Abstract
Prey monitor predator movements to assess risk, as required to make escape decisions and avoid being captured unaware. For prey that exhibit behavioral signs that they have detected predators, alert distance is the predator-prey distance when the prey performs the behavior and then continuously monitors the predator’s approach. Many other prey do not usually give any indication of having detected a predator prior to fleeing. This is especially likely in prey having laterally placed eyes that are approached from one side, as in typical studies of lizards. We conducted field trials to detect overt signs of monitoring by zebra-tailed lizards, Callisaurus draconoides, which usually exhibit no signs of monitoring. When a researcher walked in an arc starting at some distance from a lizard’s side and continuing until he was directly in front of or behind it, the lizard cocked its head and/or reoriented its body or fled and then reoriented. These behaviors allowed lizards to keep the researcher in view as he passed out of a monocular visual field. The findings demonstrate that monitoring occurs in these lizards, suggest that monitoring is so important that lizards risk being detected by moving, and suggest a possible method for studying effects of alert distance in prey that do not perform alerting behaviors when approached in full view. Alerting responses have been observed infrequently in lizards because researchers are in one of the wide lateral visual fields when they start to approach. Unless the predator moves out of view, prey with limited or no binocular vision have no need for postural adjustment to focus on the predator.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monitoring behavior is an important component of a prey’s anti-predatory defense that is required to assess predation risk as a predator moves nearby. When an immobile prey detects a predator, it should monitor the predator’s movements to gain the information to assess risk and make decisions about whether and when to flee. Prey must monitor the predator to prevent it from moving out of sight where it might move close to the prey before being detected again. Vigilance is important because it prevents predators from approaching dangerously close before being detected. Many birds, mammals, and some other prey overtly express vigilance by scanning their environments periodically (Lima 1994; Caro 2005) and either monitor predators that they detect or flee. After detecting a predator, many prey orient toward it to facilitate monitoring. Even if a prey has been aware of the predator earlier, orienting demarcates a shift from vigilance with little or no monitoring to continuous monitoring.
Reorientation of the prey’s head and/or body to face the predator is called alerting, and the predator-prey distance at which alerting occurs is alert distance (Stankowich and Coss 2007). The flush early and avoid the rush (FEAR) hypothesis predicts that as alert distance increases, prey flee sooner during the predator’s approach (Blumstein 2010; Cooper and Blumstein 2014). Flight initiation distance (FID = predator-prey distance when fleeing begins) in many species increases as alert distance increases, confirming the FEAR hypothesis and showing the importance of monitoring for escape decisions (Stankowich and Coss 2007; Samia et al. 2013; Cooper and Sherbrooke 2015).
If prey does not overtly alert during approaches, monitoring is indistinguishable from other behaviors that may occur simultaneously, such as scanning the environment for food. Many prey, including most ambush-foraging lizards, usually show no external signs of detecting or monitoring predators before fleeing. Monitoring can be inferred post hoc from escape decisions, but lizards usually do not perform postural movements that orient their bodies to place predators in their visual fields (Cooper 2008).
Most ambush-foraging lizards spend 1 % or less of their time moving; they remain immobile while foraging except to attack prey or shift ambush posts (Huey and Pianka 1981; Perry 1999; Cooper 2005a, 2007). While a lizard is motionless, an approaching predator may initially be in clear view but then begin to move into a position directly in front of or behind the lizard. In either position, it may be more difficult for the prey to monitor the predator or the predator may pass out of the lateral visual field on the side nearer the predator (Cooper 2008). The idea that lizards have difficulty detecting predators in these positions is suggested by the findings that FID is shorter in Sceloporus virgatus when the predator approaches from directly ahead of or behind a lizard rather than from one side (Cooper 2008).
In the only previous study of monitoring by ambushing lizards, Cooper (2008) found that when a predator moved out of view, S. virgatus and Sceloporus jarrovii cocked their heads, turning them to the side to maintain visual contact. During our long field experience, we have noted that these lizards sometimes turn their trunks or entire bodies to view us as we approach, as does the skink Emoia impar (McGowan et al. 2014). We examined these relationships by ourselves simulating a nearby predator that move out of clear view to positions directly in front of or behind zebra-tailed lizards (Callisaurus draconoides), demanding that they reorient to continue monitoring (Cooper 2008).
Methods
Animals, field site, and conditions
C. draconoides is a small, insectivorous phrynosomatid lizard (101-mm snout-vent length) (Stebbins 2003). As an ambusher forager, this lizard moves infrequently: the closely related and behaviorally similar Cophosaurus texanus is immobile 98 % of the time (Cooper et al. 2001). The study site was a 10-km strip along the north side of the Rillito River Park in Tucson, Arizona, USA. On both sides of a paved path in the park, native desert vegetation is maintained by irrigation. Among the plants are palo verde (Parkinsonia spp.), mesquite (Prosopis spp.), saguaro cactus (Carnegiea gigantea), and yuccas (Yucca spp.). We conducted the study in late May to early June 2013 in areas where vegetative cover was sparse. We collected data only for lizards observed on open ground, excluding those on rocks or beneath plants.
We made all observations in mid-morning to early afternoon when lizards were fully active on warm, sunny days. Because flight initiation distance does not differ between sexes of C. draconoides (Bulova 1994), which suggests that monitoring may be similar in the sexes, we did not record sex. Data were collected only for adults.
Design, data collection, and analysis
We conducted an experiment in which a human simulated predator moved in an arc around a motionless lizard, starting from a position parallel to the lizard’s longitudinal axis and continuing until the predator moved directly ahead or directly behind the lizard. Our movements simulated a predator moving close to and in full view of prey without approaching but then passing out of the prey’s view. Simulation of an approaching predator by a human investigator is a method widely used in escape studies (Blumstein 2003; Stankowich and Blumstein 2005; Samia et al. 2015) and in the only study of predator monitoring by lizards (Cooper 2008). Using a human surrogate predator permits rapid data collection, a major advantage because naturally occurring predator-prey encounters are rare. Behavior of researchers during trials is easily standardized, but that of natural predators is not. Human beings can approach efficiently in irregular terrain inaccessible to terrestrial mechanized predator models. Use of human surrogate predators also avoids ethical concerns about attacks on prey by natural predators.
Before each trial, an investigator walked slowly (0.5 m/s) along a transect while searching visually for a lizard. Upon detecting one, he moved very slowly (ca. 0.3 m/s) into position facing in the direction that he would move during the trial. To begin a trial in which a simulated predator passed from a readily viewed position on one side of the lizard to a less clearly visible or invisible position directly in front of the lizard, the investigator moved slowly and directly to a position 4.6–6.1 m from the lizard and then oriented himself to face in the same direction as the lizard was facing. For similar trials in which the predator passed from a readily viewed position on one side to a less clearly visible position directly behind the lizard, the investigator initially moved to a position 4.9–7.3 m from the lizard and facing in the direction opposite from the direction the lizard was facing.
Then, the investigator walked slowly (0.5 m/s) in an arc, maintaining the same distance from the lizard until he passed directly in front or behind it. In all trials, the investigator kept the lizard in his view without looking directly at it unless and until it fled, cocked its head toward him, or reoriented its body without moving away. Trials ended when the focal lizard fled or the investigator passed in front or directly behind it. The researcher recorded whether the lizard fled before or as he passed directly in front of or behind it and whether it turned its head or body to a position presumably affording a better view of the investigator. After a lizard fled, he recorded whether it reoriented itself upon stopping to an angle from which the predator was clearly in its lateral field of view. When a trial was completed, the investigator noted the lizard’s position or used its location and direction as it fled from view to avoid pseudoreplication. The investigator then moved along the transect before conducting another trial.
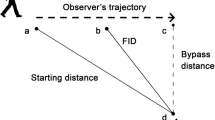
We did not record the starting distance (SD = predator-prey distance when the predator’s approach begins) from which we moved into position to begin walking an arc because SD does not affect FID in ambushing lizards, including C. draconoides approached slowly (Cooper 2005b; Cooper and Sherbrooke 2013a). There is no reason to suspect that SD differed between the two groups. Even if a slight difference in SD between groups was to have affected alerting responses, conclusions for each of the experimental groups would not be affected.
In many prey, FID increases as SD or alert distance (AD) increases (Blumstein 2003; Samia et al. 2013, 2015), which occurs for biological reasons (Cooper and Blumstein 2014) and because the mathematical constraint that SD ≥ AD ≥ FID causes a small positive correlation between FID and the other variables (Dumont et al. 2012; Cooper and Blumstein 2014). Differences in SD might affect AD due to the constraint, but we did not measure FID or SD. Furthermore, our goal was to detect alerting behaviors in a species that typically does not perform them, not to detect any difference in AD between groups. The minor difference between groups in distance maintained while walking in an arc is irrelevant.
Differences in frequencies of head cocking, reorientation, fleeing, and immobility before, as, or after the predator passed from view were assessed for significance using sign tests. For these tests, we assumed that responses were equally likely before and as the predator passed from view. This assumption is very conservative because the interval between the time required to walk from the staring position toward a lizard’s front or rear was much longer than the moment in which the researcher moved out of the lizard’s view.
We tested the significance of frequency differences between types of behaviors performed as a researcher moved out of view using binomial tests. We used Fisher exact probability tests to compare frequencies when the predator moved out of sight in front of and behind lizards. Using data pooled for both types of trials, we examine differences in frequency of lizards that fled and cocked heads versus all other responses, fled and reoriented their bodies toward the predator versus all other responses, only reoriented versus all other responses, did not react versus all other responses, and cocked heads or reoriented versus all other responses. We tested for differences among individuals that cocked their heads as the predator passed out of clear view between trial types: cocked heads only versus all other responses, cocked heads and reoriented versus all other responses, and cocked heads, reoriented, and fled versus other responses. Effect sizes are presented as r equivalent (Rosenthal and Rubin 2003). Tests were two-tailed with α = 0.05.
Results
Response when the predator passed from view
Only two lizards showed any visible reaction before the predator reached a point directly in front of them and no lizard reacted before the predator passed behind it; of the remaining 44 individuals, all but 2 performed one or more behaviors as the predator passed from clear view (Table 1). The most frequent response was cocking the head to the side, affording a monocular view of the predator. Many individuals reoriented their bodies to positions that afforded a monocular view of the predator without having to cock their heads. A few did so without fleeing, but reorientation of the body occurred more frequently at the end of escape runs. All lizards that fled when the predator passed out of view also reoriented after escape runs.
Response frequencies when the predator circled to the anterior versus posterior
No behavioral frequencies (Table 1) differed significantly between experimental groups. For tests of the frequencies from Table 1, Fisher p values ranged from 0.20 to 1.00, indicating similarity of response frequencies when the predator passed in front of and behind the lizards. Therefore, data for the two trial types were pooled for further analysis.
Head cocking was by far the most frequent response as the predator passed from view. Fleeing was infrequent before the predator passed from view (0.04) but was more frequent among the remaining lizards as the predator passed from view (0.23, Table 1). As the researcher moved from view, a large proportion (0.68) cocked their heads and 0.27 of individuals reoriented their bodies. All but two lizards that reoriented fled first. Only 2 of 44 failed to respond when the predator passed from view (0.05, Table 1).
Most lizards that cocked their heads exhibited no other responses (0.83), but some also reoriented their bodies without fleeing (0.07) and a few also fled and then reoriented (0.10). The frequency of head cocking without performing other behaviors was significantly greater than that of head cocking combined with other behaviors (reorienting without fleeing, p < 0.0001; fleeing and reorienting, p < 0.0001; reorienting or both fleeing and reorienting; p = 0.0003). Values of r equivalent for these tests were 0.66, 0.64, and 0.58, indicating intermediate effect sizes.
Response frequencies before and as the predator passed from view
Almost all responses occurred as the predator passed directly in front of or behind lizards. The probability of fleeing or showing any reaction was far lower before than as the predator passed from view (2 of 46 before, 42 of 44 as; sign test, n = 44, p < 0.0001, r equivalent = 0.80). Compared with the frequencies before the researcher passed from view, lizards were significantly more likely to perform behaviors allowing monitoring as the researcher passed from view: cock their heads (2 of 46 before, 30 as, sign test, n = 32, p < 0.0001, r equivalent = 0.77), flee and reorient (2 before, 10 as of 46; sign test, n = 12, p = 0.032, r equivalent = 0.30), and either head cock or reorient before or after fleeing (42 as, 0 before of 46; sign test, n = 42, p < 0.0001, r equivalent = 1.00). Differences between frequencies of reorienting without other response and of no reaction before and as the predator passed from view were not significant (p > 0.10 each). Several of these effect sizes in are large, indicating marked change in behavior as the predator passed from view.
The most frequent response as the predator passed from view was head cocking, followed by fleeing and reorienting (Table 1, pooled data). As the predator passed from view, lizards were significantly more likely to cock their heads than not (30 of 44, binomial p = 0.013, r equivalent = 0.34). Among lizards that did not flee as the predator passed from view (Fig. 1), head cocking was more often a sole response than part of a combination with other responses: cocked plus reoriented (2 versus 25 that cocked only; sign test, p < 0.0001; r equivalent = 0.74); cocked, fled, and reoriented (3 versus 25 cocked only; sign test, p < 0.0001; r equivalent = 0.69); cocked and fled plus cocked, fled, and reoriented (5 versus 25 that cocked only; sign test, p = 0.0003, r equivalent = 0.59).
Response frequencies by Callisaurus draconoides as the predator moved out of view in front of or behind them (pooled data). CO cocked head or oriented, C cocked head only, O oriented body toward predator only, N neither cocked head nor oriented. The error bars represent 1 standard error of a proportion
Discussion
The response pattern of C. draconoides suggests that they continuously monitor a predator moving nearby but usually remain motionless while the predator is in view at a distance long enough not to require fleeing, i.e., longer than the FID. Neither of the two lizards that fled before the predator passed from view performed any alerting responses but presumably monitored the predator. Over 95 % of lizards that did not flee while the predator remained in view moved immediately when it began to pass directly in front of or behind them. Among lizards that did not flee when they lost visual contact with the predator, a large majority (88 %) cocked their heads, bringing the predator into view; a small proportion of them (6 %) did not head cock but reoriented their bodies in directions that placed the predator in view. Of all lizards that fled or remained in place as the predator passed from view, 95 % adjusted their body orientations so that the predator was in view either at their initial positions or where they stopped after fleeing.
Ambush-foraging lizards remain immobile much of the time (Perry 1999; Cooper 2007) and are able to detect and monitor predators in their wide lateral visual fields (New and Bull 2011). A broad streak of retinal ganglion cells in laterally placed eyes in the scincid Tiliqua rugosa and many other lizards enables clear vision over a wide angle without moving the head (New and Bull 2011). In prey that have well-developed binocular vision, alerting responses presumably improve depth perception by placing the predator in the binocular field and by using their foveas to maximize acuity. Prey that lack or have minimally developed binocular vision depend on lenticular deformation (i.e., bending of biconvex lenses) for accommodation (Schwalb 2012). Such species, including most lizards, may not be able to improve their monitoring ability by orienting their heads directly toward the predator. On the contrary, by orienting directly toward a predator, a prey lacking or having limited binocular vision may lose sight of it or encounter focusing problems. However, the ability of many lizards to catch prey located directly in front of them (e.g., Ott et al. 2004) emphasizes the need for studies of lizard visual fields.
Differences in visual fields may explain in part the taxonomic distribution of alerting behavior but provides no help in establishing the occurrence or importance of monitoring behavior in prey that do not exhibit overt alerting responses. Models of escape behavior, including those that predict FID (Ydenberg and Dill 1986; Cooper and Frederick 2007, 2010) and latency to flee (Martín et al. 2009; Cooper et al. 2012; Cooper and Sherbrooke 2013b), assume ongoing monitoring of predators. Our findings show that monitoring occurs in the absence of alerting behavior but strictly applies only immediately before and as the predator move out of the prey’s visual field.
Nevertheless, there are strong reasons to believe that continuous monitoring occurs in the absence of alerting. Our findings that C. draconoides and S. virgatus (Cooper 2008, this paper) take overt action to maintain visual contact with predators suggest that the lizards had been monitoring the predator’s movements. Because their postural adjustments were immediate, lizards must have been monitoring predators closely, satisfying the assumptions of monitoring in economic models of FID and latency to flee (Ydenberg and Dill 1986; Cooper and Frederick 2007; Martín et al. 2009; Cooper 2012; Cooper et al. 2012; Cooper and Sherbrooke 2013a, b).
Predictions of these models about effects of factors affecting costs of fleeing and costs of not fleeing have been extensively confirmed in diverse prey, including species that do not exhibit alerting behavior (Stankowich and Blumstein 2005; Martín et al. 2009; Cooper 2010, 2012; Cooper et al. 2012; Cooper and Sherbrooke 2013a, b). Such verification cannot have occurred without monitoring. Vigilance enhances the ability of prey to detect and escape rapidly (Lima 1994), and monitoring is crucial for risk assessment when immediate escape is not required.
In birds, escape behavior varies with eye and brain size. Birds with larger eyes presumably can detect predators at longer distances and have longer FID than birds with smaller eyes (Møller and Erritzøe 2014). Birds having larger brains, which may indicate greater ability to assess risk while monitoring, have shorter FIDs than species with smaller brains (Møller and Erritzøe 2014). Correlated evolution between escape decisions and eye and brain sizes suggests that monitoring and risk assessment may have coevolved not only with each other but also with sensory and neural capacities.
Our findings show that monitoring can be detected in prey that do not perform alerting responses while a predator remains in view, but only when it begins to pass out of the prey’s monocular field of view, requiring postural adjustment, i.e., alerting, to keep the predator in view. These results and confirmation of hypotheses assuming monitoring allow us to infer that continuous monitoring occurs in the absence of alerting responses, as required by a wide range of predictions of escape theory.
We can be confident that prey having the requisite sensory capacities monitor predators, but the present findings do not permit researchers to detect when and where the prey becomes aware of and begins to monitor a predator. Knowing the predator-prey distance when the prey becomes alert, i.e., the alert distance, is essential for testing both the prediction of the FEAR hypothesis that FID increases as alert distance increases and the recently proposed effects of monitoring on risk assessment (Blumstein 2010; Cooper and Blumstein 2014). For prey lacking alerting behaviors, physiological monitoring may be required to detect the onset of monitoring.
Starting distance is often a useful proxy for alert distance when prey do not perform alerting responses. Although movements unrelated to a predator may occur, especially before prey becomes aware of the predator, their effects are often minimal and can be eliminated by using quantile regression (Chamaillè-Jammes and Blumstein 2012). Nevertheless, it is to be hoped that future research will discover means of detecting the onset and continuance of monitoring. Head cocking distance might be a useful measure of alert distance if lizards cock their heads at sufficiently long distances. A study in which the distance maintained while walking in an arc varies should reveal the maximum distance at which the lizards cock their heads as the predator passes out of view. This distance can be used as an indicator of the maximum distance at which continuous monitoring begins and can be used as an estimate of alert distance by prey that do not perform alerting behaviors when approached from one side.
References
Blumstein DT (2003) Flight-initiation distance in birds is dependent on intruder starting distance. J Wildl Manag 67:852–857
Blumstein DT (2010) Flush early and avoid the rush: a general rule of antipredator behaviour? Behav Ecol Sociobiol 21:440–442
Bulova SJ (1994) Ecological correlates of population and individual variation in antipredator behavior of two species of desert lizards. Copeia 1994:980–992
Caro TM (2005) Antipredator defenses in birds and mammals. University of Chicago Press, Chicago
Chamaillè-Jammes S, Blumstein DT (2012) A case for quantile regression behavioral ecology: getting more out of flight initiation distance data. Behav Ecol Sociobiol 66:985–992
Cooper WE Jr (2005a) The foraging mode controversy: both continuous variation and clustering of foraging movements occur. J Zool 267:179–190
Cooper WE Jr (2005b) When and how does starting distance affect flight initiation distance. Can J Zool 83:1045–1050
Cooper WE Jr (2007) Foraging modes as suites of coadapted movement traits. J Zool 272:45–56
Cooper WE Jr (2008) Visual monitoring of predators: occurrence, cost and benefit for escape. Anim Behav 76:1365–1372
Cooper WE Jr (2010) Economic escape. In: Breed MD, Moore J (eds) Encyclopedia of animal behavior, vol 1. Academic, London, pp 588–595
Cooper WE Jr (2012) Risk, escape from ambush, and hiding time by the lizard Sceloporus virgatus. Herpetologica 68:505–513
Cooper WE Jr, Blumstein DT (2014) Novel effects of monitoring predators on costs of fleeing and not fleeing explain flushing early in economic escape theory. Behav Ecol 25:44–52
Cooper WE Jr, Frederick WG (2007) Optimal flight initiation distance. J Theor Biol 244:59–67
Cooper WE Jr, Frederick WG (2010) Predator lethality, optimal escape behavior, and autotomy. Behav Ecol 21:91–96
Cooper WE Jr, Sherbrooke WC (2013a) Effects of recent movement, starting distance and other risk factors on escape behaviour by two phrynosomatid lizards. Behaviour 150:447–469
Cooper WE Jr, Sherbrooke WC (2013b) Risk and cost of immobility in the presence of an immobile predator: effects on latency to flee or approach food or a potential mate. Behav Ecol Sociobiol 67:583–592
Cooper WE Jr, Sherbrooke WC (2015) FEAR and DREAD: starting distance, escape decisions and hiding time in refuge. Behaviour. doi:10.1163/1568539X-00003283
Cooper WE Jr, Vitt LJ, Caldwell JP, Fox SF (2001) Foraging modes of some American lizards: relationships among measurement variables and discreteness of modes. Herpetologica 57:65–76
Cooper WE Jr, López P, Martín J, Pérez-Mellado V (2012) Latency to flee from an immobile predator: effects of risk and cost of immobility for the prey. Behav Ecol 23:790–797
Dumont F, Pasquaretta C, Réale D, Bogliani G, Von Hardenberg A (2012) Flight initiation distance and starting distance: biological effect or mathematical artefact. Ethology 118:1051–1062
Huey RB, Pianka ER (1981) Ecological consequences of foraging mode. Ecology 62:991–999
Lima SL (1994) On the personal benefits of vigilance. Anim Behav 48:734–736
Martín J, Luque-Larena JJ, López P (2009) When to run from an ambush predator: balancing crypsis benefits with costs of fleeing in lizards. Anim Behav 78:1011–1018
McGowan MM, Patel PD, Stroh JD, Blumstein DT (2014) The effect of human presence and human activity on risk assessment and flight initiation distance in skinks. Ethology 120:1–9
Møller AP, Erritzøe J (2014) Predator-prey interactions, flight initiation distance and brain size. J Evol Biol 27:34–42
New STD, Bull M (2011) Retinal ganglion cell topography and visual acuity of the sleepy lizard (Tiliqua rugosa). J Comp Physiol A 197:703–709
Ott M, Ostheim J, Sherbrooke WC (2004) Prey snapping and visual distance in Texas horned lizards, Phrynosoma cornutum. J Evol Biol 207:3067–3072
Perry G (1999) The evollution of search modss: ecological versus phylogenetic perspectives. Am Nat 153:99–109
Rosenthal R, Rubin DB (2003) r equivalent: a simple effect size indicator. Psychol Methods 8:492–496
Samia DSM, Nomura F, Blumstein DT (2013) Do animals generally flush early and avoid the rush? A meta-analysis. Biol Lett 9:20130016
Samia DSM, Blumstein DT, Stankowich TS, Cooper WE Jr (2015) Fifty years of chasing lizards: new insights advance optimal escape theory. Biol Rev. doi:10.1111/brv.12173
Schwalb IR (2012) Evolution’s witness: how eyes evolved. Oxford University Press, Oxford
Stankowich T, Blumstein DT (2005) Fear in animals: a meta-analysis and review of risk assessment. Proc R Soc Lond B 272:2627–2634
Stankowich T, Coss RG (2007) Effects of risk assessment, predator behavior, and habitat on escape behavior in Columbian black-tailed deer. Behav Ecol 18:358–367
Stebbins RC (2003) A field guide to western reptiles and amphibians, 3rd edn. Houghton Mifflin, Boston
Ydenberg RC, Dill LM (1986) The economics of fleeing from predators. Adv Study Behav 16:229–249
Ethical standards
The experiments comply with the current laws of the USA and were conducted according to research protocol 11120042015 approved by the IACUC of Purdue University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Madsen
Rights and permissions
About this article
Cite this article
Cooper, W.E., Sherbrooke, W.C. Monitoring by prey that does not reveal awareness by turning toward approaching predators. Behav Ecol Sociobiol 69, 1377–1382 (2015). https://doi.org/10.1007/s00265-015-1951-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1951-8