used as a reference
a–d Circular histograms indicating responses and mean directional vectors of females to choruses in the field. Each dot represents a female exiting the arena between the flags staked at 10° arc segments, relative to a vector oriented directly at the chorus. All orientation distributions significantly differ from a random distribution (V test, p ≤ 0.01, Batschelet 1981)
Phonotaxis in the laboratory
The percentage of females reaching the arena wall in the laboratory ranged widely, from a low of 38% in response to noise to a high of 96% in response to conspecific control stimulus (Table 2). Chorus signals elicited increasing activity with decreasing chorus distance.
Phonotaxis scores were low for the noise stimuli, with values of 0.14 ± 0.4 and 0.02 ± 0.01, for wall- and speaker-contact, respectively (Table 2; Fig. 4). For the chorus sounds, responses were generally robust at short chorus distances (1–8 m), and became progressively weaker with increasing distance (Table 2; Fig. 4). Both wall- and speaker-derived scores showed significant differences in the responses to the various stimuli (Kruskal–Wallis one-way ANOVA: F 8,252 = 1.98; p ≤ 0.05; post-hoc pairwise comparisons). Scores from the 1–16 m stimuli (wall 0.54–0.43; speaker 0.47–0.27) indicate the quickest wall- and speaker-contacts. With stimuli recorded at increasing distances, phonotaxis scores dropped rapidly, with wall- and speaker-contact scores of 0.26–0.25 and 0.12–0.10, respectively, from 32 to 100 m. The phonotaxis scores at the furthest distances (50 and 100 m) were not significantly different from responses to noise. Only two stimuli, the 1 and 8-m chorus distances, encompassed the theoretical normalized control value of 1.0 within their 25 and 75% confidence intervals of the mean scores, suggesting that the other stimuli were significantly less attractive.
Phonotaxis scores (mean ± SEM) for control and experimental acoustic stimuli from pooled data for all chorus exemplars. The circle and square symbols represent phonotaxis scores calculated using the time to reach the arena wall (irrespective of the location on the wall) and the time to reach the loudspeaker (within 10 cm), respectively. Horizontal bars indicate Bonferroni multiple comparison grouping (p ≤ 0.05) after Kruskal–Wallis one-way ANOVA test. Speaker-derived scores whose 25 and 75% confidence intervals for the mean which did not include 1.0 (corresponding to the positive control stimuli) are denoted by asterisks
Females’ phonotactic trajectories for the various stimuli are shown in Fig. 5. When presented with attractive stimuli, females moved in short bouts interspersed with pauses; females often re-oriented their trajectories in-between movement bouts, giving rise to ‘zig-zag’ movement patterns described previously (Feng et al. 1976). In response to the conspecific control stimulus, females rapidly and accurately localized the loudspeaker (Fig. 5a). Accurate localization was also seen for the 1 and 8-m chorus stimuli (Fig. 5c, d). With increasing distance (Fig. 5e–f), however, wall contacts became more random and less accurate.
a–e Phonotaxis trajectories of female treefrogs for: a synthetic Hv call (n = 35), b band-limited noise (n = 25), c 1 m chorus (n = 31), d 8 m chorus (n = 30), e 32 m chorus (n = 23), f 50 m chorus (n = 25). All traces have been aligned with respect to the acoustic stimulus presented from the top of each graph
When presented with noise stimulus, many females moved a short distance and stopped (Fig. 5b). Those females that moved for longer distances tended to wander with random orientations, and more females who left the cage failed to touch the arena wall compared to the other stimuli (Fig. 5b, unpublished data).
Analysis of the wall-contact data (Fig. 6a–f) showed a similar trend to the phonotaxis score data. Orienting responses to the synthetic conspecific calls were significantly non-random (V test, p ≤ 0.01), while those to noise were essentially random. Responses to the chorus stimuli (Table 2; Fig. 6c–f) were non-random for distances from 1 to 32 m, whereas at 50 and 100 m, responses were random. Mean vector lengths (r) were similar at the closest chorus distances, e.g., 0.8 at 1 m and 0.77 at 8 m (Table 2), and decreased with increasing distance (Table 2).
a–f Circular histograms showing the distributions of the locations of arena wall contacted by females (oriented with the direction of the stimulus at the top of each figure). Shown are results of phonotactic responses to a synthetic Hv call, b band-limited noise, c 1 m chorus, d 8 m chorus, e 32 m chorus, f 50 m chorus. Each dot represents a single wall contact in the corresponding 10° arena wall segment. Each open triangle represents ten contacts. Arrows represent the mean directions of all contacts, with the length corresponding to the r statistic (shown below each histogram). Solid and dotted lines represent the SD and 95% confidence interval, respectively, for the mean angle. Asterisks denote contact distributions that significantly differ from random (V test, p ≤ 0.01, Batschelet 1981)
Discussion
Close to the breeding pond, the calls of the nearest males are distinct with clear temporal structure. Recordings made at further distances show a rapid reduction in the amplitude of male calls as well as the masking and degradation of the temporal structure as formerly distinguishable calls ‘blend’ into the chorus background. Female H. versicolor orient towards choruses in the field at distances up to 100 m, but in phonotaxis tests in the laboratory females show directed orientation only to recordings made at a distance of up to 32 m.
A comparison of the field and laboratory orientation data shows that there is less variation in females’ orienting responses in the field with increasing distance, on the basis of the arena-exit times (Table 1; Fig. 1) and the distribution of the arena exit angles (Fig. 2). A number of factors likely contribute to the discrepancies between the field and laboratory data. The sources of the acoustic stimuli differ in the two experiments. In the laboratory, the chorus is broadcast from a single source—the playback loudspeaker, whereas the chorus sound in the field is a distributed source with origins over a large geographical area. In theory, a stimulus originating from a point source can be localized more easily, considering the directional nature of the anuran external ear (Feng and Shofner 1981) and the absence of competing sources and interfering signals (Jørgensen and Gerhardt 1991; Schwartz and Gerhardt 1995). In the laboratory, the fact that distant chorus stimuli did not reliably elicit orientation, despite playback from a point source, suggests that recognition, rather than localization, is likely the primary problem, and in particular the temporal structure of the species vocal signal at long distances is compromised (see Fig. 1) making it difficult to recognize.
Another significant difference between the laboratory and field experimental conditions is the sensory cue(s) available to the frogs. In the laboratory, females have only the acoustic cues for orientation, whereas in the field females may use olfactory, visual, and/or magnetic cues in addition to auditory cues. Previous studies have shown that anuran amphibians utilize any or all of the aforementioned sensory cues to localize and orient to a breeding pond when manually displaced from it (Sinsch 1990a, b; Murphy 2008). The use of other modalities does not preclude the use of distant (>32 m) chorus sound for orientation, which may act as an adjunct ‘unreliable’ signal (sensu Candolin 2003) that might aid orientation when presented in concert with other sensory cues, but by itself is not sufficient to localize distant choruses.
The fact that females oriented towards choruses with more challenging auditory characteristics (i.e. multi-dimensional vs. point) with superior accuracy suggests the important role of non-auditory cues. Future studies of the females’ acoustically guided orientation to the chorus in the field should carefully control for the other sensory modalities.
Our results demonstrate that the attractiveness of male’s calls embedded in a chorus varied with distance in a laboratory setting. Female H. versicolor reliably localize artificial conspecific calls and approach them within a short time (e.g. Diekamp and Gerhardt 1995; Bush et al. 2002; Beckers and Schul 2004). This was reflected in the high percentage of females that reached the playback loudspeaker (Table 2), the nonrandom distribution of contact angles (Fig. 6a), the statistics of the mean vector of orientation (Table 2), and the narrow distribution of movement trajectories in the arena (Fig. 5a). Responses to band-limited noise were poor. Only 38% of females contacted the arena wall and contact angles were randomly distributed, resulting in the lowest phonotaxis scores for any tested stimuli (Table 2; Figs. 4, 6b). We believe that this represents a baseline response to acoustic noise in general, and indicates a non-salient stimulus.
Female H. versicolor were attracted to playback of male’s calls embedded in a chorus recorded at short distances (1–32 m), but beyond which signal attractiveness was drastically reduced (Fig. 6c–e). Results from other anuran species have had more mixed results. A laboratory study in American toads (Bufo americanus) and sibling gray treefrogs (Hyla chrysoscelis) also showed a reduction in attraction and orientation to chorus sounds recorded from 20 to 80 m (Swanson et al. 2007). Barking treefrogs (Hyla gratiosa) were also attracted to chorus stimuli recorded at distances of up to 40 m (Gerhardt and Klump 1988b). Rana sylvatica showed attraction only at shorter distances <15 m (Bee 2007a, b). Evolutionary and ecological factors may have a role in determining if a species responds to distant acoustic stimuli (Gerhardt and Klump 1988b; Bee 2007a, b). Together, these results indicate that attenuation of the amplitude and/or degradation of temporal features of male advertisement calls at longer distances must reach a level that negatively impacts the female’s ability to detect, discriminate, or locate the call (see Lang 2000).
The gray treefrog’s phonotaxis scores to chorus sounds were lower than the scores to call models of individual males tested in a similar experimental setup (Bush et al. 2002; Beckers and Schul 2004). The mean speaker-derived phonotaxis score for the chorus sound at 1 m (that most closely resembles an isolated single male call), at a playback level of 83–86 dB SPL, was 0.42; Beckers and Schul (2004) could obtain the same score with a synthetic call at a playback level of just 43 dB SPL. The difference in experimental results is likely attributed to the difference in the sound stimuli; previous studies used synthetic H. versicolor calls with high signal-to-noise ratios (Fig. 3a) as opposed to calls embedded in a chorus in the present study (Fig. 3c–h).
In the laboratory phonotaxis studies, the response at 4 m measured by phonotaxis score and orientation show a marked decrease from the 1 and 8-m responses (Table 2; Fig. 4). This trend was consistent across animals and exemplar dates (data not shown). We believe these responses can be explained by local topography. The majority of chorus recordings were made roughly in an area with a downward slope extending from 3 to 6 m from the pond edge, which led away from any potentially interfering choruses. At the 4-m recording position, the microphone is shielded from the chorus by the substrate. This would act to both attenuate and filter propagating calls, which would alter the attractiveness of the call (Richards and Wiley 1980; Penna and Solis 1998). At further distances the slope no longer occluded a direct sound path to the microphone, and alterations in calls and chorus sounds would be significantly less, as shown by the increase in attraction of the 8-m calls (Fig. 4).
There is a caveat with the laboratory phonotaxis experiments. When females respond to focal male’s call in chorus sounds recorded at ‘intermediate’ distances (~4–16 m, which are distances at which the focal male’s call is perceptible over the background chorus, but at which there are some other males calling), we cannot determine the particular calls (e.g., those made by the ‘focal’ male, or near neighbors, or all) to which the females are responding. To assess the relative contribution of individual calls to female responses, future studies should incorporate more advanced sound recording techniques (e.g., an array-microphone recording technique, such as in Jones and Ratnam 2009) whereby the calls of individual callers that have the highest signal-to-noise ratios can be observed along with female responses in real time.
The current study is the first to investigate the change in signal attractiveness as one moves away from a single calling male in a chorus. With distance, both temporal and spectral signal properties of the call are increasingly affected by the ambience (Fig. 3), resulting from substrate–signal interactions, reflection, spectral absorbance, thermal effects, and the presence of competing biotic and abiotic sounds (Richards and Wiley 1980; Dabelsteen et al. 1993; Forrest 1994; Gerhardt and Klump 1988a; Holland et al. 1998, Wollerman 1999; Wollerman and Wiley 2002; Luther and Wiley 2009). If we are to understand how females process acoustic signals in real-world listening conditions, it is imperative that we employ stimuli which resemble those the females encounter in the field—we feel that the current study is a valuable step in this direction. With increasing distance the individual pulses of calling males become less distinct and have lower signal-to-noise ratios due to temporal ‘blurring’ of the pulses resulting from the presence of echoes and reverberations, other calling males and biotic sounds, and turbulent noises (Feng and Schul 2007). Further studies are being undertaken to study the independent effects of changes in sound attenuation and temporal degradation of male calls on female attraction and phonotaxis. In addition, neurophysiological studies of how chorus sounds are processed in the female auditory midbrain are underway.
Results of the present study suggest a dichotomy of female’s attraction to chorus stimuli from two ranges: (1) 1–8 m range for which the attractiveness is essentially the same, (2) 16–32 m range for which the phonotaxis score decreases progressively from 0.47 to 0.1 (Table 2). It would be of interest to investigate whether neurons in the frog central auditory system also show a dichotomy of physiological responses to chorus sounds recorded from different ranges.
References
Aubin J, Jouventin P (2002) Localisation of an acoustic signal in a noisy environment: the display call of the king penguin Aptenodytes patagonicus. J Exp Biol 205:3793–3798
Batschelet E (1981) Circular statistics in biology. Academic Press, London
Beckers O, Schul J (2004) Phonotaxis in Hyla versicolor (Anura, Hylidae): the effect of absolute call amplitude. J Comp Physiol A 190:869–876
Bee MA (2007a) Sound source segregation in grey treefrogs: spatial release from masking by the sound of a chorus. Anim Behav 74:549–558
Bee MA (2007b) Selective phonotaxis by male wood frogs (Rana sylvatica) to the sound of a chorus. Behav Ecol Sociobiol 61:955–966
Bee MA, Swanson EM (2007) Auditory masking of anuran advertisement calls by road traffic noise. Anim Behav 74:1765–1776
Bush SL, Gerhardt HC, Schul J (2002) Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim Behav 63:7–14
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78:575–595
Dabelsteen T, Larsen ON, Pedersen SB (1993) Habitat-induced degradation of sound signals: quantifying the effects of communication sounds and bird location on blur ratio, excess attenuation, and signal-to-noise ration in blackbird song. J Acoust Soc Am 93:2206–2220
Diekamp B, Gerhardt HC (1995) Selective phonotaxis to advertisement calls in the gray treefrog Hyla versicolor: behavioral experiments and neurophysiological correlates. J Comp Physiol A 117:173–190
Eggert C (2002) Use of fluorescent pigments and implantable transmitters to track a fossorial toad (Pelobates fuscus). Herpetol J 12:69–74
Feng AS, Shofner WP (1981) Peripheral basis of sound localization in anurans, acoustic properties of the frog’s ear. Hear Res 5:201–216
Feng AS, Schul J (2007) Sound processing in real-world environments. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Hearing and sound communication in amphibians. Springer, New York, pp 323–350
Feng AS, Gerhardt HC, Capranica RR (1976) Sound localization behavior of the green treefrog (Hyla cinerea) and the barking treefrog (Hyla gratiosa). J Comp Physiol A 107:241–252
Forrest TG (1994) From sender to receiver: propagation and environmental effects on acoustic signals. Am Zool 34:644–654
Gerhardt HC (1975) Sound pressure levels and radiation patterns of the vocalizations of some North American frogs and toads. J Comp Physiol A 102:1–12
Gerhardt HC (1981) Mating call recognition in the green treefrog (Hyla cinerea): importance of two frequency bands as a function of sound pressure level. J Comp Physiol A 144:9–16
Gerhardt HC (2005) Acoustic spectral preferences in two cryptic species of grey treefrogs: implications for mate choice and sensory mechanisms. Anim Behav 70:39–48
Gerhardt HC, Doherty JA (1988) Acoustic communication in the gray treefrog, Hyla versicolor: evolutionary and neurobiological implications. J Comp Physiol A162:261–278
Gerhardt HC, Klump GM (1988a) Masking of acoustic signals by the chorus background noise in the green tree frog: a limitation on mate choice. Anim Behav 36:1247–1249
Gerhardt HC, Klump GM (1988b) Phonotactic responses and selectivity of barking treefrogs (Hyla gratiosa) to chorus sounds. J Comp Physiol A 163:795–802
Gerhardt HC, Schul J (1999) A quantitative analysis of behavioral selectivity for pulse rise-time in the gray treefrog, Hyla versicolor. J Comp Physiol A 185:33–40
Gerhardt HC, Tanner SD, Corrigan CM, Walton HC (2000) Female preference functions based on call duration in the gray tree frog (Hyla versicolor). Behav Ecol 11:663–669
Holland J, Dabelsteen T, Pedersen SB, Larsen ON (1998) Degradation of wren Troglodytes troglodytes song: implications for information transfer and ranging. J Acoust Soc Am 103:2154–2166
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Johnson JR, Semlitsch RD (2003) Defining core habitat of local populations of the gray treefrog (Hyla versicolor) based on choice of oviposition site. Oecologia 137:205–210
Jones DL, Ratnam R (2009) Blind location and separation of callers in a natural chorus using a microphone array. J Acoust Soc Am 126:895–910
Jørgensen MB, Gerhardt HC (1991) Directional hearing in the gray tree frog Hyla versicolor: eardrum vibrations and phonotaxis. J Comp Physiol A 169:177–183
Jouventin P, Aubin T, Lengagne T (1999) Finding a parent in a king penguin colony: the acoustic system of individual recognition. Anim Behav 57:1175–1183
Lang F (2000) Acoustic communication distances of a Gomphocerine grasshopper. Bioacoustics 10:233–258
Lohr B, Wright TF, Dooling RJ (2003) Detection and discrimination of natural calls in masking noise by birds: estimating the active space of a signal. Anim Behav 65:763–777
Luther DA, Wiley RH (2009) Production and perception of communicatory signals in a noisy environment. Biol Lett 5:183–187
Murphy CG (2008) Assessment of distance to potential mates by female barking treefrogs (Hyla gratiosa). J Comp Psychol 122:264–273
Narins PM, Zelick RD (1988) The effects of noise on auditory processing and behavior in amphibians. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 511–536
Narins PM, Feng AS, Fay RR, Popper AN (2007) Hearing and sound communication in amphibians. Springer, New York
Penna M, Solis R (1998) Frog call intensities and sound propagation in the South American temperate forest region. Behav Ecol Sociobiol 42:371–381
Ptacek MB, Gerhardt HC, Sage RD (1994) Speciation by polyploidy in treefrogs: multiple origins of the tetraploid, Hyla versicolor. Evolution 48:898–908
Richards DG, Wiley RH (1980) Reverberations and amplitude fluctuations in the propagation of sounds in a forest: implications for animal communication. Am Nat 115:381–399
Schwartz JJ, Gerhardt HC (1995) Directionality of the auditory system and call pattern recognition during acoustic interference in the gray tree frog, Hyla versicolor. Aud Neurosci 1:195–206
Schwartz JJ, Huth K, Hutchin T (2004) How long do females really listen? Assessment time for female mate choice in the grey treefrog, Hyla versicolor. Anim Behav 68:533–540
Sinsch U (1990a) Migration and orientation in anuran amphibians. Ethol Ecol Evol 2:65–79
Sinsch U (1990b) The orientation behaviour of three toad species (genus Bufo) displaced from the breeding site. In: Hanke W (ed) Biology and physiology of amphibians. Gustav Fischer Verlag, Stuttgart, pp 75–83
Slabbekoorn H, Bouton N (2008) Soundscape orientation: a new field in need of sound investigation. Anim Behav 76:e5–e6
Swanson EM, Tekman S, Bee MA (2007) Do female frogs exploit inadvertent social information to locate breeding aggregations? Can J Zool 85:921–932
Windmiller B (1996) Tracking techniques useful for field studies of anuran orientation and movement. Herpet Rev 27:13–15
Wollerman L (1999) Acoustic interference limits call detection in a Neotropical frog Hyla ebraccata. Anim Behav 57:529–536
Wollerman L, Wiley RD (2002) Background noise from a natural chorus alters female discrimination of male calls in a Neotropical frog. Anim Behav 63:15–22
Zar J (1999) Biostatistical analysis. Prentice-Hall, Upper Saddle River, NJ
Acknowledgments
We thank the Missouri Department of Conservation and the University of Missouri-Columbia for allowing studies and capture permits. Carl Gerhardt generously provided advice and material support. Vincent Marshall, Noah Gordon, Oliver Beckers, and Sarah Bush provided valuable advice and comments on the present study. Sarah Humfeld and Alison Welch aided in coordinating field activities and in providing material support. Eric Rozier provided many helpful comments and assistance with the statistical analysis. We would also like to thank the University of Missouri-Columbia undergraduate students who greatly aided in both animal collection and experimental execution: Paul Kimball, Stephanie Kleyman, and Leah Reynolds. This research was supported by a National Science Foundation Collaborative Research in Computational Neuroscience (CRCNS-0422073) grant to ASF and a National Science Foundation grant (IOB-0445286) to JS. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christie, K., Schul, J. & Feng, A.S. Phonotaxis to male’s calls embedded within a chorus by female gray treefrogs, Hyla versicolor . J Comp Physiol A 196, 569–579 (2010). https://doi.org/10.1007/s00359-010-0544-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-010-0544-2