Abstract
Carotenoid-based integument coloration is extremely widespread in animals and commonly used as an honest signal of condition in sexual selection. Besides being used for color expression, carotenoids have antioxidant and immunomodulatory activity. Being a limited resource, carotenoid allocation to competing demands generates a trade-off. Recent studies, however, suggest that the antioxidant role of carotenoids might not be as important as previously thought. To shed light on the mechanism maintaining carotenoid-based signal honesty in the black-legged kittiwake (Rissa tridactyla), we supplemented males and females with dietary yellow xanthophylls (lutein and zeaxanthin) during the chick-rearing period, when male coloration may be a good indicator of future reproductive success. The supplementation affected plasma carotenoid levels similarly in males and females, i.e., it increased the levels of lutein but decreased the levels of total astaxanthin, one of the main pigments coloring integuments in this species. Supplemented adults and their chicks had stronger immune response than controls, suggesting that yellow xanthophylls enhance the innate immune system in kittiwakes. However, supplementation caused sex-specific effects on integument coloration and oxidative stress. Supplemented males had duller integuments, but similar oxidative damages compared to control males, while supplemented females had more colorful integuments, but higher oxidative damages than control females. Because the increase in lutein was associated with a decrease in other potential antioxidants (i.e., astaxanthin and vitamin A), the role of carotenoids as antioxidants in kittiwakes remains undetermined. Our results, however, indicate that the trade-off responses to carotenoid availability are sex-specific in kittiwakes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many animals, including birds, fish, and reptiles, use carotenoid-based coloration as an honest indicator of individual quality in sexual selection (Kodric-Brown 1989; Hill 2006; Hamilton et al. 2013). In addition to providing coloration, carotenoids have the potential to act as antioxidants and immunostimulants (Blount et al. 2003; Chew and Park 2004; McGraw 2006a; Simons et al. 2012). Carotenoids cannot be synthesized de novo by vertebrates and have to be acquired through the diet. Consequently, a trade-off between allocating carotenoids to coloring signals vs. physiological functions has been assumed (Lozano 1994), and increased redness or yellowness of carotenoid-rich ornaments has been hypothesized to signal good health (von Schantz et al. 1999). The mechanisms and functions of the expressions of carotenoid-based ornaments have been particularly well investigated in birds (reviewed in Svensson and Wong 2011; Simons et al. 2012; Pérez-Rodríguez et al. 2013). However, the mechanisms maintaining honesty of the carotenoid signals are still debated. While the role of carotenoids in the good functioning of the innate immune system, albeit complex, is relatively sound, studies of the role of carotenoids as antioxidants have yielded much more contradictory results (Horak et al. 2007; Costantini and Møller 2008; Isaksson and Andersson 2008; Perez-Rodriguez 2009; Svensson and Wong 2011), and carotenoids have been suggested not to be as powerful antioxidants as previously thought (Hartley and Kennedy 2004; Costantini and Møller 2008).
Recently, several other mechanisms have been suggested to explain how carotenoid-based ornaments can still give information on oxidative stress levels. First, at high concentrations, carotenoids, such as lutein or β-carotene, can lose their antioxidant effect and acquire pro-oxidant properties (El-Agamey et al. 2004; Costantini et al. 2007a; Huggins et al. 2010). Only individuals in good health and with good noncarotenoid antioxidant systems can afford high carotenoid levels and intense carotenoid-based coloration (Zahavi and Zahavi 1997; Costantini et al. 2007a). Accordingly, supplementation with moderate to high amount of xanthophylls causes increased oxidative stress and lower body mass in kestrels (Falco tinnunculus; Costantini et al. 2007a), and higher skeletal muscle breakdown and reduced flight performance in American goldfinches (Carduelis tristis; Huggins et al. 2010). Second, as carotenoids may be bleached by oxidative processes, coloration may reflect the concentration in major colorless antioxidants (e.g., vitamins A, C, or E, or melatonin) and, hence, the healthy functioning of antioxidative systems (Hartley and Kennedy 2004). However, results have been inconsistent. Although supplementation with colorless antioxidants causes larger red bill spots in yellow-legged gulls (Larus michahellis; Pérez et al. 2008), redder bills in zebra finches (Taeniopygia guttata; Bertrand et al. 2006), and redder nuptial coloration in sticklebacks (Gasterosteus aculeatus; Pike et al. 2007), it does not affect plumage or wattle coloration in house finches (Haemorhous mexicanus), male greenfinches (Carduelis chloris) and ring-necked pheasants (Phasianus colchicus; Karu et al. 2008; Orledge et al. 2012; Giraudeau et al. 2013). More studies are therefore needed to evaluate the link between carotenoids and oxidative stress in birds.
To shed some light on the mechanism maintaining the signaling honesty of carotenoid-based ornamentation and evaluate the role of carotenoids in redox homeostasis and immune system, we supplemented chick-rearing male and female black-legged kittiwakes (Rissa tridactyla) with dietary yellow xanthophylls (lutein and zeaxanthin) and measured the response of the following variables: plasma carotenoid levels, integument color (i.e., bill, gape, tongue, and eye-ring), plasma antioxidant activity, plasma reactive oxygen metabolite concentration, and bacterial killing ability of plasma—a measure of the innate immune system activity. Carotenoids may be part of a complex system maintaining the homeostatic balance between oxidants and antioxidants (Hill and Johnson 2012; Pérez-Rodríguez et al. 2013). Animals may regulate this balance by downregulating noncarotenoid antioxidants if carotenoids are plentiful, thereby masking the contribution of the extra carotenoids on total antioxidant activity (Pérez-Rodríguez et al. 2013). We therefore also measured levels of plasma vitamins A and E to evaluate the effect of the supplementation on some components of the antioxidant system.
The first aim of this study was to evaluate whether carotenoids help maintain redox homeostasis and bolster immunity. If physiological and coloring functions do indeed trade off, unsupplemented birds being more constrained in their carotenoid allocation should show lower coloration, lower immune response, and lower antioxidant capacity, while supplemented birds should be able to allocate carotenoids to all of these functions. The second aim of this study was to determine whether carotenoid allocation between coloration and physiological functions differ between males and females. In kittiwakes, integument color is related to carotenoid levels and reproductive success in chick-rearing males (Leclaire et al. 2011a, 2011b), but not in chick-rearing females (Leclaire et al. 2011b). During the chick-rearing period, integument color is therefore suggested to be under strong selective pressure and to work as sexual advertisement only in males (Leclaire et al. 2011b). Sex-specific allocation of supplemental carotenoids is therefore expected, with males allocating more additional carotenoids to coloration compared to females.
Despite the increasing number of carotenoid studies carried out in nestlings, the positive effects of increased carotenoid availability on chicks are still controversial. The most common effects include increased immune response (Fenoglio et al. 2002; Cucco et al. 2006), increased coloration (Casagrande et al. 2007), or improved growth or condition (Cucco et al. 2006; Saino et al. 2008). Contradictory results have, however, also been found (Biard et al. 2006; Fitze et al. 2007; Marri and Richner 2014). In kittiwake chicks, bill and eye-ring are black, while gape and tongue are dull pink, a color that probably does not originate from carotenoids but rather from underlying blood flow. Therefore, in contrast to kittiwake adults, a trade-off between allocating carotenoids to coloration and physiological functions does probably not exist in chicks. In our study, supplemental dietary carotenoids provided to adults may be passed to chicks through the bolus of food that parents regurgitate to feed their chick. Therefore, to investigate the potential effects of carotenoids on physiological functions in chicks, we also measured carotenoid and vitamin levels, oxidative stress, and immune response in chicks from supplemented and unsupplemented parents.
Methods
Study site
The study was conducted from June 19th to August 2nd 2010 on a population of black-legged kittiwakes nesting on the upper walls of an abandoned U.S. Air Force radar tower on Middleton Island (59° 26′ N, 146° 20′ W), Gulf of Alaska. Nest sites were observed from inside the tower through sliding one-way windows (Gill and Hatch 2002). This enabled easy capture and monitoring of breeders and chicks. All nest sites were checked twice daily to record events such as laying, hatching, or fledging. Studied adults and chicks were sexed based on molecular methods (Merkling et al. 2012) or on copulation and courtship feeding behavior during the prelaying period (Jodice et al. 2000).
Carotenoid supplementation
We supplemented birds with lutein and zeaxanthin. Lutein and zeaxanthin are the two most common pigments in bird blood (McGraw 2006a) and were the first and third most abundant pigments in plasma of kittiwakes breeding in the 2008 season (Leclaire 2010). Carotenoids were obtained from Kemin Foods (Nantes, France; Oro Glo Layer Dry 20) in the form of a dietary supplement made of crystalline lutein derived from marigold flowers. Carotenoid concentration in the product was 1.8 % lutein and 0.2 % zeaxanthin, and both carotenoids were in free alcohol forms readily available for absorption (Biard et al. 2006).
Upon hatching of the first chick (two chicks being the modal brood size), 63 pairs were trained to eat a daily supplemental capelin (Mallotus vilosus) for 5 days. In 15 pairs, both male and female refused to eat fish throughout the 5 days of training. They were thus excluded from the experiment. The 48 remaining pairs were randomly assigned to either carotenoid supplementation treatment (SUP birds; n = 24 pairs) or to control treatment (CO birds; n = 24 pairs). Carotenoid supplementation started 5 days after hatching of the first chick and lasted for 10 days. It consisted in one daily supplemental capelin filled with 0.05 g dietary supplement diluted in 1 ml refined soybean oil (Crisco® pure vegetable oil). SUP birds therefore received daily 0.9 mg lutein and 0.1 mg zeaxanthin. Carotenoid/oil mixture was prepared each evening and stored at 4 °C and in darkness until use the following day. Control treatment consisted in one daily supplemental capelin filled with 1 ml vegetable oil. We retained only birds that ate more than five fishes during the 10-day treatment (n = 13 SUP males and n = 14 CO males, and n = 12 SUP females and n = 7 CO females). There are no data on the specific composition of carotenoids in the diet of black-legged kittiwakes. Yet in June–August 2010, capelin was the main food item in kittiwake diet (Hatch 2013). Chick-rearing black-legged kittiwakes need to consume 315 g capelin every other day to meet their energy expenditures (Gabrielsen et al. 1987). As capelin contain 93 μg lutein + zeaxanthin per 100 g of fish (Slifka et al. 2013), a 1-day supplementation amount corresponds to 6.8-folds the normal daily carotenoid intake of a kittiwake. However, this calculated normal daily carotenoid intake based on capelin consumption is probably underestimated as kittiwakes also consume a small amount of salmon and krill which are richer in carotenoids than capelins. In addition, because kittiwakes feed their young chicks with food regurgitate, supplemented parents absorbed probably less carotenoids than the amount they received. Soybean oil did not contain high dose of carotenoids as suggested by the similar lutein, zeaxanthin, and total carotenoid levels between the CO birds after 10 days of supplementation and the 2009 unsupplemented chick-rearing kittiwakes (see Leclaire et al. 2011b; values are given as means ± SE; lutein levels: 1.24 ± 0.20 vs. 1.49 ± 0.17 μg/ml; zeaxanthin levels: 7.20 ± 0.83 vs. 6.24 ± 0.68 μg/ml; total carotenoid levels: 13.62 ± 1.76 vs. 14.03 ± 1.46 μg/ml; all P > 0.38).
Experimental birds were captured at day 0 (i.e., 5 days after hatching of the first chick) and day 10 (i.e., 15 days after hatching of the first chick) of the experiment. Blood samples were collected from the alar vein with a 1 ml syringe and a 25 gauge needle (maximum amount of blood collected: 900 μl), birds were weighed to the nearest 5 g with a Pesola® scale and integument color was measured with the method described below. Blood was centrifuged just after collection and plasma samples were stored at −20 °C until analyses.
At hatching, A and B chicks (first and second hatched chick, respectively) were marked on the head with a nontoxic marker for identification. A chicks were colored in red, while B chicks were colored in blue. Chicks were weighed and measured at 5 and 15 days of age. Body mass was measured to the nearest gram with an electronic scale and tarsus length to the nearest millimeter with a stop rule. Chicks were bled at 15 and 20 days of age in order to limit the amount of blood collected at each capture. We retained only chicks from nests where the total of fish eaten by parents was more than five fishes during the 10-day treatment (20 A chicks and 7 B chicks in SUP nests, and 15 A chicks and 7 B chicks in CO nests).
Integument color measurements
At onset and end of treatment, gape, tongue, and bill colors were measured with a reflectance spectrometer (Ocean Optics USB2000), a deuterium-halogen light source (DH2000, Top Sensor System), and a 200 μm fiber optic reflectance probe held at 45° from the integument surface. Reflectance was measured using the SpectraSuite software (Ocean Optics, Inc.) and in relation to a dark and a white (Spectralon®, Labsphere) standard. The spectrometer was calibrated between each bird measurement. We measured gape color at the intersection between the upper and lower mandibles, tongue color at ca. 3 mm of the top, and bill color under the nostril on the upper mandible.
Reflectance spectra of gape, tongue, and bill have peaks in both the ultraviolet (UV) and visible wavelengths (Leclaire et al. 2011b). From the reflectance spectra, we calculated four parameters using the Avicol software (Gomez 2007). We measured (1) mean brightness as the mean summed reflectance between 300 and 700 nm, (2) UV chroma as the proportion of the total reflectance falling in the UV range (300–400 nm), (3) carotenoid chroma [(R 700 nm − R 450 nm)/R 700 nm], and (4) hue as the wavelength at maximal reflectance in the range 500–700 nm.
During the 2010 breeding season, we measured integument color three times in a row for 27 birds, allowing us to measure repeatability of color measurement. Repeatability was calculated as intraclass coefficient using the “ICC” package in R (R Core Team 2014).
The light emitted by the spectrometer is dangerous for the birds’ eyes. Eye-ring color was thus measured from digital photographs at onset and end of treatment. Pictures were taken at a standard distance of ca. 40 cm using a digital camera with flash. For each photograph, a color swatch was placed next to the bird to standardize subsequent measurements (Montgomerie 2006). All pictures were analyzed using Adobe Photoshop 7.0. For each picture, the average components of red, green, and blue (RGB system) were recorded within the whole area of the eye-ring. RGB components were then converted into hue, saturation, and brightness values. This method has already been investigated on black-legged kittiwakes (Leclaire et al. 2011a, 2011b; Blévin et al. 2014), and information obtained from digital pictures is still useful as it reveals patterns of biological meaning (Alonso-Alvarez et al. 2004; Mougeot et al. 2007b; Leclaire et al. 2011a, 2011b; Blévin et al. 2014).
Plasma carotenoid and vitamin levels
Carotenoid and vitamin (A and E) levels in plasma were analyzed at the McGraw lab (Arizona State University, USA) at the beginning 2011 using the protocol described in McGraw et al. (2008b). Briefly, we thawed and added 15 μl of plasma to a microcentrifuged tube that together with 100 μl of ethanol was vortexed for 5 s. Afterwards, we added 100 μl of tert-butyl methyl ether and vortexed again for 5 s. We then centrifuged tubes for 3 min at 12,000 rpm. We transferred the supernatant to a fresh screw cap tube and evaporated to dryness with a nitrogen evaporator in a hood. Next, we resuspended the supernatant in 200 μl mobile phase, vortexed for 5 s, and injected 50 μl into a high-performance liquid chromatograph (HPLC; Waters Alliance® Instrument, Waters Corporation, Milford, MA). We used a 5 μm Waters Carotenoid C-30 column (4.6 × 250 mm ID) to determine types and amounts of carotenoids present. Pigment concentrations were calculated based on external curves constructed from known amounts of purified reference carotenoids. In adults, carotenoid and vitamin levels were measured at onset and end of treatment, while in chicks, they were measured at 15 days of age. Eleven plasma samples turned brown, which likely indicated degradation of the samples during transport.
In addition, to examine the biochemical basis of the coloration in kittiwakes, we performed exploratory analyses. We collected bare parts (eye-ring, tongue, gape, and bill) of three unknown sex kittiwakes found freshly dead in the field and stored them at −20 °C until analyses. Analyses of carotenoids in bare parts were performed in June 2011 following the ketocarotenoid protocol for beak described in McGraw and Toomey (2010).
Oxidative stress analyses
In May 2012, oxidative stress was measured in plasma samples by using the d-reactive oxygen metabolites (d-ROM) and the oxy-adsorbent tests (Diacron International, Grosseto, Italy), as previously described in birds (Costantini and Dell’Omo 2006). Oxidative stress was measured at the end of treatment in adults and at 20 days of age in A chicks.
The d-ROM test measures plasmatic hydroperoxydes, which are reactive oxygen metabolites (ROM) resulting from the attack of reactive oxygen species on organic substrates (carbohydrates, lipids, amino acids, proteins, nucleotides). The plasma (10 μl) was first diluted in 200 μl of an acidic buffer solution (pH 4.8) and 2 μl of a chromogen and then incubated at 37 °C for 75 min. These acidic conditions favor the release of iron ions from plasma proteins, which catalyze the breakdown of hydroperoxide into alkoxyl and peroxyl radicals. These final products, in turn, react with the chromogen and produce a complex where color intensity, read with a microplate reader (505 nm, ASYS UVM340), is proportional to its concentration. The concentration of hydroperoxide was then calculated by comparison with a standard solution.
The oxy-adsorbent test measures the total plasma antioxidant capacity (OXY). This test evaluates the plasma ability to oppose the oxidative action of a hypochlorous acid (HClO) solution. The plasma (5 μl) was first diluted 1:100 with distilled water. 5 μl of this solution was then incubated with 200 μl of a tittered HClO solution at 37 °C for 10 min. Then 5 μl of chromogen (N,N-diethyl-p-phenylenediamine) was added to measure the excess of HClO in plasma. The resulting colored complex, read with a spectrophotometer (505 nm, ASYS UVM340), is inversely related to the antioxidant power. The plasmatic antioxidant capacity was then calculated by comparison with a standard solution. Six plasma samples turned brown which likely indicated degradation of the samples during transport.
Analyses of the immune system
Bactericidal capacity of plasma was tested in adults at the end of treatment. We used Escherichia coli ATCC 8739 (#RQC11003, Sigma Aldrich). This bactericidal assay gives an index of the capacity of the blood to rapidly thwart a potential bacterial pathogen. This activity is probably a combination of several mechanisms of the innate immune system, including microbicidal activities of natural antibodies, complement proteins, or lysozymes (Tieleman et al. 2005; Matson et al. 2006; Demas et al. 2011). Thus it is considered as one of the most general and integrative in vitro measurements of the constituent elements of the innate immune system (Demas et al. 2011). Bacteria were reconstituted from lyophilized pellet in 100 ml tryptic soy broth (TSB). Bacteria were grown for 16 h at 37 °C with constant agitation. Solution was then centrifuged and the bacteria pellet was diluted in phosphate-buffered saline (PBS) until attenuance at 680 nm was 0.20. This solution was diluted 1:100 to make up the bacteria working solution. 40 μl of plasma was mixed with 20 μl of bacteria working solution on 96-well plates and incubated at 41 °C for 30 min. Then 160 μl TSB was added and the mixture was incubated at 37 °C under constant agitation for 10 h. Optical density at 630 nm was read using a Perkin Elmer Victor3 plate reader. Optical densities before the 10 h incubation were used as references. Positive and negative controls were regularly interspersed. Eight plasma samples turned brown, out of which four were particularly brown and were excluded from the statistical analysis. The bactericidal assay was carried out in autumn 2014. Although freeze duration is known to decrease E. coli killing capacity of plasma (Liebl and Martin 2009), information on bactericidal capacity obtained from plasma samples stored over a period of 4 years at −30 °C still revealed patterns of biological meaning (Rubenstein et al. 2008).
Twenty-day-old A chicks were injected subcutaneously with 0.2 mg phytohemagglutinin (PHA; L8754, Sigma Aldrich) in 0.05 ml sterile PBS within the patagium. PHA injection generates acute inflammation involving cellular components such as macrophages, heterophils, and basophils (Salaberria et al. 2013; Vinkler et al. 2013). Wing web thickness was measured with a pressure-sensitive spessimeter (Teclock Co., Okaya City, Japan) with an accuracy of 0.01 mm just before injection and 24 h after injection to assess the intensity of the immune response. To reduce measurement errors, all measurements were realized three times in a row and the same observer carried out measurements at all time points for a given bird. PHA response could not be tested in adults as it required two consecutive captures with a strict time interval.
Statistical analyses
Effects of the treatment on integument coloration (mean brightness, carotenoid chroma, UV chroma, and hue of gape, tongue, and bill, and hue, saturation and brightness of eye-ring), antioxidant levels and body mass in adults were assessed using linear mixed models (LMM). Measure after treatment was the dependent variable and measure before treatment was entered as a covariate. Treatment, sex, and their interaction, as well as the number of fish eaten during the experiment, were entered as fixed effects. Nest identity was entered as a random factor. Gape and tongue brightness and hue, as well as bill carotenoid chroma and UV chroma, were log-transformed to meet normality assumption. Total lutein, zeaxanthin, anhydrolutein, and β-cryptoxanthin levels were log-transformed to meet normality assumption. Effects of the treatment on OXY, ROM, and bacterial killing ability of plasma (log-transformed) in adults were tested using similar models, except that values before treatment were not measured and were not entered as covariates. Plate identity was entered as a random factor in the bacterial killing ability, ROM and OXY analyses. Plasma color was entered as an explanatory variable in the carotenoid, vitamin, bacterial killing ability, ROM and OXY models.
In chicks, effects of the treatment on carotenoid and vitamin levels, PHA response, OXY, ROM, mass gain, and tarsus growth rate (i.e., the difference between 5 and 15 days of age for the last two variables) were assessed using LMM with treatment, sex of the chick, rank of the chicks (except for OXY and ROM, which were measured in A chicks only), and their interactions as fixed effects and nest as a random factor. Wing length was entered as a fixed effect and observer identity as a random effect in the PHA response models. Plasma color was entered as a fixed effect in the carotenoid and vitamin level models and in the OXY and ROM models. Zeaxanthin and β-cryptoxanthin levels were log-transformed, and total astaxanthin levels was transformed using a box-cox transformation (λ = −3) to meet normality assumption.
All statistical tests were performed with SAS (SAS Intitute Inc. 1999). Nonsignificant terms were backward dropped using a stepwise elimination procedure. When the minimal model was obtained, each removed term was then put back into the minimal model to assess the level of nonsignificance and to ensure that significant terms had not been inappropriately dropped. We used two-tailed type III tests for fixed effect with a significance level set to α = 0.05, and the Satterthwaite correction for the calculation of fixed-effect degrees of freedom (Littell et al. 2006). Values are expressed as least squared means ± standard error of the LS means throughout.
Results
Carotenoids in integuments
Chemical tests indicated that all the tissues analyzed contained carotenoids. Bills mainly contained lutein and other yellow xanthophylls, whereas gape, tongue, and eye-ring mainly contained orange-red ketocarotenoids like astaxanthin and canthaxanthin. All pigments, however, were highly esterified and thus did not yield quantifiable HPLC traces.
Effects of the supplementation on adults
Supplemented (SUP) and control (CO) birds ate a similar number of fish during supplementation (means ± SE: 7.80 ± 0.27 vs. 7.52 ± 0.30 fishes; range: 6–10 fishes in both treatments; F 1,43 = 0.96, P = 0.33), but females tended to eat less fish than males (means ± SE: 7.26 ± 0.32 vs. 7.96 ± 0.24 fishes; range: 6–10 fishes in both sexes; F 1,44 = 3.14, P = 0.08). SUP and CO pairs had similar brood size at onset of treatment (means ± SE: 1.32 ± 0.10 vs. 1.33 ± 0.11 chicks; F 1,43 = 0.13, P = 0.72), as well as at the end of treatment (means ± SE: 1.20 ± 0.08 vs. 1.10 ± 0.10 chicks; F 1,44 = 0.71, P = 0.41). Males were heavier than females both before (460 ± 5 and 414 ± 6 g; F 1,29.9 = 42.85, P < 0.0001) and after treatment (435 ± 5 and 390 ± 6 g; F 1,26.8 = 37.34, P < 0.0001). Supplementation did not influence male or female body mass (SUP and CO males: 435 ± 5 and 434 ± 5 g, F 1,23 = 0.01, P = 0.94; SUP and CO females: 390 ± 6 and 387 ± 8 g, F 1,15 = 0.06, P = 0.81).
SUP birds had higher lutein levels in plasma than CO birds (Table 1 and Fig. 1a), but similar levels of zeaxanthin (Table 1 and Fig. 1a). SUP birds had lower total astaxanthin levels than CO birds (Table 1 and Fig. 1a). None of the other carotenoid levels appeared to vary with treatment (Table 1 and Fig. 1a), and total carotenoid levels were similar in SUP and CO birds (7.59 ± 2.42 vs. 9.64 ± 2.01 μg/ml; F 1,32.6 = 0.44, P = 0.51). SUP birds had lower vitamin A levels in plasma compared to CO birds (Table 1 and Fig. 1a), but similar vitamin E levels (Table 1 and Fig. 1a). Carotenoid and vitamin levels did not significantly differ between sexes (Table 1), except β-cryptoxanthin levels which were higher in males than females (0.88 ± 0.17 vs. 0.45 ± 0.20 μg/ml; Table 1). β-carotene levels decreased with fish number (Table 1). Total lutein, zeaxanthin, total astaxanthin, and β-cryptoxanthin levels were lower in the plasma samples that had turned brown (Table 1).
Almost all gape color parameters varied with the interaction between treatment and sex (Table 2 and Figs. 2 and 3). SUP males had higher gape brightness (F 1,24 = 8.13, P = 0.0088; Fig. 2) and lower gape hue (622 ± 3 vs. 630 ± 3 nm; F 1,24 = 5.06, P = 0.034) and tended to have lower gape carotenoid chroma (F 1,24 = 3.32, P = 0.08; Fig. 3) than CO males. In contrast, SUP females tended to have lower gape brightness (F 1,16 = 3.50, P = 0.080; Fig. 2), but not significantly higher gape hue (629 ± 3 vs. 622 ± 4 nm; F 1,16 = 2.65, P = 0.12) than CO females. Gape UV chroma was lower in SUP females than CO females (0.20 ± 0.01 vs. 0.24 ± 0.01, F 1,16 = 7.40, P = 0.015; Fig. 2), while it did not differ between SUP and CO males (0.20 ± 0.01 vs. 0.20 ± 0.01, F 1,24 = 0.02, P = 0.88; Fig. 2). SUP females did not have different gape carotenoid chroma than CO females (F 1,16 = 0.76, P = 0.40; Fig. 3).
Tongue brightness and carotenoid chroma depended also upon the interaction between treatment and sex (Table 2). SUP males had higher tongue brightness (F 1,23 = 8.73, P = 0.0071; Fig. 2) but not significantly lower tongue carotenoid chroma than CO males (F 1,25 = 2.56, P = 0.12; Fig. 3). In contrast, SUP females did not have different tongue brightness than CO females (F 1,16 = 0.72, P = 0.41; Fig. 2), but they tended to have higher tongue carotenoid chroma than CO females (F 1,17 = 3.62, P = 0.07; Fig. 3).
Bill brightness depended upon the interaction between treatment and sex (Table 2). It was lower in SUP females than CO females (F 1,16 = 4.95, P = 0.041; Fig. 2), but it was not significantly different between SUP males and CO males (F 1,24 = 2.81, P = 0.11; Fig. 2). SUP birds tended to have higher bill carotenoid chroma than CO birds (Table 2 and Fig. 3). Bill brightness decreased with the number of fish (Table 2). Bill hue tended to be higher in females than males (549 ± 3 vs. 543 ± 2 nm; Table 2).
Eye-ring hue, brightness, and saturation did not vary with treatment (F 1,36.9 = 3, P = 0.092; F 1,43 = 0.12, P = 0.73; and F 1,38 = 1.32, P = 0.26), sex (F 1,36.3 = 0.47, P = 0.50; F 1,43 = 0.10, P = 0.75; and F 1,30.2 = 0.01, P = 0.92) or their interaction (F 1,41 = 0.46, P = 0.50; F 1,41 = 0.10, P = 0.76; and F 1,30.5 = 2.39, P = 0.13).
Reactive oxygen metabolite (ROM) levels depended upon the interaction between sex and treatment (F 1,6.54 = 5.95, P = 0.047; Fig. 4). ROM level was higher in SUP than CO females (F 1,13.1 = 9.62, P = 0.0084; Fig. 4) but did not depend upon treatment in males (F 1,16 = 0.17, P = 0.69). Anti-oxidant capacity (OXY) did not differ between supplemented and control birds (234.2 ± 20.7 mmol−1 HOCl neutralized vs. 253.3 ± 23.4 mmol−1 HOCl neutralized; F 1,30.4 = 0.55, P = 0.46) and did not vary with sex (F 1,6.42 = 0.71, P = 0.43). ROM levels and OXY did not vary with the number of fish (F 1,24.4 = 0.04, P = 0.83; and F 1,13.9 = 0.46, P = 0.51). ROM levels was lower in plasma samples that had turned brown (F 1,13.3 = 22.29, P = 0.0004), while OXY did not vary with plasma color (F 1,13.8 = 3.29, P = 0.092).
Bactericidal capacity of plasma was higher in SUP than CO birds (F 1,34 = 4.20, P = 0.048; Fig. 5a) and did not depend upon sex (F 1,33 = 0.91, P = 0.35; Fig. 5a).
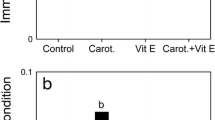
Effect of the carotenoid supplementation on the innate immune system in adults and chicks of black-legged kittiwakes. a Bacterial killing ability of plasma (% of E. coli killed) in supplemented and control adults after 10 days of treatment. b PHA-induced immune response (skin swelling in mm) in 20-day-old supplemented and control chicks. Shown are LS means ± SE
Effects of the supplementation on chicks
Supplemented A chicks and B chicks had higher lutein levels in plasma (Table 3 and Fig. 1b) but similar levels of zeaxanthin compared to control A and B chicks (Table 3 and Fig. 1b). The levels of other carotenoid pigments and vitamins did not differ between SUP and CO chicks and did not vary with sex or rank of the chicks (Table 3 and Fig. 1b), except vitamin A levels that depended upon the interaction between sex and rank (Table 3; female A and B chicks: 0.75 ± 0.14 and 0.76 ± 0.12 μg/ml; male A and B chicks: 0.50 ± 0.15 and 1.10 ± 0.20 μg/ml). SUP chicks tended to have higher vitamin E levels than CO chicks (Table 3 and Fig. 1b). Males tended to have higher total lutein levels (Table 3; 0.43 ± 0.22 and 0.10 ± 0.19 μg/ml) and β-crytptoxanthin levels than females (Table 3; 0.50 ± 0.20 and 0.15 ± 0.17 μg/ml). SUP chicks had higher total carotenoid levels than CO chicks (2.91 ± 1.17 vs. 0.57 ± 1.25 μg/ml; F 1,44 = 4.86, P = 0.033).
PHA-induced immune response was higher in SUP than CO chicks (F 1,23.5 = 7.25, P = 0.013; Fig. 5b), higher in females than males (F 1,15 = 9.77, P = 0.0069; Fig. 5b), and increased with wing length (F 1,26.5 = 6.21, P = 0.019). PHA-induced immune response did not depend upon the interaction between treatment and sex (F 1,12.7 = 0.24, P = 0.63; Fig. 5b), treatment and rank (F 2,24.6 = 2.00, P = 0.16), and rank and sex (F 1,14.2 = 0.01, P = 0.92), and it did not vary with ranks (F 1,6.92 = 1.11, P = 0.33).
Anti-oxidant capacity (OXY) and reactive oxygen metabolite levels (ROM) did not vary with treatment (OXY: F 1,29 = 1.10, P = 0.30, SUP chicks: 211.2 ± 8.2 mmol−1 HOCl-neutralized, and CO chicks: 223.4 ± 10.4 mmol−1 HOCl-neutralized; ROM: F 1,29 = 2.69, P = 0.11, SUP chicks: 17.83 ± 1.06 mg H2O2/dl, and CO chicks: 15.36 ± 1.34 mg H2O2/dl) or sex (all P > 0.35).
Mass gain between 5 and 15 days of age did not vary with treatment (F 1,42 = 0.04, P = 0.84; SUP chicks: 172 ± 6 g day−1 and CO chicks: 174 ± 6 g day−1), rank, or sex (all P > 0.15), while tarsus growth between 5 and 15 days of age did not vary with treatment (F 1,41 = 0.01, P = 0.94; SUP chicks: 8.38 ± 0.31 mm day−1 and CO chicks: 8.34 ± 0.35 mm day−1) or rank (F 1,41 = 0.40, P = 0.53) but was higher in males than females (F 1,42 = 4.81, P = 0.034).
Discussion
In kittiwakes, as in numerous bird species (Blount et al. 2003; Alonso-Alvarez et al. 2004; Baeta et al. 2008; Thorogood et al. 2008; Benito et al. 2011; Peluc et al. 2012), dietary lutein supplementation increased lutein levels in plasma. Despite zeaxanthin supplementation, no increase in zeaxanthin levels in plasma was detected. This result is in line with studies in common lizards (Lacerta vivipara; San-Jose et al. 2012), common tern (Sterna hirundo) chicks (Benito et al. 2011), and blue tit (Cyanistes caeruleus) nestlings (Larcombe et al. 2010) but contrasts with studies in some other birds (McGraw et al. 2011; Peluc et al. 2012). This lack of effect may stem from the low amount of zeaxanthin supplied to the birds, the low sample size or preferential absorption of lutein over zeaxanthin when both carotenoids are in competition. Many studies have shown that the different carotenoid pigments are in competition for their absorption in gastrointestinal mixed micelles (Kostic et al. 1995; van den Berg 1999; Tyssandier et al. 2002; Yeum and Russell 2002). Although zeaxanthin seems to outcompete lutein during intestinal absorption in American goldfinch (C. tristis; McGraw et al. 2008a), one cannot exclude that the opposite is true in kittiwakes.
Supplemented kittiwakes had lower total astaxanthin levels in plasma than control birds. Besides being acquired from a fish and crustacean diet, astaxanthin can be metabolically derived from zeaxanthin, but also from β-carotene and β-cryptoxanthin (Rodriguez et al. 1973; McGraw 2006a; LaFountain et al. 2015). The absorption of β-carotene and lutein has been suggested to depend on the same transporter (Yonekura and Nagao 2007), and, in birds, lutein absorption is known to be physiologically favored over carotene (Scheidt 1998). The lutein-rich diet of supplemented birds may therefore cause a decrease in the absorption of astaxanthin or precursors in the intestinal mucosa. Similar to kittiwakes, in Atlantic salmons (Salmo salar), lutein supplementation is associated with a weak tendency toward decreased astaxanthin levels in blood (Olsen and Baker 2006). In addition to its potential role as astaxanthin precursor, β-carotene is a precursor of vitamin A (Olson 1989). Supplemented kittiwakes had lower vitamin A levels in plasma than control birds, further suggesting a carotenoid absorption threshold and preferential absorption of lutein over β-carotene in kittiwakes.
Red and orange ketocarotenoids are the main pigments coloring gape, eye-ring, and tongue in kittiwakes (Doutrelant et al. 2013; this study). The lower plasma levels of total astaxanthin, as well as the suggested lower intestinal absorption of canthaxanthin precursor (β-carotene; LaFountain et al. 2015) in supplemented birds may therefore be expected to be associated with duller integument colors. Carotenoids are subtractive colorants, and high carotenoid levels are commonly associated with high carotenoid chroma, but low UV reflectance and low brightness of yellow orange soft integuments (Andersson and Prager 2006; Mougeot et al. 2007a; Thorogood et al. 2008; Dugas and McGraw 2011). Accordingly, supplemented males had duller gape and tongue, as showed by higher gape and tongue brightness, lower gape hue, and a tendency to lower gape carotenoid chroma, than control males. Our result is in line with a previous study on the same population of kittiwakes showing that gape and tongue color is positively correlated with vitamin A and carotenoid levels in unmanipulated chick-rearing males (Leclaire et al. 2011b).
Although the supplementation had similar effects on plasma carotenoid levels in males and females, supplemented females—in contrast to supplemented males—had lower gape UV chroma and tended to have redder gape and tongue than controls (i.e., lower gape brightness and higher tongue carotenoid chroma). Our results suggest that males and females did not differ in their carotenoid uptake from the diet, but differed in their carotenoid allocation to integuments despite the kittiwake being a monochromatic species (Leclaire et al. 2011b; Doutrelant et al. 2013). Sex-specific effects of carotenoids on coloration have also been described in common tern nestlings, where carotenoid supplementation causes an increase in feet color only in females despite similar effects on carotenoid levels in plasma in males and females (Benito et al. 2011). Carotenoid deposition in integuments is directly affected by androgen levels (Eens et al. 2000; McGraw 2006b; Mougeot et al. 2007b). Sex differences in carotenoid deposition may be due to sex differences in androgen receptor densities in the skin, as suggested in kestrels (F. tinnunculus; Casagrande et al. 2012). In addition, because formation of astaxanthin and canthaxanthin pigments from their precursors involves oxidation (LaFountain et al. 2015), the high oxidative stress in supplemented females—in contrast to supplemented males (see below)—could have increased astaxanthin formation in female bare parts (Romero-Haro and Alonso-Alvarez 2015). Similarly, high oxidative stress is associated with redder bills in zebra finches (Romero-Haro and Alonso-Alvarez 2015). Further studies including biochemical analyses of male and female soft bare parts are clearly needed to properly evaluate the effects of the different carotenoid pigments on coloration, as well as the differences in carotenoid deposition between sexes.
Plasma from supplemented males and females had higher bactericidal capacity than plasma from control birds. The bactericidal capacity of plasma is a combination of several mechanisms of the innate immune system and gives an index of the capacity of the blood to rapidly thwart a potential bacterial pathogen (Tieleman et al. 2005). The increased immunocompetence of kittiwakes following yellow xanthophyll supplementation is in line with the multiple studies showing that lutein supplementation in the diet improves the innate immune response of birds (Blount et al. 2003; McGraw and Ardia 2003; McGraw et al. 2011; Simons et al. 2012) and that individuals with higher carotenoid-based ornamentation are less parasitized than duller individuals (Martínez-Padilla et al. 2007; Baeta et al. 2008; Lumpkin et al. 2014).
In contrast to the beneficial role of carotenoids in immunity, the role of carotenoids as antioxidant in birds is currently questioned (Hartley and Kennedy 2004; Costantini and Møller 2008). In kittiwakes, we found that yellow xanthophyll supplementation did not strongly affect the antioxidant capacity of plasma. The increase in lutein being associated with a decrease in other potential antioxidants (total astaxanthin and vitamin A) prevented us from determining whether carotenoids have antioxidant function in kittiwakes. Supplemented females had higher oxidative damages than control females, whereas supplemented males had similar oxidative damages as control males. In yellow-legged gull (L. michahellis) chicks, yolk lutein increases oxidative damages in males but not in females (Saino et al. 2011). Increased oxidative damages in supplemented females may be due to pro-oxidant activities of lutein or increased reactive oxygen species (ROS) liberation associated with increased immunity. In addition, carotenoids may be important components of a complex integrated antioxidant system including, for instance, vitamins A and E, glutathione peroxidase, coenzyme Q, and uric acid (Harris 1992; Yu 1994). Although each antioxidant has specific roles, there is also a level of interaction and modulation between these different compounds (Selman et al. 2006; Hill and Johnson 2012). Increased oxidative damages in supplemented females may thus also be due to an imbalance between these different antioxidant compounds. Our result suggests sex-biased susceptibility to oxidative damages in kittiwakes. The chick-rearing period is more energetically demanding for females than males (Moe et al. 2002), and under resource limitation (i.e., low vitamin A levels, for instance), chick-rearing females may be more prone to oxidative damages than chick-rearing males. Males may also have mobilized other antioxidant compounds from storage organs to maintain redox homeostasis.
In a previous study, on the same kittiwake population, male coloration was found to be positively correlated with reproductive success, suggesting that coloration may be a signal of quality used by conspecifics in sexual selection (Leclaire et al. 2011b). Our results have shown, however, that male coloration, despite being a potential good indicator of total astaxanthin and vitamin A plasma levels, does not covary positively with antioxidant or immune capacity following yellow xanthophyll supplementation. The mechanism maintaining signal honesty remains, therefore, unknown. In this study, we supplemented birds with lutein and zeaxanthin. The key pigments for developing colorful gape and tongue in kittiwakes are, however, red ketocarotenoids. It will be valuable in future studies to manipulate levels of dietary ketocarotenoids or precursors, as well as adopting a whole animal approach by investigating changes in antioxidant levels and redox homeostasis in several tissues.
In kittiwakes, bill, in contrast to fleshy integuments, is mainly colored by yellow xanthophylls including lutein. Supplemented birds were therefore expected to have more colorful bill than control birds. We found that bill carotenoid chroma showed a weak tendency (P = 0.09) to be higher in supplemented males and females than controls and that bill brightness was lower in supplemented females than control females. The bill is a keratinized structure, and the turnover of carotenoids within it may be relatively slow compared to fleshy integuments. It may be possible that stronger effects on bill color would have been observed if the treatment would have been longer.
Chicks whose parents were supplemented with lutein had higher lutein levels than controls. In kittiwakes, young chicks are fed from food regurgitate, and the high lutein levels in chicks from supplemented parents is likely due to regurgitate made of lutein-supplemented fish. In contrast to supplemented adults, chicks from supplemented adults did not have lower total astaxanthin and vitamin A levels than controls. Carotenoid levels being much lower in chicks than in adults, astaxanthin and vitamin A precursors may not be in competition with yellow xanthophylls for their absorption in chick blood. Chicks from lutein-supplemented parents did not have higher antioxidant capacity or lower oxidative damages than control chicks. Similarly, lutein did not affect plasma antioxidant capacity in kestrel nestlings (Costantini et al. 2007b), great tit (Parus major) nestlings (Marri and Richner 2014) and yellow-legged gull chicks (Saino et al. 2011), corroborating the claim that carotenoids are not major antioxidant in birds (Hartley and Kennedy 2004; Costantini and Møller 2008). In contrast to adults, we did not detect any differences between male and female chicks, suggesting that sex-specific strategy in carotenoid allocation appears only once carotenoids are allocated to color signals. Kittiwake chicks from supplemented parents had, however, higher innate immune response as measured by the PHA skin swelling test. Similarly, lutein-supplemented yellow-legged gull chicks show similar antioxidant capacity and oxidative damages, but higher PHA response than control chicks (Lucas et al. 2014). Our results, therefore, indicate that lutein stimulates the innate immune system in chicks as in adult kittiwakes.
Altogether, we have uncovered intriguing sex-dependent effects of yellow xanthophyll supplementation on integument coloration and oxidative damages in a monochromatic seabird. Supplemented females seemed to prioritize coloration while males seemed to prioritize maintenance of redox homeostasis. This sex-specific difference poses the question of why benefits would be dissimilar for the two sexes. It is unlikely that during the chick-rearing period, integument color is under stronger selection in females than males, since during this period, males and females share parental care almost equally (Leclaire et al. 2010), and coloration seems to be an honest indicator of reproductive success only in males (Leclaire et al. 2011b). Further investigations including experimental manipulations of coloration and behavioral observations should be carried out on the functions of integument coloration in kittiwake females and males.
References
Alonso-Alvarez C, Bertrand S, Devevey G, Gaillard M, Prost J, Faivre B, Sorci G (2004) An experimental test of the dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am Nat 164:651–659
Andersson S, Prager M (2006) Quantifying colors. In: Hill GE, McGraw KJ (eds) Bird coloration, vol 1. Mechanisms and measurements. Harvard University Press, London
Baeta R, Faivre B, Motreuil S, Gaillard M, Moreau J (2008) Carotenoid trade-off between parasitic resistance and sexual display: an experimental study in the blackbird (Turdus merula). Proc R Soc Lond B 275:427–434
Benito MM, Gonzalez-Solis J, Becker PH (2011) Carotenoid supplementation and sex-specific trade-offs between colouration and condition in common tern chicks. J Comp Physiol B 181:539–549
Bertrand S, Faivre B, Sorci G (2006) Do carotenoid-based sexual traits signal the availability of non-pigmentary antioxidants? J Exp Biol 209:4414–4419
Biard C, Surai PF, Møller AP (2006) Carotenoid availability in diet and phenotype of blue and great tit nestlings. J Exp Biol 209:1004–1015
Blévin P, Tartu S, Angelier F, Leclaire S, Bustnes JO, Moe B, Herzke D, Gabrielsen GW, Chastel O (2014) Integument colouration in relation to persistent organic pollutants and body condition in arctic breeding black-legged kittiwakes (Rissa tridactyla). Sci Total Environ 470–471:248–254
Blount JD, Metcalfe NB, Birkhead TR, Surai PF (2003) Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300:125–127
Casagrande S, Costantini D, Fanfani A, Tagliavini J, Dell’Omo G (2007) Patterns of serum carotenoid accumulation and skin colour variation in kestrel nestlings in relation to breeding conditions and different terms of carotenoid supplementation. J Comp Physiol B 177:237–245
Casagrande S, Costantini D, Dell’Omo G, Tagliavini J, Groothuis T (2012) Differential effects of testosterone metabolites oestradiol and dihydrotestosterone on oxidative stress and carotenoid-dependent colour expression in a bird. Behav Ecol Sociobiol 66:1319–1331
Chew BP, Park JS (2004) Carotenoid action on the immune response. J Nutr 134:257S–261S
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Costantini D, Dell’Omo G (2006) Effects of T-cell-mediated immune response on avian oxidative stress. Comp Biochem Phys A 145:137–142
Costantini D, Møller AP (2008) Carotenoids are minor antioxidants for birds. Funct Ecol 22:367–370
Costantini D, Coluzza C, Fanfani A, Dell’Omo G (2007a) Effects of carotenoid supplementation on colour expression, oxidative stress and body mass in rehabilitated captive adult kestrels (Falco tinnunculus). J Comp Physiol B 177:723–731
Costantini D, Fanfani A, Dell’Omo G (2007b) Carotenoid availability does not limit the capability of nestling kestrels (Falco tinnunculus) to cope with oxidative stress. J Exp Biol 210:1238–1244
Cucco M, Guasco B, Malacarne G, Ottonelli R (2006) Effects of β-carotene supplementation on chick growth, immune status and behaviour in the grey partridge, Perdix perdix. Behav Process 73:325–332
Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS (2011) Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J Anim Ecol 80:710–730
Doutrelant C, Grégoire A, Gomez D, Staszewski V, Arnoux E, Tveraa T, Faivre B, Boulinier T (2013) Colouration in Atlantic puffins and blacklegged kittiwakes: monochromatism and links to body condition in both sexes. J Avian Biol 44:451–460
Dugas MB, McGraw KJ (2011) Proximate correlates of carotenoid-based mouth coloration in nestling house sparrows. Condor 113:691–700
Eens M, Van Duyse E, Berghman L, Pinxten R (2000) Shield characteristics are testosterone-dependent in both male and female moorhens. Horm Behav 37:126–134
El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ (2004) Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys 430:37–48
Fenoglio S, Cucco M, Malacarne G (2002) The effect of a carotenoid-rich diet on immunocompetence and behavioural performances in moorhen chicks. Ethol Ecol Evol 14:149–156
Fitze PS, Tschirren B, Gasparini J, Richner H (2007) Carotenoid-based plumage colors and immune function: Is there a trade-off for rare carotenoids? Am Nat 169:137–144
Gabrielsen GW, Mehlum F, Nagy KA (1987) Daily energy expenditure and energy utilization of free-ranging black-legged kittiwakes. Condor 89:126–132
Gill VA, Hatch SA (2002) Components of productivity in black-legged kittiwakes Rissa tridactyla: response to supplemental feeding. J Avian Biol 33:113–126
Giraudeau M, Sweazea K, Butler MW, McGraw KJ (2013) Effects of carotenoid and vitamin E supplementation on oxidative stress and plumage coloration in house finches (Haemorhous mexicanus). Comp Biochem Physiol A 166:406–413
Gomez D (2007) AVICOL, a program to analyse spectrometric data. Available upon request from the author at dodogomez@yahoo.fr
Hamilton DG, Whiting MJ, Pryke SR (2013) Fiery frills: carotenoid-based coloration predicts contest success in frillneck lizards. Behav Ecol 24:1138–1149
Harris ED (1992) Regulation of antioxidant enzymes. FASEB J 6:2675–2683
Hartley RC, Kennedy MW (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19:353–354
Hatch SA (2013) Kittiwake diets and chick production signal a 2008 regime shift in the Northeast Pacific. Mar Ecol Prog Ser 477:271–284
Hill GE (2006) Female mate choice for ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration, vol 2. Function and evolution. Harvard University Press, London
Hill GE, Johnson JD (2012) The vitamin A-redox hypothesis: a biochemical basis for honest signaling via carotenoid pigmentation. Am Nat 180:E127–E150
Horak P, Saks L, Zilmer M, Karu U, Zilmer K (2007) Do dietary antioxidants alleviate the cost of immune activation? An experiment with greenfinches. Am Nat 170:625–635
Huggins KA, Navara KJ, Mendonca MT, Hill GE (2010) Detrimental effects of carotenoid pigments: the dark side of bright coloration. Naturwissenschaften 97:637–644
Intitute Inc SAS (1999) SAS user’s guide, version 8. Sas Institute Inc., Cary, NC
Isaksson C, Andersson S (2008) Oxidative stress does not influence carotenoid mobilization and plumage pigmentation. Proc R Soc Lond B 275:309–314
Jodice PGR, Lanctot RB, Gill VA, Roby DD, Hatch SA (2000) Sexing adult black-legged kittiwakes by DNA, behavior, and morphology. Waterbirds 23:405–415
Karu U, Saks L, Horak P (2008) Carotenoid-based plumage coloration is not affected by vitamin E supplementation in male greenfinches. Ecol Res 23:931–935
Kodric-Brown A (1989) Dietary carotenoids and male mating success in the guppy: an environmental component to female choice. Behav Ecol Sociobiol 25:393–401
Kostic D, White WS, Olson JA (1995) Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr 62:604–610
LaFountain AM, Prum RO, Frank HA (2015) Diversity, physiology, and evolution of avian plumage carotenoids and the role of carotenoid–protein interactions in plumage color appearance. Arch Biochem Biophys (published online). doi:10.1016/j.abb.2015.01.016
Larcombe SD, Mullen W, Alexander L, Arnold KE (2010) Dietary antioxidants, lipid peroxidation and plumage colouration in nestling blue tits Cyanistes caeruleus. Naturwissenschaften 97:903–913
Leclaire S (2010) Signaux sexuels, choix du partenaire et investissement parental chez la mouette tridactyle Rissa tridactyla. PhD thesis, Université Toulouse III-Paul Sabatier
Leclaire S, Helfenstein F, Degeorges A, Wagner RH, Danchin E (2010) Family size and sex-specific parental effort in black-legged kittiwakes. Behaviour 147:1841–1862
Leclaire S, Bourret V, Wagner RH, Hatch SA, Helfenstein F, Chastel O, Danchin E (2011a) Behavioral and physiological responses to male handicap in chick-rearing black-legged kittiwakes. Behav Ecol 22:1156–1165
Leclaire S, White J, Arnoux E, Faivre B, Vetter N, Hatch SA, Danchin E (2011b) Integument coloration signals reproductive success, heterozygosity, and antioxidant levels in chick-rearing black-legged kittiwakes. Naturwissenschaften 98:773–782
Liebl AL, Martin LB II (2009) Simple quantification of blood and plasma antimicrobial capacity using spectrophotometry. Funct Ecol 23:1091–1096
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute Inc., Cary, NC
Lozano GA (1994) Carotenoids, parasites, and sexual selection. Oikos 70:309–311
Lucas A, Morales J, Velando A (2014) Differential effects of specific carotenoids on oxidative damage and immune response of gull chicks. J Exp Biol 217:1253–1262
Lumpkin DC, Murphy TG, Tarvin KA (2014) Blood parasite infection differentially relates to carotenoid‐based plumage and bill color in the American goldfinch. Ecol Evol 4:3210–3217
Marri V, Richner H (2014) Differential effects of vitamins E and C and carotenoids on growth, resistance to oxidative stress, fledging success and plumage colouration in wild great tits. J Exp Biol 217:1478–1484
Martínez-Padilla J, Mougeot F, Pérez-Rodríguez L, Bortolotti GR (2007) Nematode parasites reduce carotenoid-based signalling in male red grouse. Biol Lett 3:161–164
Matson KD, Tieleman IB, Klasing KC (2006) Capture stress and the bactericidal competence of blood and plasma in five species of tropical birds. Phys Biochem Zool 79:556–564
McGraw KJ (2006a) Mechanics of carotenoid-based coloration. In: Hill GE, McGraw KJ (eds) Bird coloration, vol. 1. Mechanisms and measurements. Harvard University Press, Cambridge
McGraw KJ (2006b) Sex steroid dependence of carotenoid-based coloration in female zebra finches. Physiol Behav 88:347–352
McGraw KJ, Ardia DR (2003) Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am Nat 162:704–712
McGraw KJ, Toomey MB (2010) Carotenoid accumulation in the tissues of zebra finches: predictors of integumentary pigmentation and implications for carotenoid allocation strategies. Physiol Biochem Zool 83:97–109
McGraw KJ, Hill GE, Navara KJ, Parker RS (2008a) Differential accumulation and pigmentation ability of dietary carotenoids in colorful finches. Physiol Biochem Zool 77:484–491
McGraw KJ, Tourville EA, Butler MW (2008b) A quantitative comparison of the commonly used methods for extracting caroteoids from avian plasma. Behav Ecol Sociobiol 62:1991–2002
McGraw KJ, Nolan PM, Crino OL (2011) Carotenoids bolster immunity during moult in a wild songbird with sexually selected plumage coloration. Biol J Linn Soc 102:560–572
Merkling T, Leclaire S, Danchin E, Lhuillier E, Wagner RH, White J, Hatch SA, Blanchard P (2012) Food availability and offspring sex in a monogamous seabird: insights from an experimental approach. Behav Ecol 23:751–758
Moe B, Langseth I, Fyhn M, Gabrielsen GW, Bech C (2002) Changes in body condition in breeding kittiwakes Rissa tridactyla. J Avian Biol 33:225–234
Montgomerie R (2006) Analyzing colors. In: Hill GE, McGraw KJ (eds) Bird coloration, vol 1. Mechanisms and measurements. Harvard University Press, Cambridge
Mougeot F, Martinéz-Padilla J, Pérez-Rodríguez L, Bortolotti GR (2007a) Carotenoid-based colouration and ultraviolet reflectance of the sexual ornaments of grouse. Behav Ecol Sociobiol 61:741–751
Mougeot F, Pérez-Rodríguez L, Martínez-Padilla J, Leckie F, Redpath SM (2007b) Parasites, testosterone and honest carotenoid-based signalling of health. Funct Ecol 21:886–898
Olsen RE, Baker RTM (2006) Lutein does not influence flesh astaxanthin pigmentation in the Atlantic salmon (Salmo salar L.). Aquaculture 258:558–564
Olson JA (1989) Provitamin A function of carotenoids: the conversion of beta-carotene into vitamin A. J Nutr 119:105–108
Orledge JM, Blount JD, Hoodless AN, Royle NJ (2012) Antioxidant supplementation during early development reduces parasite load but does not affect sexual ornament expression in adult ring-necked pheasants. Funct Ecol 26:688–700
Peluc SI, Reed WL, McGraw KJ, Gibbs P (2012) Carotenoid supplementation and GnRH challenges influence female endocrine physiology, immune function, and egg-yolk characteristics in Japanese quail (Coturnix japonica). J Comp Physiol B 182:687–702
Pérez C, Lores M, Velando A (2008) Availability of nonpigmentary antioxidant affects red coloration in gulls. Behav Ecol 19:967–973
Perez-Rodriguez L (2009) Carotenoids in evolutionary ecology: re-evaluating the antioxidant role. BioEssays 31:1116–1126
Pérez-Rodríguez L, Martínez-Padilla J, Mougeot F (2013) Carotenoid-based ornaments as signals of health status in birds: evidences from two galliform species, the red-legged partridge (Alectoris rufa) and the red grouse (Lagopus lagopus scoticus). In: Yamaguchi M (ed) Carotenoids: food sources, production and health benefits. Nova Science Publishers, Hauppauge, pp 173–198
Pike TW, Blount JD, Lindström J, Metcalfe NB (2007) Availability of non-carotenoid antioxidants affects the expression of a carotenoid-based sexual ornament. Biol Lett 3:353–356
Rodriguez DB, Simpson KL, Chichester CO (1973) The biosynthesis of astaxanthin: XVII. Intermediates in the conversion of β-carotene. Int J Biochem 4:213–222
Romero-Haro AA, Alonso-Alvarez C (2015) The level of an intracellular antioxidant during development determines the adult phenotype in a bird species: a potential organizer role for glutathione. Am Nat 185:390–405
Rubenstein DR, Parlow AF, Hutch CR, Martin LB II (2008) Environmental and hormonal correlates of immune activity in a cooperatively breeding tropical bird. Gen Comp Endocr 159:10–15
Saino N, Bertacche V, Bonisoli‐Alquati A, Romano M, Rubolini D (2008) Phenotypic correlates of yolk and plasma carotenoid concentration in yellow-legged gull chicks. Physiol Biochem Zool 81:211–225
Saino N, Romano M, Caprioli M, Rubolini D, Ambrosini R (2011) Yolk carotenoids have sex-dependent effects on redox status and influence the resolution of growth trade-offs in yellow-legged gull chicks. Behav Ecol 22:411–421
Salaberria C, Muriel J, de Luna M, Gil D, Puerta M (2013) The PHA test as an indicator of phagocytic activity in a passerine bird. PLoS ONE 8, e84108
San-Jose LM, Granado-Lorencio F, Fitze PS (2012) Dietary lipids reduce the expression of carotenoid-based coloration in Lacerta vivipara. Funct Ecol 26:646–656
Scheidt K (1998) Absorption and metabolism of carotenoids in birds, fish and crustaceans. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids: biosynthesis. Birkhauser Verlag, Basel, pp 285–355
Selman C, McLaren J, Meyer C, Duncan J, Redman P, Collins A, Duthie G, Speakman J (2006) Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech Ageing Dev 127:897–904
Simons MJP, Cohen AA, Verhulst S (2012) What does carotenoid-dependent coloration tell? Plasma carotenoid level signals immunocompetence and oxidative stress state in birds — a meta-analysis. PLoS ONE 7, e43088
Slifka KA, Wells RS, Ardente AJ, Crissey S (2013) Comparative diet analysis of fish species commonly consumed by managed and freeranging bottlenose dolphins (Tursiops truncatus). Internet J Vet Med 10:1
Svensson P, Wong B (2011) Carotenoid-based signals in behavioural ecology: a review. Behaviour 148:131–189
Thorogood R, Kilner RM, Karadas F, Ewen JG (2008) Spectral mouth colour of nestlings changes with carotenoid availability. Funct Ecol 22:1044–1051
Tieleman BI, Williams JB, Ricklefs RE, Klasing KC (2005) Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc R Soc Lond B 272:1715–1720
Tyssandier V, Cardinault N, Caris-Veyrat C, Amiot M-J, Grolier P, Bouteloup C, Azais-Braesco V, Borel P (2002) Vegetable-borne lutein, lycopene, and β-carotene compete for incorporation into chylomicrons, with no adverse effect on the medium-term (3-wk) plasma status of carotenoids in humans. Am J Clin Nutr 75:526–534
van den Berg H (1999) Carotenoid Interactions. Nutr Rev 57:1–10
Vinkler M, Svobodová J, Gabrielová B, Bainová H, Bryjová A (2013) Cytokine expression in phytohaemagglutinin-induced skin inflammation in a galliform bird. J Avian Biol 45:43–50
von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc Lond B 266:1–12
Yeum K-J, Russell RM (2002) Carotenoid bioavailability and bioconversion. Annu Rev Nutr 22:483–504
Yonekura L, Nagao A (2007) Intestinal absorption of dietary carotenoids. Mol Nutr Food Res 51:107–115
Yu BP (1994) Cellular defenses against damage from reactive oxygen species. Physiol Rev 74:139–162
Zahavi A, Zahavi A (1997) The handicap principle. Oxford University Press, Oxford, A missing piece of Darwin’s puzzle
Acknowledgments
We thank Kemin Food for kindly providing carotenoids. We are very grateful to N. Vetter, and V. Frochot for their help in the field, and F. Helfenstein for helpful discussion. We thank C. Alonso-Alvarez and two anonymous referees for their valuable comments on the manuscript. This study was financed, in part, by the French Polar Institute Paul-Emile Victor (IPEV, program 1162) and the Cambridge Infectious Diseases Consortium (CIDC). This work originated in the lab EDB as part of the ‘Laboratoire d’Excellence’ (LABEX) entitled TULIP (ANR-10-LABX-41). Any use of trade names is for descriptive purposes only and does not imply endorsement by the US government.
Ethical standards
Experiments were approved by the US Fish and Wildlife Service and State of Alaska.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Hughes
Rights and permissions
About this article
Cite this article
Leclaire, S., Bourret, V., Blanchard, P. et al. Carotenoids increase immunity and sex specifically affect color and redox homeostasis in a monochromatic seabird. Behav Ecol Sociobiol 69, 1097–1111 (2015). https://doi.org/10.1007/s00265-015-1922-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1922-0