Abstract
Purpose
It has been reported that the anterior cruciate ligament (ACL) has certain self-healing ability after acute injury or with primary suture repair. Many studies have confirmed that a remnant preservation technique with ACL reconstruction contributes to biological augmentation for ACL healing. However, it remains unclear whether mesenchymal stem cells (MSC) reside in ACL remnants in situ. The aim of this study was to investigate the methods of culture and identification of MSC derived from the remnants of ACL rupture patients and to analyse these MSC’s properties.
Methods
The cells of ACL remnants from the ACL rupture patients were isolated by the methods of enzymatic digestion and cultured in vitro to the third passage under the microscope to observe their morphology and growth status. The third passage of isolated cells was analysed for the identification of immunophenotype, osteogenic, adipogenic and chondrogenic differentiation.
Results
On the third to fifth days of in vitro culture, a few cells of long fusiform shape appeared and were adherent to the plastic walls. On the sixth to ninth days, cells clustered and colonies were observed. The third passage cells showed uniform cell morphology and good proliferation, with appearance of the typical surface markers of MSC, CD29, CD44, CD90 and CD105. The surface markers of CD34 and CD45 of haematopoietic stem cells were not expressed. Under appropriate conditions of in vitro culture, isolated cells could be differentiated into osteoblasts that deposit mineralised matrix and express early osteogenic markers, adipocytes that accumulate lipid droplets in cytoplasm and chondrocytes that secrete chondrogenic-specific matrix aggrecan and collagen II. Real-time polymerase chain reaction (PCR) analysis demonstrated that the specific mRNA expression of osteogenesis, adipogenesis and chondrogenesis increased significantly compared with the control groups at day zero.
Conclusions
Stem cells derived in situ from the human ACL stump were successfully isolated and characterised. Those isolated cells were identified as MSC according to their adherent ability, morphology, surface markers and multilineage differentiation potential. MSC derived from ACL remnants could be a potential source of seeding cells for ligament regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) injury is very common in the field of sports medicine, especially among athletes. Arthroscopic ACL reconstruction is considered the major and well-accepted treatment for ACL injury, the so-called gold standard treatment [1]. The injured ligament is removed and replaced with an autologous, allogeneic or artificial prosthetic tendon graft. The ACL graft undergoes an acute inflammatory process, followed by revascularisation, recellularisation and tissue remodelling phases, finally integrating with the host [2]. The goal of ACL reconstruction is to restore mechanical stability and re-establish knee function [3]. However, patients with ACL reconstruction may develop progressive joint degeneration and osteoarthritis of the injured knee. A prospective cohort study with long-term follow-up reported that the prevalence of radiographic osteoarthritis was up to 62 % ten to 15 years after isolated ACL reconstruction [4]. The incorporation with host and ligamentation of the graft are crucial to ACL reconstruction. Successful ACL reconstruction requires extensive biological integration of the graft with the host tissue [5]. Recently, the advances of regenerative medicine prompted researchers and clinicians to construct the bio-mimic tissue-engineered ligament substitute to replace the ACL, such as bone morphogenetic protein 7 or cyclical mechanical loading to enhance tendon-bone integration [6, 7]. Although good results have been obtained in the laboratory with regard to the composite of the three elements of tissue engineering, i.e. seed cells, scaffolds and bioactive molecules, there is no tissue-engineered ACL construct so far that has been successfully implanted in humans [8, 9].
Some studies have confirmed that the native remnant preservation technique in ACL reconstruction contributes to the biological augmentation of the graft for optimising ACL healing using a bio-enhanced method [10–15]. ACL reconstruction using remnant preservation may enhance postoperative knee stability, graft healing and proprioception recovery and improve the revascularisation and ligamentisation of tendon-bone integration. The native remnant preservation technique for ACL reconstruction could lead to biomechanical, histological and functional recovery. Besides the repaired microenvironment provided by surrounding synovial and growth factors, another important mechanism of the remnant preservation technique for ACL reconstruction may be the existence of mesenchymal stem cell (MSC)-like cells in the ACL stump. We hypothesised that MSC reside in ACL remnants in situ which would be involved in ACL repair at the cellular level using the augmentation technique with remnant preservation in ACL reconstruction.
Recently, researchers have isolated stem cells from tendon tissues, which are also called tissue-specific tendon-derived stem cells. Those cells formed adherent colonies in in vitro culture, exhibited a typical fibroblast-like morphology, expressed stem cell-related markers and showed self-renewal and multilineage differentiation potential [16, 17]. Although MSC isolated from different tissues share certain similar common stem cell characteristics, they might exhibit some tissue-specific features and functional differences [18, 19]. Do ACL remnants also contain the tissue-specific distinct cell types like tendon-derived stem cells? We hypothesised that MSC exist in ACL remnants. In this study, we isolated, identified and characterised MSC-like stem cells from ACL remnants of ACL rupture patients who underwent arthroscopy.
Materials and methods
Isolation and culture of MSC derived from ACL remnants
This study was approved by local Research Ethics Committees and written informed consents were obtained from all patients. ACL remnants were harvested from four adult patients who suffered from ACL rupture and underwent arthroscopic primary ACL reconstruction. The isolation and culture protocols of MSC derived from ACL remnants were as previously described [20]. The human adult ACL remnant tissues were rinsed with sterile phosphate-buffered saline (PBS) containing 1 % gentamicin, scraped free of any haematoma and vascular tissue. The excised tissues were minced into approximately 1 mm3 pieces, digested with 2 mg/ml collagenase type I in low-glucose Dulbecco’s modified Eagle’s medium (LG-DMEM) at 37 °C for four to six hours. The isolated cells were washed three times with PBS, suspended in complete LG-DMEM supplemented with 10 % fetal bovine serum (FBS), 2.2 g NaHCO3, 100 U/ml penicillin, 100 μg/ml streptomycin, 25 ng/ml amphotericin B and 2 mM L-glutamine, and incubated at 37 °C with 5 % humidified CO2. The non-adherent cells were washed out with PBS after 48–72 hours. The fresh medium was replaced every three to four days. When primary cultures had reached 80–90 % confluence, they were trypsinised with 0.25 % trypsin/0.1 % ethylenediaminetetraacetic acid (EDTA) and split 1:2 or 1:3 for subculturing. Passage 3 cultures (P3) were used for subsequent experiments.
Phenotypic characteristics of MSC derived from ACL remnants
MSC derived from ACL remnants at P3 were subjected to surface marker profile analysis by flow cytometry (FCM) and confocal fluorescence microscopy. The cells were first incubated with fluorescein isothiocyanate (FITC)-conjugated anti-CD29, CD44 antibody or purified primary antibodies against CD90, CD105, CD34 and CD45 for one hour, then followed by FITC-labelled secondary antibody for 30 minutes. For the isotype controls, non-special mouse IgGs were used as isotype controls to eliminate non-specific staining in each experiment. Cells were then fixed in flow buffer, washed and suspended in 0.5 ml PBS for FCM analysis using CellQuest software (BD Biosciences).
After being fixed with 4 % paraformaldehyde, MSC at P3 on cover slips were incubated for two hours with FITC-conjugated or purified primary antibodies, followed then by FITC-labelled secondary antibody for one hour. Appropriate isotype controls were prepared. Then cell nuclei were counterstained for five minutes at room temperature with Hoechst diluted 1:1,000. Slides were mounted onto cover slips using 90 % glycerol and analysed using a confocal fluorescence microscope (Leica).
Multilineage differentiation potential of MSC derived from ACL remnants
Osteogenic differentiation
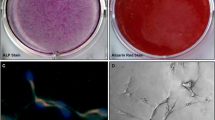
For osteogenic differentiation, MSC were grown to 80 ∼ 90 % confluency and then induced in osteogenic medium supplemented with dexamethasone, β-glycerophosphate and ascorbate incubated at 37 °C with 5 % humidified CO2 for two weeks. The medium was refreshed every three days. Osteogenesis was confirmed by early osteogenic marker alkaline phosphatase (ALP) staining and calcium deposits using Alizarin Red staining. Total RNA was harvested from the cells at different time points in monolayer culture with osteogenic medium. Expression of osteogenic genes Runx2, ALP and collagen I was analysed by real-time polymerase chain reaction (PCR).
Adipogenic differentiation
For adipogenic differentiation, MSC were induced in adipogenic medium consisting of dexamethasone, methylisobutylxanthine, insulin and indomethacin incubated at 37 °C with 5 % humidified CO2 for 14 days. The medium was refreshed every three days. Adipogenesis was evaluated by the formation of accumulated lipid vacuoles within the cytoplasm using Oil Red O staining. Total RNA was harvested from the cells at different time points in monolayer culture with adipogenic medium. Expression of adipogenic genes peroxisome proliferator-activated receptor gamma (PPARγ), lipoprotein lipase (LPL), polyadenylation signals (PAS) and acid-binding protein (aP2) was analysed by real-time PCR.
Chondrogenic differentiation
The 3D micromass pellet cultures and serum-free medium with transforming growth factor beta 3 (TGFβ3) were established to assess chondrogenic differentiation of ACL remnant-derived cells. Then 106 cells were collected in a 15-ml conical polypropylene tube and centrifuged at 480 g for ten minutes, and the cell masses were left at the bottom of the tube, cultured at 37 °C with 5 % humidified CO2. The cells in micromass were incubated in chondrogenic medium containing high-glucose DMEM, 100 × ITS, 1 mmol/l pyruvate, 0.17 mmol/l ascorbate, 0.1 μM dexamethasone, 0.35 mmol/l proline and 10 ng/ml TGFβ3 for three weeks. The medium was refreshed every three days. Pellets were harvested after 3 weeks, embedded in paraffin, sectioned and assessed for chondrogenesis by haematoxylin and eosin (H&E) for general histology and Toluidine Blue staining for aggrecan and immunohistochemistry for collagen II expression. Total RNA was harvested from the pellets after three weeks. Expressions of chondrogenic genes SOX9 and collagen II were analysed by real-time PCR.
Real-time quantitative PCR
The expression of specific markers for extracellular matrix and transcription factors was assessed by real-time PCR after induction for quantitating the differentiation capability of MSC. Total RNA from samples was extracted using RNAVzol reagent (Vigorous) according to the manufacturer’s instructions. Two micrograms of total RNA were subjected to a reverse transcription using the SuperScript First Strand Synthesis System (Invitrogen). Quantitative real-time PCR was performed with 15 μl of reaction volume consisting of 7.5 μl 2 × SYBR Green PCR Master Mix (Toyobo), 1 μl of cDNA product, 1 μl of each primer and 5.5 μl of nuclease-free water. All the PCR amplifications were performed by preheating at 95 °C for 10 min and followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C by employing ABI PRISM 7000 Sequence Detection System software. The specificity of amplicons was verified by melting curve analysis. Levels of mRNA were normalised against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the comparative cycle threshold (Ct) method.
Statistics
Expression levels of each mRNA are shown as mean ± SD (n = 4). Student’s t test or Student-Newman-Keuls (SNK) q test was used to assess significant differences. P < 0.05 was considered statistically significant.
Results
Isolation and culture of MSC derived from ACL remnants
The diagnosis of ACL rupture of the four patients was made by clinical exams and radiographic studies (Fig. 1a) confirmed arthroscopically (Fig. 1b). The nucleated cells were isolated from ACL remnants (Fig. 1c) by collagenase. The isolated round cells gradually spread and adhered to the culture dish after three days of primary plating (Fig. 1d). Next, cells exhibited typical spindle-shaped fibroblast-like morphology by five to six days. With the increase of culture time in vitro, the cells grew rapidly and demonstrated swirling or cluster appearance. By ten ∼ 15 days, 80 ∼ 90 % confluence (Fig. 1e) was reached for subculture at a ratio of 1:2 or 1:3. After the subculture, approximately four to five days were needed for each passage. Cell morphology performed homogeneously until P3 (Fig. 1f).
Magnetic resonance imaging (a) and arthroscopic examination (b) showed typical ACL rupture. ACL remnants were obtained during surgery (c). A few nucleated cells spread and adhered to the culture dish after three days of primary plating (d). The cells exhibited swirling or cluster appearance by 12 days (e) and performed homogeneously until P3 (f)
Phenotypic characteristics of MSC derived from ACL remnants
Phenotypic characteristics by FCM analysis (Fig. 2a) and confocal fluorescence microscopy (Fig. 2b) indicated that cells derived from ACL remnants at P3 highly expressed typical mesenchymal associated markers CD29, CD44, CD90 and CD105, while they were weakly negative to haematopoietic markers CD34 and CD45, which showed those cells had MSC phenotypic characteristics.
MSC derived from ACL remnants were subjected to differentiation assays in vitro
Osteogenesis
The cells began to gather and became sparse after incubation for one week. Cell morphology changed from spindle shape into a more polygonal appearance. Between one and two weeks the nodules became larger and scattered uniformly. In order to assess the osteogenic potential of MSC, mineralised matrix and osteoblast markers were determined by Alizarin Red and ALP staining after 14 days. MSC in osteogenic medium secreted bone matrices or osteoblast-specific markers, i.e. ALP, which are early markers for bone differentiation. The bone matrices became red when the cultures were stained by Alizarin Red. PCR illustrated that osteogenic-specific markers Runx2, ALP and collagen I of MSC derived from ACL remnants were significantly greater than the control on day zero (Fig. 3).
Osteogenic differentiation potential of MSC derived from ACL remnants at P3. Osteogenesis was examined by ALP (a: ×50) and Alizarin Red staining (b: ×400) after 14 days culture in osteogenic induction media in vitro. The expression of specific osteogenic genes Runx2 (c), ALP (d) and collagen I (e) was assessed by real-time PCR. Data presented as mean ± SD. *P < .05; **P < .01
Adipogenesis
After incubation with adipogenic medium for about six days, cell volume and cell nucleus became larger, and intracellular lipid droplets appeared in cytoplasm under the microscope (Fig. 4a). The lipid accumulation grew larger and increased by two weeks. MSC cultured in adipogenic medium were differentiated into adipocytes characterised by lipid vacuoles emerging in the cytoplasm, which appeared red by adding Oil Red O (Fig. 4b, c). PCR illustrated that adipogenic-specific markers PPARγ, LPL, PAS and aP2 of MSC derived from ACL remnants were significantly greater than the control on day zero (Fig. 4d–g).
Adipogenic differentiation potential of MSC derived from ACL remnants at P3. Adipogenesis was detected by the formation of neutral lipid vacuoles under inverted phase contrast microscope (a: ×200) and with Oil Red O staining (b: ×50, c: ×400) after 14 days culture in adipogenic induction media in vitro. The expression of specific adipogenic genes PPARγ (d), LPL (e), PAS (f) and aP2 (g) was assessed by real-time PCR. Data presented as mean ± SD. *P < .05; **P < .01
Chondrogenesis
The pellets changed to spheroids under serum-free conditions in three days. The micromasses became rich in aggrecan and collagen II as distinguished by their strong staining by Toluidine Blue and immunohistochemistry. H&E staining showed that the shape of cells were round as chondrocytes in lacunge-like structure. Toluidine Blue staining demonstrated a considerable degree of metachromasia. There was significantly higher mRNA expression of chondrogenic-specific markers SOX9 and collagen II after chondrogenic induction than control on day zero (Fig. 5).
Chondrogenic differentiation potential of MSC derived from ACL remnants at P3. Chondrogenesis was induced under serum-free micromass pellet culture conditions (a) and assessed by H&E staining (b) for general observation, Toluidine Blue staining (c) for aggrecan and immunohistochemical staining for specific chondrogenic matrix collagen II (d) after 21 days culture in chondrogenic induction media in vitro. Scale bars = 100 μm. The expression of specific chondrogenic genes SOX9 and collagen II was assessed by real-time PCR. Data presented as mean ± SD. **P < .01
Discussion
It has been reported that MSC could be isolated from a variety of tissues of the body [21]. However, whether MSC are located in ACL remnants in situ is not clear yet. In this study, we successfully isolated nucleated cells from the human ACL stump, which could adhere to plastic surfaces, grow well and be serially subcultured in vitro. The surface markers of those cells were similar to those previously reported for MSC [20]. Under appropriate conditions in in vitro culture, they could be induced to differentiate into osteoblasts that deposit mineralised matrix and express early osteogenic marker ALP, adipocytes that accumulate lipid droplets in cytoplasm and chondrocytes that secrete chondrogenic-specific matrix aggrecan and collagen II. Real-time PCR analysis demonstrated that the specific mRNA expression of osteogenesis, adipogenesis and chondrogenesis increased significantly compared with the control groups at day zero. Those isolated cells were identified as MSC according to adherence ability, morphology, surface markers and multilineage differentiation potential. We also confirmed that MSC existed in the ACL stump tissue.
Where do ACL stump tissue-derived MSC come from? Almost all tissues throughout the body reside in MSC. Whether these progenitor/stem cells share a common ancestor in multiple developed organs is unknown [22]. Some studies hypothesised that MSC natively lie on the blood vessel wall and originate from perivascular cells (pericytes) [23, 24]. Some researchers found that ACL tissue contained CD34+ and CD146+ vascular-derived stem cells, which showed high expansion and multilineage differentiation potential [25]. That evidence suggests that the vasculature of ACL stump tissue might harbour progenitor/stem cells. The stem cells in our study, however, were negative for CD34, which suggests that another stem cell subset could exist in ACL tissue. Those cells residing in ligament rely on the relation and regulation between stem cells and their local ACL niche microenvironment. Nohmi et al. found that the number and differentiation potential of MSC in ruptured ACL decreased over time [26]. Another source of ACL stump-derived MSC may be traced to the systemic bloodstream, and these cells are in quiescent state under healthy physiological conditions, but once injury or disorder occurs, the endogenous stem cells are mobilised from the stem cell bank into the peripheral blood, recruit and migrate to the ACL-injured site, and those cells are also differentiated and responsible during the pathophysiological process of ACL repair [27–29]. Either from local tissues or from the systemic peripheral circulation, the exact source of ACL stump-derived MSC needs to be further determined.
Recently, a lot of tissue-specific stem cells have been identified in various tissues, including articular cartilage, trabecular bone, adipose tissue, synovium and muscle etc. Some studies found that tissue-specific tendon stem cells exhibited common universal characteristics of MSC: clonogenicity, self-renewal and multi-differentiation potential [30]. MSC from various types of tissues share certain similar biological characteristics, but they also exhibit different properties according to different origins. Bi et al. also found tendon stem cells were more sensitive to bone morphogenetic protein 2 signalling [31]. ACL stump-derived MSC are tissue-specific stem cells, which may be good candidates for seed cells of ligament or tendon regeneration compared with fibroblasts and MSC derived from other sources. Tendon/ligament derived-fibroblasts exhibit very low proliferative capacity and low biological activity compared with stem cells, and they are relatively quiescent and have limited potential for further differentiation [32, 33]. In comparison with bone marrow MSC, ligament stem cells proliferate faster and maintain an undifferentiated state with basic fibroblast growth factor (bFGF) treatment, whereas under TGFβ1 treatment, ligament stem cells upregulate major tendinous gene expression and produce a robust amount of ligament extracellular matrix protein, making ligament stem cells a potential cell source in future applications of ACL tissue engineering [34, 35]. The tissue-specific, adult stem cells seem to have a higher regenerative potential for the tissue where they reside in and possess a resident stem cell population [36].
In conclusion, stem cells derived in situ from the human ACL stump were successfully isolated and characterised. Those isolated cells were identified as MSC according to their adherent ability, morphology, surface markers and multilineage differentiation potential. However, how these MSC participate in matrix deposit and tendon-bone healing and how they activate endogenous tissue-specific stem cells for ligament repair need further investigation. This tissue-specific stem cell may be a good candidate and the ACL stump may be the optimal source of seed cells for ligament or tendon regeneration.
References
Stevanović V, Blagojević Z, Petković A, Glišić M, Sopta J, Nikolić V, Milisavljević M (2013) Semitendinosus tendon regeneration after anterior cruciate ligament reconstruction: can we use it twice? Int Orthop 37:2475–2481
Sun L, Wu B, Tian M, Liu B, Luo Y (2013) Comparison of graft healing in anterior cruciate ligament reconstruction with and without a preserved remnant in rabbits. Knee 20:537–544
Tashman S, Kopf S, Fu FH (2008) The kinematic basis of ACL reconstruction. Oper Tech Sports Med 16:116–118
Oiestad BE, Holm I, Aune AK, Gunderson R, Myklebust G, Engebretsen L, Fosdahl MA, Risberg M (2010) Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med 38:2201–2210
Chen CH (2009) Graft healing in anterior cruciate ligament reconstruction. Sports Med Arthrosc Rehabil Ther Technol 1:21
Schwarting T, Benölken M, Ruchholtz S, Frink M, Lechler P (2015) Bone morphogenetic protein-7 enhances bone-tendon integration in a murine in vitro co-culture model. Int Orthop 39:799–805
Struewer J, Roessler PP, Schuettler KF, Ruppert V, Stein T, Timmesfeld N, Paletta JR, Efe T (2014) Influence of cyclical mechanical loading on osteogenic markers in an osteoblast-fibroblast co-culture in vitro: tendon-to-bone interface in anterior cruciate ligament reconstruction. Int Orthop 38:1083–1089
Leong NL, Petrigliano FA, McAllister DR (2014) Current tissue engineering strategies in anterior cruciate ligament reconstruction. J Biomed Mater Res A 102:1614–1624
Yang G, Rothrauff BB, Tuan RS (2013) Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today 99:203–222
Park SY, Oh H, Park SW, Lee JH, Lee SH, Yoon KH (2012) Clinical outcomes of remnant-preserving augmentation versus double-bundle reconstruction in the anterior cruciate ligament reconstruction. Arthroscopy 28:1833–1841
Kazusa H, Nakamae A, Ochi M (2013) Augmentation technique for anterior cruciate ligament injury. Clin Sports Med 32:127–140
Wu B, Zhao Z, Li S, Sun L (2013) Preservation of remnant attachment improves graft healing in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy 29:1362–1371
Eguchi A, Adachi N, Nakamae A, Usman MA, Deie M, Ochi M (2014) Proprioceptive function after isolated single-bundle posterior cruciate ligament reconstruction with remnant preservation for chronic posterior cruciate ligament injuries. Orthop Traumatol Surg Res 100:303–308
Lee DC, Shon OJ, Kwack BH, Lee SJ (2013) Proprioception and clinical results of anterolateral single-bundle posterior cruciate ligament reconstruction with remnant preservation. Knee Surg Relat Res 25:126–132
Kim SJ, Kim SH, Chun YM, Hwang BY, Choi DH, Yoon JY (2012) Clinical comparison of conventional and remnant-preserving transtibial single-bundle posterior cruciate ligament reconstruction combined with posterolateral corner reconstruction. Am J Sports Med 40:640–649
Lui PP (2013) Identity of tendon stem cells--how much do we know? J Cell Mol Med 17:55–64
Lui PP, Chan KM (2011) Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev 7:883–897
Fu WL, Zhou CY, Yu JK (2014) A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am J Sports Med 42:592–601
Fu WL, Zhang JY, Fu X, Duan XN, Leung KK, Jia ZQ, Wang WP, Zhou CY, Yu JK (2012) Comparative study of the biological characteristics of mesenchymal stem cells from bone marrow and peripheral blood of rats. Tissue Eng Part A 18:1793–1803
Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju YJ, Ezura Y, Umezawa A, Sekiya I (2009) Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res 27:435–441
Asari T, Furukawa K, Tanaka S, Kudo H, Mizukami H, Ono A, Numasawa T, Kumagai G, Motomura S, Yagihashi S, Toh S (2012) Mesenchymal stem cell isolation and characterization from human spinal ligaments. Biochem Biophys Res Commun 417:1193–1199
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313
da Silva Meirelles L, Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26:2287–2299
Caplan AI (2008) All MSCs are pericytes? Cell Stem Cell 3:229–230
Mifune Y, Matsumoto T, Ota S, Nishimori M, Usas A, Kopf S, Kuroda R, Kurosaka M, Fu FH, Huard J (2012) Therapeutic potential of anterior cruciate ligament-derived stem cells for anterior cruciate ligament reconstruction. Cell Transplant 21:1651–1665
Nohmi S, Yamamoto Y, Mizukami H, Ishibashi Y, Tsuda E, Maniwa K, Yagihashi S, Motomura S, Toh S, Furukawa K (2012) Post injury changes in the properties of mesenchymal stem cells derived from human anterior cruciate ligaments. Int Orthop 36:1515–1522
Kwapisz A, Chojnacki M, Domzalski M, Grzegorzewski A, Synder M (2014) Do gene expression changes in articular cartilage proteases of the synovial membrane correlate with expression changes of the same genes in systemic blood cells? Int Orthop 38:649–654
Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I (2008) Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford) 47:1137–1143
Zhang J, Pan T, Im HJ, Fu FH, Wang JH (2011) Differential properties of human ACL and MCL stem cells may be responsible for their differential healing capacity. BMC Med 9:68
Zhang J, Wang JH (2010) Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord 11:10
Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13:1219–1227
Pauly S, Klatte F, Strobel C, Schmidmaier G, Greiner S, Scheibel M, Wildemann B (2010) Characterization of tendon cell cultures of the human rotator cuff. Eur Cell Mater 20:84–97
Van Eijk F, Saris DB, Riesle J, Willems WJ, Van Blitterswijk CA, Verbout AJ, Dhert WJ (2004) Tissue engineering of ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng 10:893–903
Cheng MT, Yang HW, Chen TH, Lee OK (2009) Isolation and characterization of multipotent stem cells from human cruciate ligaments. Cell Prolif 42:448–460
Cheng MT, Liu CL, Chen TH, Lee OK (2010) Comparison of potentials between stem cells isolated from human anterior cruciate ligament and bone marrow for ligament tissue engineering. Tissue Eng Part A 16:2237–2253
Randelli P, Conforti E, Piccoli M, Ragone V, Creo P, Cirillo F, Masuzzo P, Tringali C, Cabitza P, Tettamanti G, Gagliano N, Anastasia L (2013) Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am J Sports Med 41:1653–1664
Acknowledgments
This study was funded by National Natural Science Foundation of China (No. 81301560), China Postdoctoral Science Foundation (No. 2012 M521698).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, W., Li, Q., Tang, X. et al. Mesenchymal stem cells reside in anterior cruciate ligament remnants in situ. International Orthopaedics (SICOT) 40, 1523–1530 (2016). https://doi.org/10.1007/s00264-015-2925-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2925-1