Abstract
Purpose
Patients who undergo hip hemiarthroplasty (HHA) due to traumatic femoral neck fracture frequently require red blood cell (RBC) transfusion. Although post-operative autologous blood transfusion (ABT) is well established in elective arthroplasty, its role in trauma patients remains unclear.
Methods
Two hundred twenty-nine patients with a traumatic femoral neck fracture that underwent HHA at our level-I trauma centre between 2005 and 2009 were prospectively randomized to a high-vacuum drainage or an ABT device. In this single-institution analysis, the number of RBC units as well as the amount of retransfused shed blood were recorded and compared according to study groups. Additionally, the significance of confounding factors for allogenic blood demand such as age, gender, pre-operative Hb level, surgical approach, type of prosthesis and amount of intra-operative RBC units were evaluated using multivariate analysis.
Results
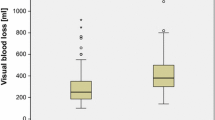
One hundred thirty-five patients were randomized in the high-vacuum group while 94 patients received an ABT device. Intention to treat analysis revealed no significant difference in post-operative RBC demand (ABT: 0.87 RBC, high-vacuum drainage: 1.01 RBC; P = 0.374). However, patients that actually received retransfusion (N = 35) had a reduced post-operative RBC demand (0.49 RBC units, P = 0.014).
Conclusion
While only one third of trauma patients treated with an ABT device during HHA actually receive retransfusion, retransfused patients seem to significantly benefit from this treatment as reflected by a reduced pos-toperative RBC demand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients routinely receive HHA (hip hemiarthroplasty) when sustaining a displaced femoral neck fracture. The operation together with the impaired medical condition of the elderly exposes the patient to numerous risks and is associated with an increased post-operative mortality [1]. Subsequently, these patients often require allogenic blood transfusions, which have been shown to increase post-operative infections after elective total hip or knee replacement [1, 2]. As traumatically injured patients that undergo surgery have been reported to be at high risk for infectious post-operative complications, this is of major clinical relevance [3, 4]. Moreover, post-operative anemia itself has been found to be associated with a prolonged hospital stay [5].
Several strategies have been reported to avoid anemia as well as the need for allogenic blood transfusions, e.g. intra-operative blood salvage and normovolemic hemodilution. Further, retransfusion of post-operatively collected filtered shed blood is commonly applied and reported to be safe and efficient in elective arthroplasty [6–9]. Patients with acute traumatic femoral neck fracture that receive HHA are often excluded from studies focusing on blood demand as blood loss is considered to be variable and unpredictable. Accordingly, comparability to elective total hip arthroplasty (THA) has been reported to be limited [10]. Moreover, comparability to elective THA of traumatic HHA patients is further limited as they occur primarily in an aged population with increased American Society of Anesthesiologists (ASA) scores [1].
As available studies frequently subsume HHA patients in a heterogeneous group of procedures the significance of retransfusion in these patients is substantially limited [11]. Accordingly, we aimed to evaluate the effect of a post-operative autologous blood transfusion (ABT) device on the need for allogenic red blood cell (RBC) transfusion among patients undergoing primary HHA for traumatic femoral neck fracture. The study was performed in a clinically relevant trauma surgery setting within a randomized prospective trial.
Material and methods
Trial design and patients
We performed a prospective randomized clinical trial including patients that underwent HHA for a traumatic displaced femoral neck fracture (type III or IV according to Garden) at our institution. Approval was obtained by the Ethics Commission of the Medical University of Vienna. We included primary and unilateral HHA procedures. Exclusion criteria were metastatic fracture, revision surgery and THA.
The primary end point of the study was the number of allogenic RBC units required upon post-operative day five. Secondary end points were adverse events during ABT transfusion as well as the number of intra-operative RBC’s. ABT related adverse events were defined as hypotension (e.g. systolic blood pressure <90 mmHg), tachycardia (e.g. >100 beats per minute), chills, as well as febrile episodes (e.g. >37.9 °C) observed during or within two hours after retransfusion.
Drainage system
The high-vacuum group consisted of patients who were treated with a closed suction drainage (Medinorm, Spiesen, Germany), which was removed 48 hours after surgery.
The ABT group was post-operatively treated with an ABT salvage device (Bellovac® ABT system Astra Tech AB, Mölndal, Sweden). The blood salvage device was used according to manufacturer’s instructions. Collected blood was retransfused to the patient within the first six post-operative hours, if a minimum amount of 100 ml was collected. If less than 100 ml was collected, the drained blood was discarded. Anemia was defined as a Hb (haemoglobin) level <13.0 g/dl for males and <12.0 g/dl for females according to WHO criteria. Allogenic RBC units were transfused if the Hb level dropped below 8 g/dl or a haematocrit <27 %. Predonated autologous blood units were not available. Intraoperative salvage of autologous blood was not performed in either group.
Surgery
HHA was performed by or under the supervision of experienced surgeons using either an anterolateral approach (Watson Jones) or a lateral approach (Bauer) [12]. Patients received either a cemented (Stryker MV) or an uncemented femoral stem (Vienna stem). The surgical drain was inserted before closure of the fascia lata and connected either to a high-vacuum drainage or to the ABT salvage device.
Statistics
Statistical analyses were carried out with SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) and were based on parametric tests. In particular, student’s t-test was used for continuous variables, and Chi-square test was used for categorical variables. A univariate regression analysis was performed to determine patient characteristics that influenced the allogenic blood demand. All parameters with a significance level of P < 0.1 upon univariate analysis were included in multivariate analysis (MVA) to identify independent predictors for post-operative RBC demand. A P-value ≤ 0.05 was considered statistically significant.
Results
A total of 229 patients (mean: 83.1 years; range: 44.7–102.5 years) were found eligible to be enrolled between May 2005 and August 2009 and randomly assigned to either receive an ABT or high-vacuum drainage system after HHA. Accordingly, 94 patients comprised the ABT group and 135 patients comprised the high-vacuum group. The groups were found to be homogenous with respect to age, gender, preoperative Hb level, surgical approach, type of prosthesis and intra-operative RBC units, as illustrated in Table 1.
Overall, 134 patients (58.5 %) post-operatively required an average of 1.63 RBC units (range, 1–4 units). In our intention to treat analysis, including all patients treated within the study we did not find a statistically significant difference regarding the post-operative RBC demand with respect to the different drainage devices. Patients in the high-vacuum group received 1.01 RBC units, whereas patients allocated to the ABT group received 0.87 RBC units (P = 0.374).
However, we further aimed to assess the RBC demand of those patients assigned to the ABT group who actually received retransfusion in a subgroup analysis. Regarding the frequency of retransfusion of filtered shed blood 35 patients (37.2 %) received retransfusion. In these patients an average amount of 184.3 ml (range, 100–400 ml) was retransfused. Importantly, patients that had received ABT had a significantly reduced RBC unit demand (mean 0.49 units; range, 0–2 units) as compared to patients in the high-vacuum group (mean 1.01; range, 0–4 units; P = 0.014). Accordingly, the percentage of patients requiring allogenic blood was 25.7 % in the ABT subgroup of retransfused patients (n = 9) versus 46.7 % in the high-vacuum drainage group (n = 63).
Reasons for missed retransfusion were ≤100 ml collected wound fluid within six hours post-operatively (52.1 %). Other reasons were accidental disconnection of the drain (4.3 %) or a missed time frame for retransfusion during the night (6.4 %).
As we had observed an association of retransfused shed blood with reduced post-operative transfusion requirements, it was of interest to investigate if retransfusion of filtered shed blood would independently predict post-operative blood demand. Therefore, we performed MVA in all recruited patients using clinically relevant parameters. All parameters that reached a statistical P-value ≤0.1 in univariate analysis were included in MVA. Accordingly, retransfusion of collected blood, Hb1 and age were included in MVA (Table 2).
Strikingly, the retransfusion of collected shed blood was independently associated with the necessity for post-operative blood transfusion (OR: 0.33, CI = 0.15–0.77; Table 2). Furthermore, Hb1 and age also independently predicted post-operative transfusion requirement (OR = 0.82, CI = 0.68–0.99; OR = 1.04, CI = 1.00–1.07; Table 2).
As side effects might be a drawback of retransfusion, we aimed to evaluate the safety profile of retransfused shed blood in our patients. Accordingly, in the ABT group, retransfusion-related symptoms were observed in three patients (8.6 %, all patients suffered from chills with subsequent fever of >38 °C after initiation of retransfusion): retransfusion was stopped and patients recovered without pharmacological interventions.
Discussion
Post-operative retransfusion of unprocessed autologous shed blood is an established procedure in elective arthroplasty [13, 14]. A recent Cochrane review emphasized the significance of peri-operative ABT in elective orthopedic surgery [15]. However, the role of retransfusion in trauma patients remains still unclear. Accordingly, we aimed at evaluating the clinical significance of retransfusion of unprocessed filtered shed blood in a homogenous cohort of trauma patients’, i.e. traumatic displaced femoral neck fracture patients undergoing HHA. We were able to demonstrate that despite only about one third of our patients actually received retransfusion, those patients that received their collected shed blood within six hours after surgery had a significantly reduced post-operative RBC demand, while side effects were rare and easily manageable.
Carless et al. recently reviewed a total of 75 trials on the use of RBC saving devices, documenting a significant benefit in terms of post-operative blood cell demand [15]. While we did not observe a reduction of post-operative RBC demand in our intention to treat analysis, we could however observe similar results in patients that actually receive retransfusion of their filtered shed blood. In particular, we were able to demonstrate a reduction of post-operative RBC demand of about 50 % in retransfused patients. Nonetheless, Thomassen et al. reported results on the use of ABT that failed to demonstrate a beneficial effect of retransfusion. In more detail, they used ABT in an elective joint replacement (knee and hip replacement) setting and found no difference in post-operative RBC demand. Intriguingly, they reported a very low post-operative RBC transfusion rate of 6 to 8 % [16]. This is in clear contrast to our collective that required post-operative RBC transfusion in 58.5 %. This seems to be attributable to substantial differences in patients’ collectives, presumably primarily caused by given differences in traumatic and elective patients. First the collective studied by Thomassen et al. was substantially younger reporting a mean age of 68 years. Further, patients had been pre-operatively optimized with erythropoietin if the pre-operative Hb was <13 g/dl reflected by a mean pre-operative Hb of 14.2 g/dl. In clear contrast our patients were a mean of 15 years older (mean age: 83.1 years) and also the pre-operative Hb was substantially lower (mean Hb1: 12.8 g/dl). These differences are also confirmed in other studies using ABT devices in elective surgery. Accordingly, Horstmann et al. reported preoperative Hb levels of 14.1 g/dl in 118 patients undergoing THA at an average of 68.5 years [17]. This nicely demonstrates that patients who sustain acute femoral neck fractures substantially differ compared to patients scheduled for elective surgery, which might explain the differences observed by Thomassen et al. as well as nicely illustrates the clinically necessity of our study.
Horstmann et al. used a salvage device collecting intra- and post-operative drained blood in 56 patients undergoing primary elective THA [17]. Eighty-two percent of their patients were found eligible for ABT and a mean of 254 ml shed blood was transfused six hours after the surgery. As compared to our results, ABT seemed substantially more effective in their elective setting as we report a mean retransfusion rate of only 36 % with an average of 184.3 ml transfusion volume. Accordingly, their post-operative RBC transfusion rate was by far lower than in our collective. Of note, Horstmann et al. reported on concomitant intra-operative blood collection, which might explain the increased available post-operative drainage volume, which might also result in an increased post-operative retransfusion rate. Furthermore, higher pre-operative Hb levels in their cohort might also explain the lower post-operative RBC demand. Nonetheless, the adaption of concomitant intra-operative blood drainage might render the ABT drainage device in traumatic patients more effective.
Pre-operative Hb levels have been reported to be a significant predictor of post-operative RBC demand in elective THA patients [2, 18, 19]. Indeed, also in our setting dealing with traumatic displaced femoral neck fracture patients, 74.6 % of patients that were anemic pre-operatively ultimately required post-operative RBC units. When we compared patients that received retransfusion and patients treated with the conventional high-vacuum drainage system within MVA, we were able to provide evidence that indeed pre-operative Hb levels independently predict post-operative blood demand (P = 0.041). Moreover, upon MVA we were further able to demonstrate that retransfusion of the filtered shed blood was associated with a reduced risk to require post-operative RBC transfusion (P = 0.01). Ultimately, age was also an independent predictor of post-operative RBC demand, demonstrating the relevance of these basic patients’ characteristics, age and pre-operative Hb respectively.
Several aspects have to be considered regarding the efficacy of the ABT device. In particular, one of the main findings of our study was that only 37.2 % of patients treated with an ABT device were actually transfused with their filtered shed blood. This is of major clinical relevance as singularly these patients benefit in terms of a reduction of allogenic blood requirements. Drain dislocation, narrow time frame for retransfusion and a small amount of drained shed blood were the obstacles preventing retransfusion. In particular, most of the patients (52.2 %) simply drained too little wound fluid (<100 ml), while drain dislocation and missed time frame occurred in only 4.3 and 6.4 % respectively. Missing the critical threshold of 100 ml might be due to the fact that the ABT device works as a passive drain, with only intermittent suction pressure up to 90 mmHg. Missing the critical threshold reflects a major drawback regarding the systems effectiveness in everyday practice. The narrow time frame of six hours was another reason impeding retransfusion. This illustrates that retransfusion might be difficult to implement in the daily routine of a trauma department. Especially when patients are operated on at night, retransfusion six hours post-operatively is easily overlooked. Of note, since patients and surgeons are aware of the possible transmission of potentially life-threatening infections and other serious adverse events during RBC transfusion, retransfusion might be a beneficial tool to lower RBC transfusion demand despite its relatively low efficiency. Importantly, efficiency in everyday practice might be increased if retransfusion was performed more often. This is consistent with Moonen et al. who expects limited efficacy of the retransfusion systems in every day practice when compared to prospective study protocols that focus on blood management [10].
Although shed blood is an autologous product, reported side effects have to be considered. Studies report on occasional febrile reactions, with an incidence of 2–9 % if patients received shed blood collected within six hours post-operatively [13, 20]. Nevertheless, amounts up to 1800 ml were reported to be retransfused without serious adverse events [13]. We observed potentially retransfusion-related side effects in 8.6 %, which resolved without pharmacological intervention.
Some potential limitations of our study should be discussed. One limitation is the relatively small number of patients who were actually eligible for retransfusion which in turn reduces the statistical power of our analysis. Further, our indication for RBC transfusion was a Hb level <8 g/dl or a haematocrit <27 %. We use this fairly liberal transfusion trigger at our institution with regard to the advanced age of traumatic displaced femoral neck fracture patients. However, transfusion triggers might differ between institutions which should be taken into account when interpreting the results of this study. Furthermore, we should stress that HHAs were performed by numerous different surgeons at our level-I trauma centre, which might represent a possible confounding factor. However, it should also be stated that all operations were performed by or under the supervision of experienced trauma surgeons. As the ABT device has a different appearance as compared to a regular suction drainage, blinding of the investigators after randomization was ultimately not possible. This might have introduced a potential confounder within our study (e.g. higher threshold to transfuse in the ABT group). However, using clear transfusion triggers for allogenic RBC transfusion in all patients (Hb level <8 g/dl or a haematocrit <27 %), we aimed to minimize this potential confounder.
In conclusion, the value of an autologous erythrocyte source in arthroplasty is evident. With regard to limited pre-operative options, post-operative autologous retransfusion of filtered shed blood seems to be a feasible method to decrease allogenic blood requirements in patients who undergo HHA for traumatic femoral neck fractures.
References
Muller F, Galler M, Zellner M, Bauml C, Fuchtmeier B (2015) The fate of proximal femoral fractures in the 10th decade of life: an analysis of 117 consecutive patients. Injury 46:1983–1987
Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB (1999) An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg (Am Vol) 81:2–10
Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP (2003) Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma 54:908–914
Carson JL, Altman DG, Duff A, Noveck H, Weinstein MP, Sonnenberg FA, Hudson JI, Provenzano G (1999) Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion 39:694–700
Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM (2002) Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 102:237–244
Ritter MA, Keating EM, Faris PM (1994) Closed wound drainage in total hip or total knee replacement. A prospective, randomized study. J Bone Joint Surg (Am Vol) 76:35–38
Strumper D, Weber EW, Gielen-Wijffels S, Van Drumpt R, Bulstra S, Slappendel R, Durieux ME, Marcus MA (2004) Clinical efficacy of postoperative autologous transfusion of filtered shed blood in hip and knee arthroplasty. Transfusion 44:1567–1571
Moonen AF, Knoors NT, van Os JJ, Verburg AD, Pilot P (2007) Retransfusion of filtered shed blood in primary total hip and knee arthroplasty: a prospective randomized clinical trial. Transfusion 47:379–384
Spahn DR (2010) Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology 113:482–495
Moonen AF, Thomassen BJ, van Os JJ, Verburg AD, Pilot P (2008) Retransfusion of filtered shed blood in everyday orthopaedic practice. Transfus Med 18:355–359
Rizzi L, Bertacchi P, Ghezzi LM, Bellavita P, Scudeller G (1998) Postoperative blood salvage in hip and knee arthroplasty. A prospective study on cost effectiveness in 161 patients. Acta Orthop Scand 69:31–34
Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn TA, Graves DT (2007) Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res Off J Am Soc Bone Miner Res 22:560–568
Faris PM, Ritter MA, Keating EM, Valeri CR (1991) Unwashed filtered shed blood collected after knee and hip arthroplasties. A source of autologous red blood cells. J Bone Joint Surg (Am Vol) 73:1169–1178
Sturdee SW, Beard DJ, Nandhara G, Sonanis SV (2007) Decreasing the blood transfusion rate in elective hip replacement surgery using an autologous drainage system. Ann R Coll Surg Engl 89:136–139
Carless PA, Henry DA, Moxey AJ, O’Connell D, Brown T, Fergusson DA (2010) Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 18:CD001888
Thomassen BJ, den Hollander PH, Kaptijn HH, Nelissen RG, Pilot P (2014) Autologous wound drains have no effect on allogeneic blood transfusions in primary total hip and knee replacement: a three-arm randomised trial. Bone Joint J 96-B:765–771
Horstmann WG, Swierstra MJ, Ohanis D, Rolink R, Kollen BJ, Verheyen CC (2014) Favourable results of a new intraoperative and postoperative filtered autologous blood re-transfusion system in total hip arthroplasty: a randomised controlled trial. Int Orthop 38:13–18
Jones HW, Savage L, White C, Goddard R, Lumley H, Kashif F, Gurusany K (2004) Postoperative autologous blood salvage drains--are they useful in primary uncemented hip and knee arthroplasty? A prospective study of 186 cases. Acta Orthop Belg 70:466–473
Nuttall GA, Santrach PJ, Oliver WC Jr, Horlocker TT, Shaughnessy WJ, Cabanela ME, Bryant S (1996) The predictors of red cell transfusions in total hip arthroplasties. Transfusion 36:144–149
Blevins FT, Shaw B, Valeri CR, Kasser J, Hall J (1993) Reinfusion of shed blood after orthopaedic procedures in children and adolescents. J Bone Joint Surg (Am Vol) 75:363–371
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Starlinger, J., Schmidt, R. & Machold, W. Post-operative retransfusion of unwashed filtered shed blood reduces allogenic blood demand in hip hemiarthroplasty in traumatic femoral neck fractures—a prospective randomized trial. International Orthopaedics (SICOT) 40, 2575–2579 (2016). https://doi.org/10.1007/s00264-016-3143-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-016-3143-1