Abstract
Sexual conflict is common in nature, but detailed behavioral studies on the role female resistance behavior plays in shaping mating patterns are rare. I manipulated female resistance to examine its effects on pairing dynamics in two ecologically different freshwater amphipods. I found evidence for female behavior playing a role in both the outcome of pre-pairing interactions and the initiation of pairing in both species. In these species, the male optimum pairing duration is greater than the value preferred by females or compromised pairing durations observed under natural conditions, thus indicating sexual conflict. Furthermore, the proportion of male–female encounters producing male grasping was greater and the duration of such interactions was longer when female resistance was reduced. Thus, sexual conflict over pairing duration may select simultaneously for female resistance and for male persistence both of which mediate the outcome of pre-pairing interactions in Hyalella. Contact precopulatory mate guarding and the interactions that precede it are common components of crustacean and insect mating systems, suggesting that such conflicts may play an important role in the evolution of mating traits in many taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual conflict over mating is expected to be common in nature (Chapman et al. 2003; Arnqvist and Rowe 2005), although its detection and analysis can be difficult in practice. Rowe and Day (2006) recently proposed three elements for demonstrating that sexually antagonistic selection is acting within a population: (1) identification of the trait over which conflict occurs (shared trait), (2) identification of the traits in each sex that mediate the outcome of conflict over the shared trait (antagonistic traits), and (3) an understanding of the fitness consequences of the antagonistic traits for each sex (Thornhill 1980). Although point 3 makes uncovering sexually antagonistic selection a daunting task, significant progress has been made for some groups, particularly species in which females store sperm and sexes experience conflict over mating rates (reviewed in Arnqvist and Rowe 2005). In many of these taxa, researchers have identified traits that mediate the outcome of conflict over mating rate, and the effect of these traits on fitness components has been demonstrated in many cases (reviewed in Arnqvist and Rowe 2005). However, evidence for pre-mating sexual conflicts is far less common and often incomplete.
Local ecological conditions across a species’ range can result in variation in sexual conflict dynamics and lead to divergence in mating patterns across populations. The opportunity for, or intensity of, sexual conflict varies across populations. Population structure, i.e. density and sex ratios, will determine male–female encounter rates, and thus play an important role in determining the degree of sexual conflict within populations. In fact, population structure is often manipulated in sexual conflict studies to vary the opportunity for conflict. These studies have shown increases in resistance and persistence behaviors in treatments with more male–female interactions (Arnqvist 1992; Martin and Hosken 2003), and decreases in female fitness components under high-density situations (Martin and Hosken 2004). Furthermore, predation risk and environmental resource levels can affect a female’s willingness to mate, thereby affecting the value of male persistence traits (Rowe et al. 1994; Magurran and Seghers 1994a).
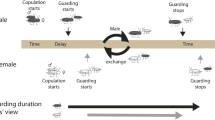
In many crustaceans, a female’s receptivity to fertilization is limited to a brief period after she molts, a temporal restriction that automatically produces a male-bias in the operational sex ratio. This skew is thought to have created fitness incentives for male precopulatory mate guarding (also known as precopula, pairing, passive phase, or amplexus), which is widespread in the group (Ridley 1983). In species that form precopulatory pairs, sexual conflict occurs because males and females are likely to disagree about the optimal duration of pairing (Parker 1979). Sex-specific costs while paired may be associated with predation risk (Cothran 2004), foraging efficiency (Robinson and Doyle 1985), depletion of stored energy (Jormalainen et al. 2001), and missed mating opportunities (reviewed in Jormalainen 1998), any or all of which may affect the relative value being paired. Similarly, the value of entering precopula at a particular time may differ greatly between the sexes because of sex differences in the ability to find a mate (Jormalainen et al. 1994). This topic has received considerable theoretical treatment and conflict is generally predicted to be most intense near the middle of a female’s molt cycle (Jormalainen et al. 1994; Yamamura and Jormalainen 1996). Models have shown that, over a range of conditions, males have more to gain from pairing earlier in the female’s molt cycle than do females.
Freshwater amphipods in the genus Hyalella are common inhabitants of permanent freshwater habitats in North America (Bousfield 1958). Two ecomorphs are commonly found regionally: a small-bodied ecomorph (hereafter “small species”) found in habitats with visual, size-selective predators (e.g. Lepomis spp.), and a large-bodied ecomorph (hereafter “large species”) found in habitats where they experience little or no fish predation (Wellborn 1994). These ecomorphs represent reproductively isolated, undescribed species and each ecomorph is represented by multiple species within the Hyalella azteca species complex (Wellborn et al. 2005). Each ecomorph has morphological, behavioral, and life history phenotypes that are adaptive in their respective environment (Wellborn 1994).
Like many crustaceans, Hyalella mating behavior is tightly linked to the female’s molt cycle. Females are unreceptive to all male pairing attempts early in their molt cycle and become increasingly receptive as the molt approaches (Strong 1973). Pairing durations are variable within Hyalella likely due to differences in predation costs driven by disparate predator regimes (Strong 1973). For the large species, pairing decreases predation risk by larval dragonflies, a common predator in large species habitats, whereas, it increases predation risk by bluegill sunfish, Lepomis machrochirus, a common predator in small species habitats (Cothran 2004). For the species studied here, the large and small species pair for 3 and 1.4 days, respectively. Asymmetries in the costs females pay while paired also may translate into different levels of sexual conflict over the initiation of pairing between the two species. Although detailed analyses of oviposition and fertilization have not been carried out in Hyalella, the end of the pairing phase is behaviorally consistent with observations on Gammarus amphipods. In Gammarus, the pairing phase ends with oviposition of eggs into a ventral brood chamber (marsupium), where the male fertilizes them coincident with the female molt (Sutcliffe 1992). Embryos develop in the female’s marsupium and are released as early instars near the end of the female’s subsequent molt cycle. Females are iteroparous and generally produce a clutch of eggs at each molt. Females do not store sperm and thus require the presence of a male at each molt. Within a population, female molt cycles, and thus the timing of receptivity, are typically asynchronous.
In this study, I manipulated female behavior to determine the potential for sexual conflict over the start of pairing (a shared mating trait) in large and small species of Hyalella. Females were lightly sedated and pairing and several behavioral interactions were recorded. An earlier pairing when females were sedated would indicate that females normally have some degree of control over the initiation of precopula. Also, I tested whether female behavior is important in determining the outcome of pre-pairing interactions, including the frequency and duration of male-initiated grasping behavior, in both Hyalella species.
Materials and methods
Amphipods used in this study were collected in late May. Large species of Hyalella were collected from a spring seep adjacent to the flowing portion of Cowen Creek, Marshal County, Oklahoma (33° 55′ N, 96° 51′ W). Small species of Hyalella were collected from the vegetated littoral region, mostly composed of Potamogeton and Chara, of a farm pond at the University of Oklahoma Kessler Farm Field Laboratory, McClain County, Oklahoma (35°03′ N, 97°32′ W). Amphipods were kept in 80 l aquaria at the University of Oklahoma Biological Station greenhouse, using water and vegetation from their source habitats.
Female behavior and pairing dynamics
Males and females were randomly assigned as pairs to 150 ml beakers. Each of these females had recently deposited eggs into their marsupium (recently fertilized eggs are dark green and easily distinguished from older embryos) a requirement ensuring that females experienced males for nearly an entire molt interval and that standardized the reproductive condition of females used in the experiment. Each beaker contained beach sand plus water from the animals’ source habitats. At 12-h intervals (between 0800 and 1200 and 2000 and 0000 h) beakers were checked for precopulatory pairs. At each check, unpaired females were removed from beakers and placed in small dishes (5-cm diameter) filled with either lake water (control females) or lake water containing a sedative (experimental females; see below). Females remained in these dishes for 5 min, after which they were returned to their respective 150-ml beaker. Sedated females were immobile for several minutes after treatment and a reduction in activity was noticeable throughout the experiment. Beakers were checked for pairs 30 min after females were treated. I recorded two response variables to compare pairing dynamics between treatments. First, I recorded the time remaining to the female molt when pairing (defined as the male grasping the female with his anterior gnathopods in the precopula position; Borowsky 1984) was first observed, even if the pairing was transient, for each pair of amphipods. This was necessary because the first pairing was often unstable (when a pair separated before the female molt, the pair was scored as having had a switch in pairing state) in the sedated female treatment. I also recorded pairing duration, defined as the interval between stable (no observed switch in pairing state) pair formation and separation of the pair coinciding with the female molt and oviposition. For each individual, I measured head length, a measure of body size (Edwards and Cowell 1992; Pickard and Benke 1996), and male posterior gnathopod width, a sexually dimorphic appendage in Hyalella, using a dissecting microscope fitted with an ocular micrometer.
For the large species, water treated for 10 min with a constant supply of CO2 was used to sedate females. For the small species, mortality was high for females exposed to CO2-treated water; therefore, a clove oil solution (0.002 ml clove oil per milliliter, 0.001 ml ethanol per milliliter, water solution) was used in its place. The clove oil solution and CO2 had similar effects on female behavior. Female mortality was higher in the sedated treatment for both the large (control 4.7% vs. sedated 44.2%; \(\chi _1^2 = 18.21\), P < 0.001) and small (control 7.1% vs. sedated 26.2%; \(\chi _1^2 = 5.49\), P = 0.019) species. Only trials where females survived were used in analyses.
Within treatments, I first tested for correlations between response variables and female body size, male body size, and male gnathopod size. For male gnathopod size, partial correlations were used to control statistically for the covariance between male body size and gnathopod size. I then tested for an effect of reduced female activity level on pairing dynamics using independent samples t tests or Welch’s t when homogeneity of variances could not be achieved via transformation of data (Quinn and Keough 2002). First, I tested whether the reduction in female activity level affected the timing of the first pairing (even if transient). For this analysis, the time remaining to the female molt when the first pairing was observed was divided by the total time (time that elapsed between the start of the experiment and the female molt) of the trial. This correction was necessary because the total time determines the maximum possible pairing duration and was correlated with the timing of the first pairing in the sedated female treatment. The total time did not differ between treatments for either the large or small species, and ranged from 120–240 h in the large species and 120–264 h in the small species. Pearson Chi-squared tests were used to compare the proportion of trials for which a switch in pairing state was observed for control and sedated female treatments. Finally, I tested whether female activity level affected stable pairing duration. This response variable was not corrected for the total trial time because there was no correlation between these two variables.
Pre-pairing behavioral observations
Behavioral observations were performed on a random subset (half of the pairs set up for each treatment) of beakers each day during either the morning or evening observation (alternated for each beaker each day). During each 5 min observation I recorded each case of physical contact between the sexes, whether this led to an interaction (defined as the male grasping the female in an attempt to pair), the duration of each interaction, and pairings. Data used in analyses represent mean values for all observations recorded for each pair of amphipods.
From behavioral observations, I compared the proportion physical contacts that led to a male grasping the female as well as the duration of these interactions for females with normal (control females) and reduced (sedated females) activity levels using independent samples t tests or Welch’s t when homogeneity of variances could not be achieved via transformation of data.
Results
Pairing dynamics differed between treatments for both the large and small species. First pairings were observed earlier in the experiment when female activity levels were reduced for both the large (152% earlier: Welch’s t 30.42 = 6.41, P < 0.001) and small (125% earlier: t 68 = 3.74, P < 0.001) species (Fig. 1). These initial pairings were often transient in the sedated female treatment, as indicated by the fact that switches in pairing state were much more common in this treatment than the control treatment for both the large (control: 1/41, sedated: 15/24; \(\chi _1^2 = 29.43\), P < 0.001) and small (control: 3/39, sedated: 13/31; \(\chi _1^2 = 11.49\), P = 0.001) species. Stable pairing (i.e., pairs that did not separate before oviposition) duration was longer for sedated females than control females for both the large (49% longer: t 63 = 2.57, P = 0.012) and small (43% longer: t 68 = 2.9, P = 0. 005; Fig. 2) species. For the small species, there was a positive correlation between the first site of pairing and male body size in the control but not the sedated treatment. For both species, correlations for male body size and both the first site of pairing and stable pairing duration were greater in the control than the sedated treatment. Male size-corrected gnathopod size and female body size were not correlated with the first site of pairing or stable pairing duration in either species (Table 1).
Proportion of time remaining to the female molt when the first pairing was observed for control vs. sedated females. These results include cases where the initial pairing was unstable, which was often the case in the sedated treatment. Each box represents the 25th and 75th percentiles. Whiskers represent the 10th and 90th percentiles. The dashed line represents the mean and the solid line the median. Closed circles represent outliers. Sample sizes are given above each box
Stable pairing durations for control vs. sedated females. Symbols as in Fig. 1
Sedated females experienced a higher proportion of encounters that led to their being grasped in the large species (Welch’s t 13.64 = 7.62, P < 0.001), and tended to do so in the small species (Welch’s t 19.08 = 1.96, P = 0.07; Fig. 3). Interactions between the female and male lasted longer when females were sedated for both the large (t 29 = 5.67, P < 0.001) and small (t 34 = 2.26, P = 0.03; Fig. 4) species. There were no significant correlations between response variables and male body and size-corrected gnathopod size and female body size for pre-pairing behavioral data. For the large species larger males grasped females at a higher rate than smaller males in the control group but this was not statistically significant (Table 2).
Proportion of encounters that resulted in the male grasping control vs. sedated females. Symbols as in Fig. 1
Average male grasp durations for control vs. sedated females. Symbols as in Fig. 1
Discussion
In this study, phenotypic manipulation of female activity level, including the capacity to resist male pairing attempts, revealed disagreement between the sexes over paring duration in Hyalella amphipods. In the sedated female treatment, pairings occurring early in the female molt cycle were often transient, probably because females eventually recover from sedation and invest in resistance behavior when paired too early. The first site of pairing occurred earlier in the experiment when females were unable to resist male pairing attempts (Fig. 1), indicating that male optimal pairing durations are longer than those preferred by females. This result has now been documented in several peracarid crustaceans (Jormalainen and Merilaita 1995; Jormalainen and Shuster 1999; Sparkes et al. 2000), and highlights the importance of female behavior in what was traditionally considered to be a male decision-making process (Jormalainen 1998).
An alternative interpretation for these results is that males use the early stages of precopula to assess females, and in the sedated treatment they did not receive necessary feedback to continue pairing. Although this interpretation cannot be rejected, available evidence suggests that this form of male assessment is largely lacking in Hyalella. The transient pairings observed when female resistance behavior was reduced are anomalies that rarely occur under more natural conditions (Strong 1973; for this study, results from control treatments: large species 1/41 and small species 3/39 trials), a pattern that is inconsistent with males using such an assessment strategy. In general, there is little evidence that males assess females in that they attempt to pair with individuals regardless of size, sex, age, and even whether they are dead or alive, and sometimes succeed in doing so (Holmes 1903; Strong 1973; Wellborn 1995, personal observation).
Theoretical and empirical studies suggest that local ecological conditions will affect the opportunity for sexual conflict within populations (Gavrilets 2000; Martin and Hosken 2004; Härdling and Kaitala 2005). High population density and male-biased operational sex ratios result in high intersexual encounter rates and thus increased male harassment of females (Krupa and Sih 1993; Magurran and Seghers 1994b). The structure of Hyalella populations is conducive for intersexual conflict over pairing duration in these key respects. Both the small and large species occur at high densities (small species from 8,300 to 18,100; large species from 700 to 8,400 individuals per square meter; Wellborn 1994), which combined with male-biased operational sex ratios driven by temporally restricted and asynchronous female receptivity (Wellborn and Cothran 2007), sets the stage for intense male–male competition for mating opportunities. Under these circumstances, the probability of finding a female that is receptive to fertilization is low, which favors precopulartory mate guarding as a male time investment strategy (Parker 1974a; Jormalainen 1998). Although females need a male near the end of their molt to fertilize their eggs, they are expected to pay costs for prolonged guarding durations (Jormalainen et al. 2001; Cothran 2004). Therefore, optimal duration for mate guarding is expected to be longer for males than for females. Results from this study are congruent with these predictions. For both the large species and small species, pairing duration increased when female resistance was limited (Fig. 2), suggesting that females prefer shorter paring durations than males. The extent to which male and female interests differ, however, will depend on the costs associated with pairing for each sex.
Intersexual asymmetries in the costs of pairing in the small species may result in intense sexual conflict, whereas, the opportunity for conflict appears to be weaker in the large species. In Hyalella, predation risk associated with pairing differs between species. In the small species, pairing increases male and female susceptibility to predation by Lepomis sunfish, which are size-selective predators preferring larger prey items (Strong 1972; Wellborn 1994). The magnitude of this cost is greater for females than males because females are not as susceptible to fish predation while single compared to males (Cothran 2004). On the other hand, female large species are less likely to fall prey to larval dragonflies while paired than when single. This is probably due to lower activity levels while paired, and thus decreased encounter rates with these sit-and-wait predators (Cothran 2004). These results suggest that asymmetries between the sexes in predation costs are less likely to play a significant role in sexual conflict over pairing duration in the large species (Cothran 2004). Therefore, female small species may have more to lose and are expected to invest more in resistance behaviors than female large species. This argument is in agreement with observations of field guarding durations where female large species pair earlier in their molt cycle than female small species (Wellborn 1995; Wellborn and Bartholf 2005). In this study, such interspecific differences in the benefits of female resistance behavior should manifest as a greater increase in pairing duration in the small species compared to the large species. However, the increase in pairing duration when female resistance was limited was similar in the large species and small species (large species: 49% increase, small species: 43% increase; Fig. 2). These results suggest that other factors, perhaps interspecific variation in the social and environmental context in which pairing decisions take place (Dick and Elwood 1996), drive variation in field pairing durations in Hyalella amphipods.
Female resistance behavior may be important in moderating the negative effects of male guarding attempts. Pre-pairing interactions with males may be costly for females resulting in decreased energy reserves and fecundity, as has been observed in the isopod Idotea baltica (Jormalainen et al. 2001). Furthermore, pre-pairing interactions involve considerable movement, which may increase the conspicuousness of the interacting pair to predators. These costs are magnified by the fact that Hyalella occur at high densities (Wellborn 1994). In this study, a higher proportion of encounters led to the male grasping the female in the large species (Fig. 3) and these grasps lasted longer when females were unable to resist in both the large and small species (Fig. 4). Thus, control females were more efficient at avoiding male grasps and quickly dislodging males compared to sedated females, suggesting that female behavior is important in mediating pre-pairing interactions in Hyalella.
To understand the evolutionary implications of conflict over precopula requires a functional understanding of the traits that mediate its outcome and knowledge about how these traits impact the fitness of each sex (Pizzari and Snook 2003; Rowe and Day 2006). Mating biases are common in Hyalella, with both male body size and posterior gnathopod size increasing male pairing success in some populations (reviewed in Wellborn and Cothran 2007). Sexual conflict is likely to be resolved in favor of the sex with the most physical power/best fighting ability (Yamamura and Jormalainen 1996; Jormalainen 1998), which often depends on body size (see references in Parker 1974b; Thornhill and Alcock 1983). Results from this study suggest that male body size may mediate the outcome of sexual conflict in Hyalella populations. For the small species, larger males were more successful than smaller males at overcoming female resistance; however, when female resistance was reduced, male size had no effect on initiation of precopula. In addition, in the large species larger males tended to grasp females at a higher rate than smaller males in the control but not the sedated treatment. These results are both consistent with larger male body size increasing persistence ability in conflicts over pairing duration, and may explain why large male mating biases are commonly found in Hyalella populations.
It is likely that sexual conflict has played at least an indirect role in the evolution of nonrandom mating in Hyalella. Clearly, females resist male pairing attempts early in their molt cycle to avoid the costs of early pairing (Jormalainen 1998). Thus, natural selection has favored the phenotype in females, resistance to pairing, which is responsible for filtering male phenotypes later in the female molt cycle. In addition to resistance early in the molt interval, however, females may also practice selective resistance (i.e. favoring some male phenotypes over others) during the period they are receptive to male guarding. If this is the case, then the mating biases that emerge from such a process are best explained as a form of traditional sexual selection via female choice.
In the large species, females receive fitness benefits from mating with large males with large gnathopods (Cothran, submitted), suggesting that traditional sexual selection through female choice is important in maintaining mating biases in large species populations. In the small species, mating biases with respect to male body and posterior gnathopod size are weaker. Intermediate and larger males have equal pairing success and large gnathopods increase pairing success only for smaller males (Wellborn 1995; Wellborn and Bartholf 2005). Currently, we do not know how male traits influence female fitness in the small species, but because sexual conflict is expected to be most intense in this species, this issue certainly deserves attention. Sexual conflict over pairing duration has potential to shape mating traits in Hyalella species; therefore, studies on the fitness consequences of intersexual interactions in this group are necessary to shed light on the evolutionary potential of this conflict.
References
Arnqvist G (1992) Pre-copulatory fighting in a water strider: inter-sexual conflict or mate assessment. Anim Behav 43:559–567
Arnqvist G, Rowe L (2005) Sexual conflict. Princeton University Press, Princeton, New Jersey
Borowsky B (1984) The use of the males’ gnathopods during precopulation in some gammaridean amphipods. Crustaceana 47:245–250
Bousfield EL (1958) Fresh-water amphipod crustaceans of glaciated North America. Can Field-Nat 72:55–113
Chapman TG, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47
Cothran RD (2004) Precopulatory mate guarding affects predation risk in two freshwater amphipod species. Anim Behav 68:1133–1138
Dick JTA, Elwood RW (1996) Effects of natural variation in sex ratio and habitat structure on mate-guarding decisions in amphipods (Crustacea). Behaviour 133:985–996
Edwards TD, Cowell BC (1992) Population dynamics and secondary production of Hyalella azteca (Amphipoda) in Typha stands of a subtropical Florida lake. J North Am Benthol Soc 11:69–79
Gavrilets S (2000) Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403:886–889
Härdling R, Kaitala A (2005) The evolution of repeated mating under sexual conflict. J Evol Biol 18:106–115
Holmes SJ (1903) Sex recognition among amphipods. Biol Bull 5:288–292
Jormalainen V (1998) Precopulatory mate guarding: male competitive strategy and intersexual conflict. Q Rev Biol 73:275–304
Jormalainen V, Merilaita S (1995) Female resistance and duration of mate-guarding in 3 aquatic peracarids (Crustacea). Behav Ecol Sociobiol 36:43–48
Jormalainen V, Shuster SM (1999) Female reproductive cycle and sexual conflict over precopulatory mate-guarding in Thermosphaeroma isopods. Ethology 105:233–246
Jormalainen V, Tuomi J, Yamamura N (1994) Intersexual conflict over precopula duration in mate guarding Crustacea. Behav Process 32:265–283
Jormalainen V, Merilaita S, Riihimäki J (2001) Costs of intersexual conflict in the isopod Idotea baltica. J Evol Biol 14:763–772
Krupa J, Sih A (1993) Experimental studies on water strider mating dynamics: spatial variation in density and sex ratio. Behav Ecol Sociobiol 33:107–120
Magurran AE, Seghers BH (1994a) Sexual conflict as a consequence of ecology—evidence from guppy, Poecilia reticulata, populations in Trinidad. Proc R Soc Lond B Biol Sci 255:31–36
Magurran AE, Seghers BH (1994b) A cost of sexual harassment in the guppy, Poecilia reticulata. Proc R Soc Lond B Biol Sci 258:89–92
Martin OY, Hosken DJ (2003) The evolution of reproductive isolation through sexual conflict. Nature 423:979–982
Martin OY, Hosken DJ (2004) Reproductive consequences of population divergence through sexual conflict. Curr Biol 14:906–910
Parker GA (1974a) Courtship persistence and female-guarding as male time investment strategies. Behaviour 48:157–184
Parker GA (1974b) Assessment strategy and evolution of fighting behaviour. J Theor Biol 47:223–243
Parker GA (1979) Sexual selection and sexual conflict. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in insects. Academic, New York
Pickard DP, Benke AC (1996) Production dynamics of Hyalella azteca (Amphipoda) among different habitats in a small wetland in the southeastern U S A. J North Am Benthol Soc 15:537–550
Pizzari TR, Snook R (2003) Perspective: Sexual conflict and sexual selection: chasing away paradigm shifts. Evolution 57:1223–1236
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Ridley M (1983) The explanation of organic diversity: the comparative method and adaptations for mating. Clarendon, Oxford
Robinson BW, Doyle RW (1985) Trade-off between male reproduction (amplexus) and growth in the amphipod Gammarus lawrencianus. Biol Bull 168:482–488
Rowe L, Day T (2006) Detecting sexual conflict and sexually antagonistic coevolution. Philos Trans R Soc Lond B 361:277–285
Rowe L, Arnqvist G, Sih A, Krupa JJ (1994) Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol Evol 9:289–293
Sparkes TC, Keogh DP, Haskins KE (2000) Female resistance and male preference in a stream-dwelling isopod: effects of female molt characteristics. Behav Ecol Sociobiol 47:145–155
Strong DR (1972) Life history variation among populations of an amphipod (Hyalella azteca). Ecology 53:1103–1111
Strong JDR (1973) Amphipod amplexus, the significance of ecotypic variation. Ecology 54:1383–1388
Sutcliffe DW (1992) Reproduction in Gammarus (Crustacea, Amphipoda): basic processes. Freshw Forum 2:102–129
Thornhill R (1980) Rape in Panorpa scorpionflies and a general rape hypothesis. Anim Behav 28:52–59
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press, Cambridge
Wellborn GA (1994) Size-biased predation and prey life histories: a comparative study of freshwater amphipod populations. Ecology 75:2104–2117
Wellborn GA (1995) Determinates of reproductive success in a freshwater amphipod species that experience different mortality regimes. Anim Behav 50:353–363
Wellborn GA, Bartholf SA (2005) Ecological context and the importance of body and gnathopod size for pairing success in two amphipod species. Oecologia 143:308–316
Wellborn GA, Cothran RD (2007) Evolution and ecology of mating behavior in freshwater amphipods. In: Duffy E, Thiel M (eds) Evolutionary ecology of social and sexual systems: crustaceans as model organisms. Cambridge University Press, Cambridge
Wellborn GA, Cothran R, Bartholf S (2005) Life history and allozyme diversification in regional species of the Hyalella azteca (Crustacea: Amphipoda) species complex. Biol J Linn Soc 84:161–175
Yamamura N, Jormalainen V (1996) Compromised strategy resolves intersexual conflict over pre-copulatory guarding duration. Evol Ecol 10:661–68
Acknowledgments
This research was funded by grants from The University of Oklahoma Graduate Student Senate, and scholarships from the University of Oklahoma, College of Arts and Sciences (Sturgis Scholarship), Department of Zoology (Blanche Adams Memorial Scholarship) and Biological Station (Summer Fellowship). My graduate advisor Gary Wellborn provided insightful comments and encouragement throughout the preparation of the manuscript. I thank my advisory committee Wayne Elisens, Ola Fincke, Rosemary Knapp, and Douglas Mock for guidance and constructive criticisms. Veijo Jormalainen and an anonymous reviewer offered constructive comments on an earlier version of the manuscript. This manuscript formed part of the requirements for a Doctor of Philosophy degree at the University of Oklahoma. The experiments herein comply with the current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Backwell
Rights and permissions
About this article
Cite this article
Cothran, R.D. Phenotypic manipulation reveals sexual conflict over precopula duration. Behav Ecol Sociobiol 62, 1409–1416 (2008). https://doi.org/10.1007/s00265-008-0570-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0570-z