Abstract
Mate preference for conspecifics does not necessarily lead to assortative mating in cases where mating outcomes also depend on preferences based on mate quality and on individual competitiveness. We tested how such traits affected mate choice among genetically divergent lineages (called molecular operational taxonomic units; MOTU) of the amphipod Gammarus fossarum. We presented males with two females, including one from its own MOTU. Females also potentially differed in body size, and therefore fecundity, and in time before reproduction, two traits previously recognized as important in male mate choice. Males generally preferred females from their own MOTU when females originated from highly divergent MOTUs (17 % genetic divergence), but not when they were more closely related (3.5 % genetic divergence). Contrary to expectations, they did not prefer larger females, but they consistently paired with the female closest to reproduction. A second experiment involving duos of males of different MOTUs in competition for a female also revealed that males consistently won the competition over pairing with females of their own MOTU. Overall, these results reveal a strong influence of genetic divergence on mate recognition and reproductive isolation between sympatric MOTUs. However, male preference for females that are close to being available for reproduction also potentially results in hybridization among closely related MOTUs. We examine these results in the light of field mating patterns observed in a previous study of G. fossarum and discuss the importance of considering competitiveness and preferences for mate quality signals when studying evolutionary consequences of secondary contact between divergent lineages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Populations separated by geographical barriers tend to accumulate genetic and phenotypic differences through genetic drift or local adaptation to the two isolated environments. Genetic divergence may eventually lead to partial or total reproductive isolation among these populations (Mayr 1988). Traits linked to mate choice may differ to the point that females (or males) do not recognize individuals of the other population as suitable for mating. In case of secondary contact, when geographical barriers have receded, this can lead to assortative mating among sympatric individuals of the two original populations; mating occurs exclusively among individuals of the same original population/genetic lineage (Panhuis et al. 2001; Mendelson and Shaw 2012).
Several different traits are usually involved in mate choice and mate access. Two main groups of traits are thought to play major roles in mating decisions. First, necessary mate recognition signals (sensu Mendelson and Shaw 2012) are used to discriminate among individuals that are potential mates and those that are not (Ryan and Rand 1993). If these traits have diverged between two allopatric populations, animals may not recognize sympatric individuals from the other population as potential mates in case of secondary contact. Second, quality signals are traits informing individuals about the direct or indirect fitness benefits associated with a particular mate (i.e. mate quality; Andersson 1994). These two types of traits can conflict during mate choice (Ryan and Rand 1993; Pfennig 1998). Individuals may not recognize heterospecifics as suitable mates on the basis of necessary mate recognition signals but may still engage in mating if heterospecifics display traits associated with high-quality mates. Depending on which signals prevail in mating decisions, conflict between recognition and quality traits can lead to various levels of hybridization (Pfennig 2000; Mendelson and Shaw 2012). For instance, in a swordtail fish species, females show strong preferences for larger males and sympatric heterospecifics males are on average larger than conspecifics (Hankison and Morris 2002). Female preference has been shown to prevail over necessary mate recognition of conspecifics and females regularly mate with larger heterospecific males (Hankison and Morris 2003). The relative importance of mate recognition and quality traits on mate choice is likely to vary among and within species. Simultaneously measuring the effects of both trait groups on mating decisions should provide important insights into natural variation in levels of assortative mating among genetically divergent populations found in sympatry.
Male–male competition for access to females is another important but largely unexplored factor potentially affecting assortative mating among sympatric species (Crespi 1989). For example, if males of two species living in sympatry do not discriminate between conspecific or heterospecific females, the mating pattern is expected to be random. However, if males of both species outcompete heterospecific males for access to their conspecific females, hybridization rates should decrease (Howard et al. 1998). Alternatively, male–male competition may be asymmetrical; males outcompete heterospecifics in only one of the two species. Thus, everything else being equal, competitive males are expected to hybridize whereas males from the other species should mate only with conspecific females (Lengagne et al. 2006). Here, we assessed the relative importance of mate recognition and quality signals and the effects of male–male competition on mate choice and mating patterns among three genetically divergent lineages of the amphipod Gammarus fossarum.
A recent study by Lagrue et al. (2014) revealed important cryptic diversity in amphipods of eastern France, identifying eight morphologically similar but genetically distinct molecular operational taxonomic units (i.e. MOTU; Floyd et al. 2002) of G. fossarum. Genetic determination based on mitochondrial DNA (COI sequences) revealed large genetic divergences among MOTUs (up to 18 %) which is thought to result from multiple events of geographical separation and lasting isolation among G. fossarum populations. These genetically divergent MOTUs have experienced secondary contacts, leading to a complex geographical distribution and multiple situations where divergent MOTUs occur in sympatry. Here, we tested mating behaviour among three MOTUs of G. fossarum with variable degrees of genetic divergence (Lagrue et al. 2014; Galipaud et al. 2015a). In amphipods, mate recognition is thought to occur via chemical cues (Hartnoll and Smith 1980; Thiel 2011). These signals may diverge among isolated MOTUs, to the point where individuals from divergent MOTUs are not recognized as suitable mates. The probability for premating isolation may therefore increase with genetic divergence among MOTUs (Wong et al. 2004). Accordingly, in natural populations, sympatric G. fossarum MOTUs diverging by less than 3.5 % seemed to mate randomly (Lagrue et al. 2014). Contrastingly, highly divergent MOTUs almost never engaged in inter-MOTUs mating (Lagrue et al. 2014; Galipaud et al. 2015a). However, under laboratory conditions, males presented with a single (no-choice situation, sensu Dougherty and Shuker 2015), highly divergent female (17 %) still mated in about 50 % of the trials (although the proportion of observed mating was still significantly higher when males were presented with a more closely related female; Lagrue et al. 2014). Discrepancies observed between field and laboratory mate choice patterns suggest that other factors may influence mating decisions in amphipods.
Mate choice in male amphipods has been shown to be influenced by two other traits linked to female quality. First, larger females carry more eggs and males have been shown to prefer larger and more fecund females over smaller ones (Elwood et al. 1987; Franceschi et al. 2010). Second, egg fertilization can occur only for a short period, just after female moulting (Jormalainen 1998). As a result, males engage in long-lasting precopulatory guarding (also called precopula or amplexus) of females before they moult (Grafen and Ridley 1983; Jormalainen 1998). Because guarding may result in missed mating opportunities, males preferentially pair with females close to moulting (Birkhead and Clarkson 1980; Dick and Elwood 1989; Dunn 1998; Lemaître et al. 2009). Female quality signals may still be detected by males in females from genetically divergent MOTUs and override necessary mate recognition, thus inducing hybridization in cases where divergent females are perceived as being of better relative quality than females of the same MOTU. In G. fossarum, MOTUs have been shown to differ in mean body size (Galipaud et al. 2015a). This could lead males to choose large hetero-MOTU females over small females of their own MOTU. Body size differences among MOTUs could also affect cross-MOTU hybridization patterns if linked to male–male competition for female access. Larger male amphipods have a competitive advantage over smaller individuals during mating. They are capable of stealing females already paired with smaller male competitors (Ward 1983; Elwood et al. 1987). They also generally have a stronger hold on females and can maintain the amplexus for longer (Adams and Greenwood 1983; Elwood and Dick 1990). Finally, it has also been suggested that larger males are preferred by females over smaller males because they provide higher protection against predators and higher mating success to male offspring (Cothran 2008a; Cothran et al. 2012). Males from larger MOTUs should thus be able to defend their own females (females of their own MOTU) against males from other, smaller MOTUs or to outcompete them, thereby engaging in hetero-MOTU mating, i.e. mating with females from other MOTUs.
The present study had three objectives. First, we tested the prediction that male probability of choosing females of their own MOTU over females from different MOTUs increased with increasing genetic divergence between MOTUs. Second, we tested whether male preference for large females and females close to moulting affected their likelihood of mating with females from divergent MOTUs. Third, we assessed the capacity of males to outcompete genetically divergent males for access to females and how this may affect hybridization patterns among MOTUs.
Material and methods
Amphipod sampling and housing
We collected amphipods from three MOTUs and originating from five isolated rivers/populations (Gf-I: Norge, Meuzin and Doulonne rivers; Gf-II: Orain river; Gf-VII: Résurgence du Vivier; Fig. 1). Molecular operational taxonomic units Gf-I and Gf-II are genetically divergent by about 3.5 % and are both genetically divergent from Gf-VII by 17 % (Lagrue et al. 2014, Fig. 1). All amphipod populations used in this study consist of a single MOTU (Lagrue et al. 2014). There was thus no need for genetic identification of individuals used in our experiments. Also, due to the limited dispersal abilities of amphipods, no migration is likely to occur among populations from different rivers (Lagrue et al. 2014). Although isolated in our experiment, MOTUs used here also occur naturally in sympatry in other rivers of eastern France and are therefore likely to experience mate choice and male-male competition in the field (see Lagrue et al. 2014 for a more extensive account of the phylogeographical history of G. fossarum MOTUs).
MOTUs used in experiments. Male and female amphipods from five single MOTU sampling sites/populations were used (Gf-I, Gf-II and Gf-VII). Gf-I amphipods from different sites/populations were used to test male mate choice between sympatric females and allopatric females of the same MOTU. Gf-I (Norge River), Gf-II and Gf-VII amphipods were used to test male mate choice between females of their own MOTU and females from genetically divergent MOTUs (by either 3.5 or 17 %)
Amphipods used in experiments were captured by “kick sampling”, consisting of moving rocks from the river bottom with a foot and collecting the dislodged amphipods downstream (Hynes 1954). Amphipods from each population were kept in separate stock tanks filled with aerated stream water and maintained at 13 ± 1 °C with a 12-h day/12-h night photoperiod. Amphipods were fed ad libitum with elm leaves until required for experiments. Only individuals found already in amplexus in stock tanks were selected to ensure sexual maturity. In mate guarding crustacean, females of some species have been shown to resist male precopula attempts that occur too early in their moulting cycle (Jormalainen and Merilaita 1995; Cothran 2008b). Using only females already found paired for our experiments thus ensured that they were receptive to pairing and were less likely to resist precopula attempts. Males and females were then gently separated from their current partner to be used for experiments. Pairing trials were conducted in small glass containers (diameter, 6 cm; height, 3.2 cm; volume, 45 mL). Trials where one or more individual(s) died were excluded from the data set. At the end of each experiment, individual amphipod body size was estimated by measuring the height of their fourth coxal plate under a Nikon SMZ microscope using the Lucia G 4.8.1 software (Galipaud et al. 2015a).
Mate choice
We tested male mate choice by presenting one male with two females originating from different MOTUs. Except for their MOTU, females were otherwise randomly picked. This allowed us to test for male mate choice on female body size and time before moulting while avoiding correlations between females’ characteristics and their respective MOTU in each trial. One of the two females was tagged with a dot of white paint to be able to distinguish each female in the trial. In a series of trials, we alternated tagging so that females from different MOTUs were tagged the same number of times. First, one male was introduced in the glass cup but confined in a small wire cylinder (500-μm mesh size) and left to acclimatize for 1 h. Two females were then simultaneously introduced in the container, outside of the male’s enclosure, and were given a 15-min acclimatization period. The wire enclosure was then removed, freeing the male, and amphipods were left to interact. After 12 h, we recorded which female was paired with the male. Trials where one or more individual(s) moulted during the 12 h (which was easily observable by the presence of an exuvia in the cup) were removed from the dataset. After each trial, the mating pair was left in the cup until the female moulted. The other female was put in another cup with a male of its own MOTU until moult. This procedure provided a precise, a posteriori, evaluation of each female’s time before moulting at the start of each trial.
Of the two females presented to a male in each trial, one was of the male’s own MOTU (hereafter referred to as the resident female) and the other was from one of the two other MOTUs (hereafter referred to as the challenger female). This allowed us to test for male preferences in all six possible combinations of resident and challenger females, genetically diverging either by 3.5 or 17 %. Two combinations involving the same two MOTUs but differing in which MOTU was the resident were considered opposite combinations. For example, (i) Gf-I males presented with a Gf-I resident female and a Gf-VII challenger female is the opposite combination to (ii) Gf-VII males presented with a Gf-VII resident female and a Gf-I challenger female. This experiment therefore involved three pairs of opposite combinations.
To control for a potential role of geographical origin on mate choice, we also tested male preferences for Gf-I females originating from different sampling sites/populations (i.e. allopatric; Fig. 1). Using the same protocol as described above, we considered all six possible combinations. They involved a male Gf-I from a given population presented with a resident female of the same population and a challenger female from one of the two other Gf-I populations (Fig. 1).
In the 12 combinations described above (6 testing for male mate choice between genetically divergent females and 6 testing for mate choice between genetically identical females from geographically isolated populations), we quantified the number of trials where males paired with their resident female. For each combination, we then used a binomial test to assess deviation from random mating, when the proportion of trials where the male paired with the resident female was significantly higher or lower than 0.5. We also tested for differences in male probability to pair with their resident female between opposite combinations using Fisher’s exact tests.
We tested male preferences based on female body size and time left before moulting to further understand the relative roles of female quality traits and MOTU of origin on male mate choice. For the analysis, we first randomly picked a focal female in each trial (either the resident or the challenger). Using a generalized linear model for a binomial distribution, we then tested for an effect of the following predictor variables on the focal female’s probability of being chosen by the male: the difference in body size between the two females, the difference in time before moulting between the two females, and the focal female’s status (either resident or the challenger). We also considered the interaction between body size difference and focal female’s status as well as the interaction between difference in time before moulting and focal female’s status in the model. If mating is random according to female status but non-random according to other female characteristics, it means that these characteristics play a greater role in mate choice than females’ MOTU. In addition, if interactions between female characteristics and female status (resident or challenger) are non-significant, it means that the magnitude to which males discriminate among females based on their body size or their time before moult is the same regardless of the female’s MOTU. As females differ in mean body size among MOTUs (Table 1), it may seem difficult to disentangle possible mate choice based on body size and mate choice based on MOTU. Males could simply pair with resident females because they also are the largest. This can happen for some combinations, but it is reversed in their opposite combinations, with resident females being the smallest. If in these opposite combinations males keep pairing with the resident female (even though it is smaller than the challenger), mate choice is likely to be mainly based on female MOTU. Otherwise, if males pair with the larger challenger female, mate choice is likely to be based on body size. We therefore relied on the comparison between opposite combinations described above to further disentangle the relative role of female MOTU and female body size on mate choice when trials involved females from MOTUs differing in mean body size.
Male–male competition
We assessed the likelihood of a male (resident male) to pair with a female of its own MOTU when competing against a male from another MOTU (competitor male) genetically distant by either 3.5 or 17 % (Fig. 1). In each trial, we placed two males from different MOTUs in a glass cup and let them acclimatize for 1 h before adding a single female. One of the two males was marked with a dot of white paint for easy identification. We alternated the marking between the two males among trials to avoid biases. After 12 h, we counted the number of trials where the resident male was paired with the female.
We considered all six combinations of resident and competitor males, among which were three pairs of opposite combinations (e.g. (i) Gf-I resident males versus Gf-VII competitor males and its opposite combination (ii) Gf-VII resident males versus Gf-I competitor males). To control for the potentially confounding effects of geographical origin of MOTUs, we assessed the competitive ability of Gf-I males to pair with sympatric females when facing competition from Gf-I males from other populations (Fig. 1). This experiment also involved six different combinations. We tested for deviation from random pairing in resident males’ probability of pairing using binomial tests for each considered combination. Between opposite combinations, we also tested for differences in resident males’ probability of pairing using Fisher tests.
We then tested for potential effects of male body size on mating probabilities. We randomly picked one of the two males in each trial as the focal male for analysis. We then tested the effect of male body size, male status (resident or competitor) and their interaction on the focal male probability to pair using a generalized linear model with a binomial link function. As described above, we also relied on comparisons between opposite combinations to further disentangle the relative role of male body size and male MOTU on their pairing probability when males of different MOTUs differed in mean body size. If male body size increases competitiveness, large males should always win the competition over pairing with the female, whether resident (competing for access to a female of their own MOTU) or competitor (competing for access to a female of another MOTU).
Results
Mate choice
Males showed no preference for one or the other female based on their geographical origin, i.e. among females from the same MOTU but different populations (Table 2). Similarly, males paired without discrimination with the resident or the challenger female when the two females originated from MOTUs genetically diverging by 3.5 % (Table 3). However, males paired preferentially with the female of their MOTU when the two available females were from MOTUs genetically distant by 17 %, except in one combination; Gf-VII males paired randomly with either Gf-VII or Gf-II females (Table 3).
Among combinations involving MOTUs genetically divergent by 3.5 %, males did not seem to pair with the largest females (χ 2 = 0.25, df = 20, P = 0.61), whether resident or challenger (non-significant interaction between females’ difference in body size and female’s status χ 2 = 0.88, df = 18, P = 0.34). Females’ relative body size also did not affect male mate choice among combinations involving MOTUs genetically divergent by 17 % (no effect of the difference in female body size, χ 2 = 0.26, df = 69, P = 0.60; non-significant interaction between difference in female body size and female status, χ 2 = 0.37, df = 67, P = 0.54). In addition, although differing in body size (Table 1), resident females had the same probability to be chosen by males between some opposite combinations (Gf-I versus Gf-II resident females: Fisher test, P = 0.25; Gf-I versus Gf-VII resident females: Fisher test, P = 0.52). Finally, contrary to what would be expected under mate choice for female body size, in combinations involving Gf-VII and Gf-II MOTUs, Gf-II males seemed more eager to pair with the smallest of the two females (Gf-II females, probability of choosing = 0.95, Table 3) and Gf-VII males paired randomly with either the larger (Gf-VII, probability of choosing = 0.63, Table 3) or the smaller female (Gf-II, probability of choosing = 0.37, Table 3).
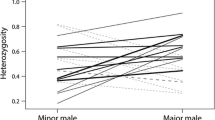
Females’ time before moulting influenced male mate choice. For 3.5 and 17 % divergence combinations, males tended to pair with the female closest to moulting (for 3.5 %, χ 2 = 7.80, df = 20, P < 0.01; for 17 %, χ 2 = 3.90, df = 69, P = 0.04; Fig. 2), regardless of whether it was a resident or a challenger (non-significant interaction between females’ difference in time left to moult and female status: for 3.5 % χ 2 = 1.09, df = 18, P = 0.29; for 17 % χ 2 = 0.04, df = 67, P = 0.84). Note that among combinations involving females genetically divergent by 17 %, males mostly paired with females of their own MOTU. However, in the few cases where they paired with the challenger female (Gf-VII males pairing with Gf-II females; Table 3), the challenger female was closer to moulting than the resident female. We found no effect of the white paint tagging on female probability of being chosen (chi-square test, mate choice experiment between MOTUs: χ 2 = 0.21, df = 1, P = 0.65; mate choice experiment between population of Gf-I: χ 2 = 0.90, df = 1, P = 0.34).
Female’s probability of being chosen by a male as a function of the difference between its time before moult and that of the other female in the trial. Trials involving females originating from MOTUs genetically divergent by 3.5 % are represented by black filled dots and the black curve. Trials involving females originating from MOTUs genetically divergent by 17 % are represented by white dots and the gray curve. Curves are fitted from a generalized linear model for binomial data. Although the response could only take values of either 0 or 1, we added a jitter to each data point for representation purposes
Male–male competition
Generally, resident Gf-I males did not pair more often than expected under random pairing when competing against males of the same MOTU but from different populations/sampling sites (Table 4). However, males from the Doulonne River lost their females to males from the Meuzin River more often than expected (Table 4). They were also unable to pair with females from the Meuzin River when competing against a resident male (Table 4). Note that this deviation from random pairing is unlikely to have biased our competitive experiment among males of different MOTUs in which only males from the Norge River were used for Gf-I (Fig. 1).
When males diverged by 3.5 %, resident males did not outcompete challenger males more often than expected under random pairing (Table 5). However, when competing with a male from a MOTU diverging by 17 %, resident males were found paired significantly more often than expected under random pairing (Table 5). Although always greater than random, the resident male probability of pairing varied between some opposite combinations. In trials with Gf-II resident males and Gf-VII challenger males, Gf-II males had a significantly lower probability to access their females (probability of winning = 0.76) than in trials of the opposite combination, where resident Gf-VII completely excluded Gf-II males from accessing Gf-VII females (probability of winning = 1; Fisher test, P = 0.047). In other opposite combinations, resident males from different MOTUs did not differ in their pairing probability (Gf-I versus Gf-II resident males: Fisher test, P = 0.062; Gf-I and Gf-VII resident males had both an exact probability of pairing of 1). Male body size did not affect their ability to win the competition over pairing (3.5 % divergence trials: χ 2 = 0.11, df = 37, P = 0.50; 17 % divergence trials: χ 2 = 0, df = 77, P = 0.95). Males’ probability of pairing was also not affected by the white paint tagging (chi-square test, χ 2 = 0.0004, df = 1, P = 0.99).
Discussion
Mate choice on mate recognition signals
Genetic divergence among potential mating partners seemed to be of primary importance for mate choice among individuals from divergent MOTUs. Males generally avoided pairing with females genetically distant by 17 % but did not seem to discriminate between females genetically distant by 3.5 % and females of their own MOTU (i.e. genetically similar). These results are consistent with mating patterns observed in the field, where pairing among closely related MOTUs is frequently observed but pairing among more distant MOTUs is rare (Lagrue et al. 2014). Data also substantiate the previous hypothesis formulated by Lagrue et al. (2014) of a threshold of genetic divergence below which no premating barriers to hybridization seem to exist among MOTUs. An increase in assortative preferences with increasing genetic divergence among populations/MOTUs has long been hypothesized, but little evidence exists for its occurrence in natural populations (but see de Kort and ten Cate 2001; Wong et al. 2004). In amphipods, phylogenetically distant species tend to interbreed at lower rates (Kolding 1986). However, most studies lack evidence for interbreeding among taxa, showing almost perfect assortative mating among species occurring in sympatry (Kinne 1954; Dick and Elwood 1992). We present here the first evidence for the existence of a threshold of genetic divergence among cryptic MOTUs of a single morphological species above which individuals are not preferred as mates anymore.
Male–male competition and mate choice on mate quality signals
Beyond genetic divergence, competitive traits and traits related to mate quality also seem to play a role in mate choice among MOTUs. Although the majority of combinations involving MOTUs diverging by 17 % led to almost strict assortative mating, Gf-VII males showed no preference for either resident Gf-VII or challenger Gf-II females. The lack of selectivity from Gf-VII males towards females from a highly divergent MOTU is consistent with previous findings from laboratory experiments (Lagrue et al. 2014). It suggests that, contrary to other combinations, traits used by Gf-VII males for mate selection may not differ enough between Gf-II and Gf-VII females to prevent mating between these two MOTUs. Contrastingly, Gf-VII male–Gf-II female pairs were never observed where these two MOTUs occur naturally in sympatry (Lagrue et al. 2014). Male-male competition for female access may explain this discrepancy and the maintenance of assortative mating among these MOTUs. Despite them being smaller on average, Gf-II males tended to outcompete Gf-VII males for access to Gf-II females, hence partially preventing Gf-VII male–Gf-II female mating. This contrasts with the general finding that large males outcompete smaller males for mating access (Ward 1983; Elwood et al. 1987). Alternatively, if Gf-II males are preferred by Gf-II females over Gf-VII males, Gf-II males might have an advantage in mating access. Gf-II females might resist and/or escape pairing with Gf-VII males, hence resulting in a relatively higher probability of Gf-II males to pair. Physical resistance as a form of female mate choice has been observed in some mate guarding crustacean species (Sparkes et al. 2002; Cothran 2008a). However, it is still unclear whether females from the Gammarus genus actually resist pairing attempts (Dick and Elwood 1989; Jormalainen and Merilaita 1995). Further investigations are needed on G. fossarum female selective resistance behaviours and their ability to discriminate among males from divergent MOTUs.
MOTUs distant by 3.5 % may not have diverged enough to display strong differences in mate recognition signals. Males may therefore not consider females from closely related MOTUs as distinct from females of their own MOTU, thus mating indiscriminately with females from either MOTU. This, however, contrasts with experimental pairing patterns observed when presenting only one female from a closely related MOTU to one male. Under such conditions, males showed a twofold decrease in their propensity to pair with the only available female compared to when presented with a female of their own MOTU (i.e. genetically identical; Lagrue et al. 2014). The possibility for males to compare females not only on the basis of their MOTU but also on their respective quality as mates is likely to account for such a discrepancy between results presented here and those of Lagrue et al. (2014). Males showed a strong preference for females closer to moulting in our experiments and this preference may prevail over preference for females of their own MOTU. Prevailing preference based on time before moulting may explain the overall random pairing we observed among MOTUs with 3.5 % divergence.
Contrary to time before moulting, female body size did not seem to influence male mate choice among MOTUs. This was particularly obvious in trials involving females from highly distant MOTUs (17 % genetic divergence) which generally did not result in pair formation, even though challenger females could be on average larger than resident females. The lack of effect of body size on mate choice among MOTUs may be due to a hierarchy in assessment of cues used during mate choice. Males may reject females on the basis of mate recognition signals before assessing female body size. In amphipods, assessment of MOTU identity is likely to occur via chemical signals, shortly following first contact with the female (Hartnoll and Smith 1980; Thiel 2011). In contrast, body size assessment is thought to happen only secondarily, when males manipulate the female during amplexus (Dick and Elwood 1989). Mate recognition signals may thus prevail over body size assessment in mating decision and lead to assortative mating among MOTUs rather than hybridization due to mate choice on body size. Alternatively, it is also possible that male G. fossarum simply do not discriminate females on body size. In amphipods, male preference for larger females does not always occur, potentially due to constraints on mate choice imposed by the strong competition among males for access to unpaired females (e.g. Birkhead and Clarkson 1980; Elwood and Dick 1990; Galipaud et al. 2015b). Here, female body size differences among MOTUs did not seem to lead to mate choice for larger females and hybridization.
Concluding remarks on evolutionary consequences in sympatry
Documenting how mating behaviours affect pairing among genetically divergent populations experiencing secondary contact is important for understanding subsequent selection on mating traits occurring in sympatry. In case of low hybrid fitness, selection should favour individuals possessing traits (preferences and signals) allowing or reinforcing mate recognition and premating isolation among divergent MOTUs (Liou and Price 1994; Servedio and Noor 2003). Theory predicts that it should occur through selection of individuals possessing phenotypes most distinct from individuals of the other population/MOTU (Bacquet et al. 2015). Results presented here show that mating behaviours can also affect reinforcement of reproductive isolation, either enhancing or constraining it. Competition among males for access to females can prevent hybridization, by preventing males from mating with heterospecifics (here females from different MOTUs). Under low hybrid fitness, male competitive abilities should thus be selected for. In contrast, strong preferences for mate quality traits (such as female time before moulting) can prevail over mate recognition and lead to hybridization if heterospecifics are perceived as good quality mates. In such case, individuals with a weaker inclination to discriminate mates on quality traits would presumably be selected. They would indeed only choose mates based on recognition signals and therefore avoid hybridization. In the present study, we did not investigate mating behaviours of MOTUs in sympatry. According to Lagrue et al. (2014), in G. fossarum, hetero-MOTU mating produces the same amount of viable fertilized eggs as homo-MOTU mating. However, nothing is known about offspring survival beyond the earliest stages of development and their subsequent mating success. In consequence, it remains hard to predict the selective pressures acting on mating behaviours of individuals from sympatric MOTUs to avoid hybridization. However, studying mating behaviours among allopatric, genetically divergent MOTUs provides an important baseline for comparison with behaviours observed when these MOTUs experience secondary contact. The amount to which mating behaviours differ between allopatric and sympatric populations would provide clues about the action of selection to prevent (or favour) hybridization in sympatry. More research is needed to understand how the interplay between mate recognition, competition for mates and mating preference based on mate quality signals affect mating patterns and subsequent evolution of mating behaviours of genetically divergent MOTUs in sympatry.
References
Adams J, Greenwood PJ (1983) Why are males bigger than females in pre-copula pairs of Gammarus pulex? Behav Ecol Sociobiol 13:239–241
Andersson M (1994) Sexual selection. Princeton University Press
Bacquet PMB, Brattström O, Wang H-L, Allen CE, Löfstedt C, Brakefield PM, Nieberding CM (2015) Selection on male sex pheromone composition contributes to butterfly reproductive isolation. Proc R Soc B 282:20142734
Birkhead TR, Clarkson K (1980) Mate selection and precopulatory guarding in Gammarus pulex. Z Tierpsychol 52:365–380
Cothran RD (2008a) Direct and indirect fitness consequences of female choice in crustacean. Evolution 62:1666–1675
Cothran RD (2008b) Phenotypic manipulation reveals sexual conflict over precopula duration. Behav Ecol Sociobiol 62:1409–1416
Cothran RD, Chapman K, Stiff AR, Relyea RA (2012) “Cryptic” direct benefits of mate choice: choosy females experience reduced predation risk while in precopula. Behav Ecol Sociobiol 61:905–913
Crespi BJ (1989) Causes of assortative mating in arthropods. Anim Behav 38:980–1000
de Kort SR, ten Cate C (2001) Response to interspecific vocalizations is affected by degree of phylogenetic relatedness in Streptopelia doves. Anim Behav 61:239–247
Dick JTA, Elwood RW (1989) Assessments and decisions during mate choice in Gammarus pulex (Amphipoda). Behaviour 109:235–246
Dick JTA, Elwood RW (1992) Coexistence and exclusion among Gammarus species: behavioural avoidance of interspecific precopulation by male G. pulex (Amphipoda). Oikos 64:541–547
Dougherty LR, Shuker DM (2015) The effect of experimental design on the measurement of mate choice: a meta-analysis. Behav Ecol 26:311–319
Dunn A (1998) The role of calceoli in mate assessment and precopula guarding in Gammarus. Anim Behav 56:1471–1475
Elwood RW, Dick JTA (1990) The amorous Gammarus: the relationship between precopula duration and size-assortative mating in G. pulex. Anim Behav 39:828–833
Elwood RW, Gibson J, Neil S (1987) The amorous Gammarus: size assortative mating in G. pulex. Anim Behav 35:1–6
Floyd R, Abebe E, Papert A, Blaxter M (2002) Molecular barcodes for soil nematode identification. Mol Ecol 11:839–850
Franceschi N, Lemaître J-F, Cézilly F, Bollache L (2010) Size-assortative pairing in Gammarus pulex (Crustacea: Amphipoda): a test of the prudent choice hypothesis. Anim Behav 79:911–916
Galipaud M, Bollache L, Wattier R, Dubreuil C, Dechaume-Moncharmont F-X, Lagrue C (2015a) Overestimation of the strength of size-assortative pairing in taxa with cryptic diversity: a case of Simpson’s paradox. Anim Behav 102:217–221
Galipaud M, Bollache L, Oughadou A, Dechaume-Moncharmont F-X (2015b) Males do not always switch females when presented with a better reproductive option. Behav Ecol 26:359–366
Grafen A, Ridley M (1983) A model of mate guarding. J Theor Biol 102:549–567
Hankison SJ, Morris MR (2002) Sexual selection and species recognition in the pygmy swordtail, Xiphophorus pygmaeus: conflicting preferences. Behav Ecol Sociobiol 51:140–145
Hankison SJ, Morris MR (2003) Avoiding a compromise between sexual selection and species recognition: female swordtail fish assess multiple species-specific cues. Behav Ecol 14:282–287
Hartnoll RG, Smith SM (1980) An experimental study of sex discrimination and pair formation in Gammarus duebenii (Amphipoda). Crustaceana 38:253–264
Howard DJ, Gregory PG, Chu J, Cain ML (1998) Conspecific sperm precedence is an effective barrier to hybridization between closely related species. Evolution 52:511–516
Hynes HBN (1954) The ecology of Gammarus duebeni Lilljeborg and its occurrence in fresh water in western Britain. J Anim Ecol 23:38–84
Jormalainen V (1998) Precopulatory mate guarding in crustaceans: male competitive strategy and intersexual conflict. Q Rev Biol 73:275–304
Jormalainen V, Merilaita S (1995) Female resistance and duration of mate-guarding in three aquatic peracarids (Crustacea). Behav Ecol Sociobiol 36:43–48
Kinne O (1954) Interspezifische sterilpaarung als konkurren zokologischer Faktor bei Gammariden (Crustacea, Peracarida). Naturwissenschaften 18:434–435
Kolding S (1986) Interspecific competition for mates and habitat selection in five species of Gammarus (Amphipoda: Crustacea). Mar Biol 91:491–495
Lagrue C, Wattier R, Galipaud M, Gauthey Z, Rullmann J-P, Dubreuil C, Rigaud T, Bollache L (2014) Confrontation of cryptic diversity and mate discrimination within Gammarus pulex and Gammarus fossarum species complexes. Freshw Biol 59:2555–2570
Lemaître J-F, Rigaud T, Cornet S, Bollache L (2009) Sperm depletion, male mating behaviour and reproductive «time-out» in Gammarus pulex (Crustacea, Amphipoda). Anim Behav 77:49–54
Lengagne T, Grolet O, Joly P (2006) Male mating speed promotes hybridization in the Rana lessonae–Rana esculenta waterfrog system. Behav Ecol Sociobiol 60:123–130
Liou LW, Price TD (1994) Speciation by reinforcement of premating isolation. Evolution 48:1451–1459
Mayr E (1988) The why and how of species. Biol Philos 3:431–441
Mendelson TC, Shaw KL (2012) The (mis)concept of species recognition. Trends Ecol Evol 27:421–427
Panhuis TM, Butlin R, Zuk M, Tregenza T (2001) Sexual selection and speciation. Trends Ecol Evol 16:364–371
Pfennig KS (1998) The evolution of mate choice and the potential for conflict between species and mate-quality recognition. Proc R Soc Lond B 265:1743–1748
Pfennig KS (2000) Female spadefoot toads compromise on mate quality to ensure conspecific matings. Behav Ecol 11:220–227
Ryan MJ, Rand AS (1993) Species recognition and sexual selection as a unitary problem in animal communication. Evolution 47:647–657
Servedio MR, Noor MAF (2003) The role of reinforcement in speciation: theory and data. Annu Rev Ecol Evol Syst 34:339–364
Sparkes TC, Keogh DP, Orsburn TH (2002) Female resistance and mating outcomes in a stream-dwelling isopod: effects of male energy reserves and mating history. Behaviour 139:875–895
Thiel M (2011) Chemical communication in peracarid crustaceans. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer Verlag, New York, pp 199–218
Ward PI (1983) Advantages and a disadvantage of large size for male Gammarus pulex (Crustacea: Amphipoda). Behav Ecol Sociobiol 14:69–76
Wong BBM, Keogh JS, Jennions MD (2004) Mate recognition in a freshwater fish: geographical distance, genetic differentiation, and variation in female preference for local over foreign males. J Evol Biol 17:701–708
Acknowledgments
We thank Aude Balourdet and Christine Dubreuil for field assistance and Thierry Rigaud for insightful discussions on the project and manuscript. The manuscript was significantly improved by very constructive comments from two anonymous reviewers.
Funding
Clément Lagrue was funded by a postdoctoral grant from the regional council of Burgundy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with Ethical Standards
ᅟ
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by T. Breithaupt
Rights and permissions
About this article
Cite this article
Galipaud, M., Gauthey, Z., Turlin, J. et al. Mate choice and male–male competition among morphologically cryptic but genetically divergent amphipod lineages. Behav Ecol Sociobiol 69, 1907–1916 (2015). https://doi.org/10.1007/s00265-015-2003-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-2003-0