Abstract
In lizards, males are predicted to sprint faster and run for longer than females by virtue of higher testosterone levels and differences in morphology. Consequently, escape behaviour is also predicted to be associated with sex and locomotor performance, yet these links have rarely been explored. Here, we tested whether escape behaviour is associated with locomotor performance in the toad-headed agama, Phrynocephalus vlangalii, and whether it is sex-dependent. This species is also characterized by elaborate tail displays, which we examined as a potential pursuit-deterrent signal. Tail waves were performed by a very small proportion (2/58, 3 %) of individuals during predatory trials, suggesting that tail signalling functions exclusively in a social context. To understand the relationships between sex, escape behaviour and performance, we first measured escape behaviour (flight initiation distance, flight distance—measured differently compared to previous studies of lizard escape behaviour, and refuge use) in the field before measuring maximal sprint speed and endurance on the same individuals in the laboratory. Flight initiation distance did not differ between the sexes and was unrelated to performance capacity (maximal endurance and sprint speed) but was positively related to body size with larger individuals fleeing earlier. Males fled farther than females, but flight distance was also unrelated to either endurance or sprint speed. Interestingly, faster females were less likely to enter a refuge than slower females, whereas sprint speed and the probability of taking refuge were unrelated for males. Our results suggest that when males and females are not obviously sexually dimorphic, they are more likely to overlap in escape tactics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physiological performance capacity, how fast and for how long an organism is able to run or how hard an animal is able to bite, is predicted to be shaped by both natural and sexual selection because of their effect on escape behaviour and mating success (Husak and Fox 2008; Irschick et al. 2008). Therefore, selection on performance traits is frequently predicted to be sex-dependent (Lailvaux 2007; Irschick et al. 2008; Van Damme et al. 2008; Noble et al. 2014), and this may in turn influence escape behaviour in different ways for males and females. Typically, males are predicted to delay fleeing and run shorter distances in escaping because males are usually reported to run faster and longer than females by virtue of differences in morphology and higher testosterone levels (Klukowski et al. 1998; Lailvaux et al. 2003; Noble et al. 2014). Conversely, females may adjust their escape behaviour and rely more on crypsis if their performance capacity is reduced (Vanhooydonck et al. 2007; Goodman et al. 2008). We currently have few tests of sex-based differences in escape behaviour or performance capacity in lizards (Lailvaux et al. 2003; Martin et al. 2009; Noble et al. 2014). For example, Lailvaux et al. (2003) found sexual differences in sprint speed but no differences in endurance in the lizard Platysaurus intermedius wilhelmi, and males were found to take refuge earlier and flee farther than females.
Various optimality models have been proposed to predict when an animal should flee a predator (Cooper and Frederick 2007). The most popular of these is an economic model that qualitatively predicts that prey should flee when the risk of predation is equivalent to the cost of escaping (Ydenberg and Dill 1986). In contrast, other optimality models predict that escape behaviour should be flexible and adjusted based on predator search speed and prey locomotor performance (Broom and Ruxton 2005; Cooper and Frederick 2007). The key difference between these models lies in the escape cost. In the Ydenberg and Dill (1986) economic model, the escape cost is the lost opportunity associated with foraging and courtship, whereas in other optimality models, it is alerting the predator to the presence of the prey (Broom and Ruxton 2005; Cooper and Frederick 2007). The latter model best explains flight initiation distance in cryptic species because the likelihood of a predator detecting cryptic prey increases as it gets closer to the prey. Nevertheless, the act of fleeing will alert the predator; therefore, the prey will have to trade-off the benefits of remaining stationary against the cost of being detected (Broom and Ruxton 2005). No matter the model, escape decisions are critical to an individual’s fitness (Cooper and Frederick 2007) and these decisions may depend on an individual’s intrinsic capacity to escape predation (Clobert et al. 2000; Kullberg et al. 2002; Lailvaux et al. 2003).
Avoiding predation and escaping from predators may incur a variety of costs including lost foraging and mating opportunities. One way to reduce these costs is to communicate with predators using pursuit-deterrent displays (Dial 1986; Cooper 2001). For example, Anolis cristatellus use push-up displays to convey information about their endurance to a predator (Leal 1999) and wall lizards Podarcis muralis communicate with predators using foot shake displays (Font et al. 2012). Alternatively, animals may freeze or make escape decisions through risk assessment. A typical metric of risk assessment is flight initiation distance, which is frequently adjusted in response to risk (Broom and Ruxton 2005; Cooper and Frederick 2007). For example, conspicuous individuals are predicted to flee earlier than cryptic ones if escape costs are low (Martin et al. 2009). Additional measures of risk assessment during escape include flight distance and latency to enter a refuge (Martin and Lopez 2000; Lailvaux et al. 2003; Martin and Lopez 2010). Locomotor performance capacity (sprint speed and endurance) is potentially a key driver of escape behaviour because of its obvious influence on evading capture by a predator. For example, individuals with higher sprint speed are predicted to flee shorter distances (Lailvaux et al. 2003; Broom and Ruxton 2005). Also, lizards such as Lacerta schreiberi adjust their refuge use based on sprint speed, with faster lizards decreasing their reliance on refuges (Martin and Lopez 2010). Because of differences and constraints in reproductive state (Schwarzkopf and Shine 1992; Olsson et al. 2000; Cooper and Wilson 2007; Vanhooydonck et al. 2007), escape behaviour is frequently sex-specific. For example, male striped plateau lizards, Sceloporus virgatus, pay more attention to courtship and social interactions during the mating season, thus delaying escape from predators (Cooper and Wilson 2007). In the southern water skink, Eulamprus tympanum, compared to males and nongravid females, gravid females allow predators to approach more closely (Schwarzkopf and Shine 1992). Presently, little is known about the general relationship between sex, performance and escape behaviour, especially outside the mating season.
We used an Asian agamid lizard, Phrynocephalus vlangalii, to test the relationship between escape behaviour, sex and maximal locomotor performance capacity. Because both male and female P. vlangalii are territorial and use complex tail displays during social interactions, we first tested whether their tail displays function as a pursuit-deterrent signal. Males and females are similarly conspicuous with the exception of the underside of the tail tip (orange in females, black in males) precluding a role for sex-specific conspicuousness in escape behaviour. Males tend to be larger than females in both body length and head size, although not by much (Qi et al. 2012). Given that female lizards typically have lower sprint speed and endurance than males (Lailvaux et al. 2003; Noble et al. 2014), we predicted that males will permit predators to approach closer, will flee shorter distances and will be less likely to enter a refuge than females. With respect to locomotor performance capacity, we predicted that sprint speed would be inversely related to flight initiation distance, flight distance and refuge use because faster runners are predicted to be at a lower risk of predation and can more easily escape from predators (Irschick et al. 2008). Similarly, we predicted that individuals with higher endurance would flee sooner (longer flight initiation distance), run longer distances and be less likely to seek refuge (Leal 1999; Le Galliard and Ferriere 2008; Martin and Lopez 2010).
Methods
Study area
We conducted fieldwork during mid-June/July (summer) in 2011 near Xiaman Conservation Station in the Zoige Wetland Nature Reserve in southwestern China (33° 43′ 25.0″ N, 102° 29′ 04.0″ E), at an elevation of ca. 3464 m.a.s.l. P. vlangalii occupy patchily vegetated sand dunes at a density of ca. 0.32/m2 (Yin Qi, unpublished data). The vegetation on and around these sand dunes is predominantly composed of the grasses Kobresia humilis, Kobresia prattii and Elymus natans and a shrub (Salix sclerophylla).
Escape behaviour
We quantified responses to a predatory threat by using a human ‘predator’ that directly approached each lizard. For consistency, the same person performed all approaches from roughly 15 m (comfortably beyond maximum flight initiation distance) and always wore the same clothes. In order to avoid sampling the same individual twice, we systematically moved through a 3-km2 area and did not work in the same area more than once. To ensure that approach speed was consistent (0.5 m/s), we conducted 17 timed practice trials over 10 m. Our protocol consisted of walking directly towards a lizard and dropping a marker when the lizard fled at least 10 cm (to distinguish this from a postural adjustment) before stopping at the lizard’s initial location, after which, we dropped a second marker. Unlike many other studies of lizard escape behaviour, we continued our approach because otherwise lizards fled very short distances that were insufficient to relate to physiological performance capacity. We calculated flight initiation distance (FID) as the distance between markers one and two. We waited 10 s before dropping a third marker at the lizard’s final location where it paused for at least 2 s in order to calculate flight distance (FD). We used 2 s between moves to distinguish a single movement from a series. We also recorded the lizard’s final destination [refuge or open which we called refuge use (RU) in the following analysis]. All measurements were to the nearest 1 cm. Finally, we also scored whether lizards tail-waved in response to being approached in the event that this behaviour might be a pursuit-deterrent signal. We then caught the focal lizard by noose or by digging a pitfall trap in front of the burrow in which it had sought refuge. All captured lizards were brought back to the field station in order to measure their morphology and maximal performance capacity (sprint speed and endurance).
Morphology and performance capacity
We measured snout-vent length (SVL) from the tip of the snout to the posterior edge of the cloaca to the nearest 1 mm using a clear plastic ruler.
We quantified two measures of performance capacity: sprint speed and endurance. We first warmed lizards to a body temperature of ca. 30 °C (actual range 22–37.5 °C) by floating them in plastic bags on warm water. Prior to all performance measurements, we measured the lizard body temperature using a cloacal thermometer (Miller-Weber, USA) to the nearest 1 °C and we used these measurements to statistically control for any effects temperature had on performance. After measuring body temperature, lizards were enticed to sprint down a 1.5-m racetrack by lightly touching them with an artist’s paintbrush on the base of their tail (Noble et al. 2014). Four 100-W incandescent lights were suspended above the racetrack to provide illumination. Fine lightly compacted sand from their habitat was used as substrate to provide natural traction for the lizard. The floor of the racetrack was marked every 25 cm, and all runs were recorded using a high-speed digital camera [Casio High Speed Exlim camera (EX-FH100), 240 fps].
Immediately following sprinting, lizards were placed in a circular racetrack (circumference 180 cm, width 13 cm) with a sand substrate to measure endurance. Lizards were enticed to run around this track by gently touching them on the base of their tail using an artist’s paintbrush. We regarded the lizard as exhausted if after ten light taps with the paintbrush, the lizard no longer ran. The time the lizard started running until the last tap was considered the lizard’s endurance.
Each lizard’s sprinting speed and endurance were measured once/day over three consecutive days before releasing them at their point of capture. We used the lizard’s maximal performance capacity in all analyses because lizards can perform highly variably based on motivation and other factors (Losos et al. 2002).
Data analysis
All data analyses were carried out in R software version 2.14 (R-Core-Team 2013). Before analysis, we excluded lizards without locomotor performance and/or which did not have temperature data. Sample sizes for each of the analyses are indicated throughout. To test whether the sexes differed significantly in sprint speed and running endurance, we compared the difference in log sprint speed and log endurance between the sexes using analysis of covariance (ANCOVA) after controlling for log body temperature and SVL. We included interactions between sex and SVL and temperature because the relationships between these variables and performance appeared to vary between the sexes. Models were then simplified by excluding interactions and testing model fit using F tests. We tested for a trade-off between sprint speed and endurance along with log FD and log FID using a Pearson’s product-moment correlation test.
We tested the relationships between escape behaviours (FID, FD and RU), sex and physiological performance capacities (sprint speed and endurance) by creating models that included all main effects (sprint speed, endurance, sex) and important interactions related to our questions. We also included SVL as a covariate in models because body size has been linked with escape behaviours in many ectotherms (Cooper 2000; Miller et al. 2011; Sagonas et al. 2013). Given that temperature affected endurance measurements, and this varied between the sexes, we used residuals from a model with sex, SVL, temperature and a sex*temperature interaction as our measure of endurance in the FID, FD and RU models. Given that none of these variables affected sprint speed, we included our raw sprint speed data as a predictor. Only sex*residual endurance and sex*sprint speed interactions were tested because we were explicitly interested in whether the relationships between performance and escape behaviours differed between males and females. We used log-transformed FID and FD and assumed a Gaussian error distribution (identity link function) whereas we modelled RU using a binomial error distribution (logit link function). We tested whether over-dispersion affected inferences in RU models by fitting a quasi-binomial model; however, this had little effect on estimates. Using backwards elimination procedures, we excluded higher-order interactions (P > 0.15) from the global models using F tests (FID and FD) or likelihood ratio tests (refuge use). We present estimates from the full main effects models regardless of significance because these parameters directly related to our predictions. Using our models, we predicted the response surface to test how model predictions were related to our verbal predictions. Model residuals in both the FID and FD models were normally distributed; however, there was a highly influential point in FID models. Exclusion of this point did not affect the overall inferences from this model, and we include it in our final analysis. All continuous predictor variables were standardized (mean = 0, SD = 1) to make model parameters more comparable and to make main effects easier to interpret in the presence of interactions. To quantify the goodness of fit, we also calculated the R 2 of our models (Nakagawa and Schielzeth 2013). All means are reported as mean ± 1 SE.
Results
We found no support for tail waving as a pursuit-deterrent signal in P. vlangalii. Only two P. vlangalii tail-waved upon approach [2/58 total 3 %, males (N = 1/31) and female (N = 1/27)], most likely due to other factors (possibly other lizards not seen by us).
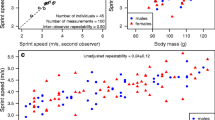
Males had a tendency to be larger (mean = 56.29 ± 0.43 mm, n = 31), on average, over females (mean = 54.70 ± 0.77 mm, n = 28), but this was not significant (Wilcoxon test: W = 300.5, P = 0.07). Sprint speed was not significantly different between the sexes (male mean 0.54 ± 0.03 m/s, n = 28; female mean 0.50 ± 0.03 m/s, n = 22), when body size and body temperature were controlled for (sex: F = 0.45, P = 0.50; SVL: F = 0.72, P = 0.40; body temperature: F = 1.98, P = 0.17; Table 1), and there was no evidence that the relationship varied between the sexes (SVL*sex: F = 0.07, P = 0.79; log temp*sex: F = 0.67, P = 0.42). Endurance was also not significantly different between the sexes (male mean 39.40 ± 2.60 s, n = 29; female mean 42.84 ± 2.02 s, n = 19; Table 1), when body size and body temperature were controlled. Temperature positively influenced log endurance, and its effects on endurance varied between the sexes (sex*log temp: F = 6.56, P = 0.01) with temperature more strongly affecting males than females (Table 1).
Mean FID for males was 268.23 ± 36.56 cm (n = 31) and for females 248.44 ± 33.07 cm (n = 28) (Fig. 1a). We found no evidence for an interaction between sex*sprint speed (F = 0.02, P = 0.90) or sex*endurance (F = 0.84, P = 0.37). FID did not differ significantly between males and females (F = 0.01, P = 0.92; Table 2) and was not related to endurance (F = 0.57, P = 0.46; Table 2) or sprint speed (F = 0.02, P = 0.89; Table 2); however, it was positively related to SVL (F = 5.42, P = 0.03; Table 2; Fig. 2a).
Relationship between a log flight initiation distance and snout-vent length (SVL) and b predicted probability of taking refuge and sprint speed. The filled circles and solid line represent males, while the clear triangles and dashed line represent females along with standard errors (dotted lines). Model predictions are based on models in Table 2
Mean FD for males was 105.97 ± 20.81 cm (n = 31) and for females 59.00 ± 14.21 cm (n = 28) (Fig. 1b). We found no evidence for a significant sex*endurance interaction (F = 0.58, P = 0.45) and a marginally nonsignificant sex*sprint speed interaction (F = 3.36, P = 0.07; Table 2). FD was significantly greater in males than in females (Table 2, Fig. 1b) but was not a function of individual endurance (F = 1.30, P = 0.26; Table 2) or SVL (F = 0.41, P = 0.53; Table 2).
Seventeen of 31 (54.8 %) males and 16 of 27 (59.26 %) females took refuge in a burrow when approached (Fig. 1c). The probability of refuge use was not a function of individual endurance, and there was no evidence that this differed between the sexes (sex*residual endurance: χ 2 = 0.24, P = 0.62; residual endurance: χ 2 = 2.00, P = 0.16; Table 2). The probability of refuge use was also not related to SVL (χ 2 = 0.04, P = 0.85; Table 2). The relationship between the probability of taking refuge and sprint speed depended on sex (sex*sprint speed: χ 2 = 6.21, P = 0.01; Table 2), and this was true even after accounting for over-dispersion (quasi-binomial GLM—sex*sprint speed: F = 4.73, P = 0.035). Slower females were predicted to take refuge with a higher probability compared to faster females, whereas there was no strong relationship between the probability of taking refuge and sprint speed for males (Fig. 2b).
Log sprint speed was not associated with log endurance (r = 0.09, P = 0.52, n = 51). Log FID was negatively associated with log FD, but the relationship was not significant (r = −0.12, P = 0.39, n = 58).
Discussion
P. vlangalii did not tail-wave in response to simulated predatory approaches and therefore does not use any obvious pursuit-deterrent signals. In terms of performance capacity, maximal sprint speed and maximal endurance were not significantly different for males and females. Also, males and females exhibited similar escape behaviour with one exception: males fled farther than females. Escape behaviours were, however, related to body size and locomotor performance. First, flight initiation distance increased as SVL increased. Second, the association between refuge use and sprint speed was sex-dependent such that slower females were more likely to take refuge than faster females whereas the same was not true for males.
Tail displays do not have an anti-predator function
Both male and female P. vlangalii frequently use complex tail waves during social interactions. The tail tip is orange in females and black in males, and because the tail is prehensile, they are able to dorsally curl and then uncurl their tails. Tail waving is conspicuous and could therefore function as a pursuit-deterrent signal to potential predators to discourage pursuit (Dial 1986; Cooper 2001). However, a very low percentage of individuals tail-waved during our predatory approaches, and as such, the tail display is only likely to be used in social signalling. While it is possible that some aspect of our approach failed to elicit tail waving, this protocol has been used to successfully test pursuit-deterrent signals in lizards in the past (Font et al. 2012). Cooper (2001) suggested that the lizard Callisaurus draconoides tail-waved based on predator approach distance, with fewer individuals signalling at longer or shorter distances. We think this is unlikely for P. vlangalii because we began approaching from a distance beyond which they respond (ca. 15 m), and the very low incidence of tail waves simply suggests that this behaviour has no clear link to anti-predatory behaviour.
Sex-dependent escape behaviour and influence of locomotor performance
No sexual differences were apparent in mean flight initiation distance and refuge use; however, flight distance was significantly greater for males than females. The lack of any difference in flight initiation distance and refuge behaviour might be a consequence of the cryptic coloration of both sexes. Males are larger than females, but this relationship is not significant and only the underside of the tail tip is sexually dimorphic. Therefore, it is likely that they experience similar predator-driven selection pressures as a consequence of their shared degree of conspicuousness. At the time of this study, female P. vlangalii were likely to be in an early stage of pregnancy while males were still defending territories. The similarity of flight initiation distance and refuge use between the sexes suggests an absence of any effect of reproductive state (at that point in time) on escape behaviour unless sex-specific effects cancel each other out. Conversely, the longer flight distance of males may be related to some difference in physiological state.
Individuals are predicted to adjust their flight initiation distance based on the risk of capture and the cost of initiating a chase as a function of their locomotor performance (Broom and Ruxton 2005; Cooper and Frederick 2007; Martin et al. 2009). Running too soon may be costly because it alerts predators, and being able to run faster or longer would be beneficial in escaping predators (Irschick et al. 2008; Le Galliard and Ferriere 2008). We found no evidence of associations between flight initiation distance and sprint speed or endurance in P. vlangalii, but larger individuals ran earlier when scared compared with smaller individuals. We tentatively suggest this might be because of the relationship between body size and heating rates. Ydenberg and Dill (1986) predicted that flight initiation distance and body temperature should be inversely related because of higher predation risk at lower temperature. Therefore, larger lizards might flee earlier because they are compensating for lower body temperature, and this behavioural anti-predator response has been shown in multiple species (e.g. Anolis lineatopus) (Rand 1964). Nonetheless, this needs to be tested in the field.
The relationship between sprint speed and flight distance was marginally nonsignificant and likely influenced by low statistical power. Nonetheless, there is good theoretical reason to believe that individuals should adjust their flight distance based on locomotor performance (Lailvaux et al. 2003), such that individuals that run faster and/or longer may be more likely to evade capture (Clobert et al. 2000; Irschick et al. 2008; Le Galliard and Ferriere 2008). Running too far can be energetically costly, while not running far enough can increase predation risk (Ydenberg and Dill 1986; Cooper and Frederick 2007), and these trade-offs are predicted to vary depending on sex. Different selection pressures as a consequence of differences in reproductive state may shape these trade-offs (Irschick et al. 2008). However, future work will be required to rigorously test this hypothesis in P. vlangalii.
The association between refuge use and sprint speed was sex-dependent. Taking refuge leads to a loss of foraging time and carries thermal costs (Martin and Lopez 2000; Martin and Lopez 2003; Blumstein and Pelletier 2005; Martin and Lopez 2010), and as such, individuals are predicted to make decisions on refuge use based on their locomotor performance (Martin and Lopez 2010). In P. vlangalii, faster females were less likely to enter burrows when scared compared with slower females, whereas there did not appear to be a strong relationship between performance and refuge use in males.
In summary, we found no evidence that tail displays function as pursuit-deterrent signals in P. vlangalii. Male and female lizards had similar sprint speed and endurance capacity. Sex differences in escape behaviour appear limited to flight distance (males run farther), while there were no differences in flight initiation distance and refuge use. Our study addresses the potentially important role of physiological performance capacity in responding to predation risk and demonstrated that when both sexes are cryptically coloured and territorial (aggressive), there is likely to be a significant overlap in both locomotor capacity and escape behaviour.
References
Blumstein DT, Pelletier D (2005) Yellow-bellied marmot hiding time is sensitive to variation in costs. Can J Zool 83:363–367
Broom M, Ruxton GD (2005) You can run—or you can hide: optimal strategies for cryptic prey against pursuit predators. Behav Ecol 16:534–540
Clobert J, Oppliger A, Sorci G, Ernande B, Swallow JG, Garland T (2000) Trade-offs in phenotypic traits: endurance at birth, growth, survival, predation and susceptibility to parasitism in a lizard, Lacerta vivipara. Funct Ecol 14:675–684
Cooper WE (2000) Effect of temperature on escape behaviour by an ectothermic vertebrate, the keeled earless lizard (Holbrookia propinqua). Behaviour 137:1299–1315
Cooper WE (2001) Multiple roles of tail display by the curly-tailed lizard Leiocephalus carinatus: pursuit deterrent and deflective roles of a social signal. Ethology 107:1137–1149
Cooper WE, Frederick WG (2007) Optimal flight initiation distance. J Theor Biol 244:59–67
Cooper WE, Wilson DS (2007) Sex and social costs of escaping in the striped plateau lizard Sceloporus virgatus. Behav Ecol 18:764–768
Dial BE (1986) Tail display in two species of iguanid lizards: a test of the 'predator signal' hypothesis. Am Nat 127:101–111
Font E, Carazo P, Pérez i de Lanuza G, Kramer M (2012) Predator-elicited foot shakes in wall lizards (Podarcis muralis): evidence for a pursuit-deterrent function. J Comp Psychol 126:87–96
Goodman BA, Miles DB, Schwarzkopf L (2008) Life on the rocks: habitat use drives morphological and performance evolution in lizards. Ecology 89:3462–3471
Husak JF, Fox SF (2008) Sexual selection on locomotor performance. Evol Ecol Res 10:213–228
Irschick DJ, Meyers JJ, Husak JF, Le Galliard J-F (2008) How does selection operate on whole-organism functional performance capacities? A review and synthesis. Evol Ecol Res 10:177–196
Klukowski M, Jenkinson NM, Nelson CE (1998) Effects of testosterone on locomotor performance and growth in field-active northern fence lizards, Sceloporus undulatus hyacinthinus. Physiol Zool 71:506–514
Kullberg C, Houston DC, Metcalfe NB (2002) Impaired flight ability—a cost of reproduction in female blue tits. Behav Ecol 13:575–579
Lailvaux SP (2007) Interactive effects of sex and temperature on locomotion in reptiles. Integr Comp Biol 47:189–199
Lailvaux SP, Alexander GJ, Whiting MJ (2003) Sex-based differences and similarities in locomotor performance, thermal preferences, and escape behaviour in the lizard Platysaurus intermedius wilhelmi. Physiol Biochem Zool 76:511–521
Le Galliard JF, Ferriere R (2008) Evolution of maximal endurance capacity: natural and sexual selection across age classes in a lizard. Evol Ecol Res 10:157–176
Leal M (1999) Honest signalling during prey-predator interactions in the lizard Anolis cristatellus. Anim Behav 58:521–526
Losos JB, Creer DA, Schulte JA (2002) Cautionary comments on the measurement of maximum locomotor capabilities. J Zool 258:57–61
Martin J, Lopez P (2000) Costs of refuge use affect escape decisions of Iberian rock lizards Lacerta monticola. Ethology 106:483–492
Martin J, Lopez P (2003) Ontogenetic variation in antipredator behavior of Iberian rock lizards (Lacerta monticola): effects of body-size-dependent thermal-exchange rates and costs of refuge use. Can J Zool 81:1131–1137
Martin J, Lopez P (2010) Thermal constraints of refuge use by Schreiber’s green lizards, Lacerta schreiberi. Behaviour 147:275–284
Martin J, Luque-Larena JJ, Lopez P (2009) When to run from an ambush predator: balancing crypsis benefits with costs of fleeing in lizards. Anim Behav 78:1011–1018
Miller BM, McDonnell LH, Sanders DJ, Lewtas KLM, Turgeon K, Kramer DL (2011) Locomotor compensation in the sea: body size affects escape gait in parrotfish. Anim Behav 82:1109–1116
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Noble DWA, Fanson KV, Whiting MJ (2014) Sex, androgens and whole organism performance in an Australian lizard. Biol J Linn Soc 111:834–849
Olsson M, Shine R, Bak-Olsson E (2000) Locomotor impairment of gravid lizards: is the burden physical or physiological? J Evol Biol 13:263–268
Qi Y, Noble DWA, Fu JZ, Whiting MJ (2012) Spatial and social organization in a burrow-dwelling lizard (Phrynocephalus vlangalii) from China. PLoS ONE 7:e41130
Rand AS (1964) Inverse relationship between temperature and shyness in the lizard Anolis lineatopus. Ecology 45:863–864
R-Core-Team (2013) R: a language and environment for statistical computing
Sagonas K, Meiri S, Valakos ED, Pafilis P (2013) The effect of body size on the thermoregulation of lizards on hot, dry Mediterranean islands. J Therm Biol 38:92–97
Schwarzkopf L, Shine R (1992) Costs of reproduction in lizards: escape tactics and susceptibility to predation. Behav Ecol Sociobiol 31:17–25
Van Damme R, Entin P, Vanhooydonck B, Herrel A (2008) Causes of sexual dimorphism in performance traits: a comparative approach. Evol Ecol Res 10:229–250
Vanhooydonck B, Herrel A, Irschickt DJ (2007) Determinants of sexual differences in escape behavior in lizards of the genus Anolis: a comparative approach. Integr Comp Biol 47:200–210
Ydenberg RC, Dill LM (1986) The economics of fleeing from predators. Adv Study Behav 16:229–249
Acknowledgments
We thank Li Hua and Cuoke for helping with field data collection. This work was supported by the National Natural Science Foundation of China (31201723) and the Chinese Academy of Sciences (Y2C3041).
Ethical standards
Handling and processing of lizards followed approved protocols from the Chengdu Institute of Biology of the Chinese Academy of Sciences and the Animal Behavior Society (ABS)/Association for the Study of Animal Behaviour (ASAB) Guidelines for the treatment of animals in behavioural research and teaching. The Chengdu Institute of Biology approved this project, and permission for fieldwork was provided by the Forestry Department of the Sichuan Provincial Government and the Management Office of the Zoige Nature Reserve.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Madsen
Rights and permissions
About this article
Cite this article
Qi, Y., Noble, D.W.A., Wu, Y. et al. Sex- and performance-based escape behaviour in an Asian agamid lizard, Phrynocephalus vlangalii . Behav Ecol Sociobiol 68, 2035–2042 (2014). https://doi.org/10.1007/s00265-014-1809-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1809-5