Abstract
Purpose
This study was performed to evaluate one-stage anterior cruciate ligament (ACL) reconstruction using a semitendinosus tendon graft injected with bone morphogenetic protein 2 (BMP-2) in a rabbit model.
Methods
We injected recombinant human BMP-2 (rhBMP-2) in the experimental group and phosphate-buffered saline in the control group at two sites of the semitendinosus tendon (15 μg in each site) to replace tendon with bone in the bone tunnel. Twenty minutes later, the injected tendon graft was transplanted for ACL reconstruction by passing the graft through the bone tunnel. The animals were harvested at four, eight, or 12 weeks postoperatively and examined by histological and biomechanical methods.
Results
Histological analysis revealed that the tendon graft was replaced with new bone in the tunnel of the experimental group. Characteristic features identical to the regenerated direct insertion morphology at the bone–tendon junction were acquired at eight or 12 weeks in the experimental group. Biomechanical pull-out testing revealed greater stiffness in the experimental than control group at 12 weeks, although the maximum load to failure showed no significant difference between the two groups at four, eight, or 12 weeks.
Conclusion
These results indicate the potential for ACL reconstruction with regenerated direct insertion morphology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anterior cruciate ligament (ACL) is an important stabilizer of physiological knee motion. ACL reconstruction has become a common procedure for ACL-deficient knees. Successful ligament reconstruction necessitates effective osteointegration of tendon grafts. In ACL reconstructive surgery, hamstring grafts or bone–patellar tendon–bone (BTB) grafts have mainly been used to replace the injured ACL by graft fixation through bone tunnels. Because the ACL has a direct insertion transitionally comprising a ligament, noncalcified fibrocartilage, calcified cartilage, and bone, a BTB graft may be suitable as the direct insertion in ACL reconstruction with bone-to-bone integration between the graft bone plug and tunnel wall [1, 2]. In ACL reconstruction using tendon grafts, collagen fibres are re-established between the grafted tendon and the walls of the bone tunnel in the form of Sharpey’s fibres. The tendon–bone interface is weak, exhibiting a woven bone formation. Furthermore, the tendon graft always remains within the bone tunnel and itself never replaces the bone in the tunnel [3].
Previous studies have found no differences in clinical results between BTB and hamstring grafts [4, 5]. In some studies, the BTB group exhibited a significantly higher incidence of kneeling-induced pain and anterior knee pain than did the hamstring group [6, 7]. On the other hand, enlargement of bone tunnels after ACL reconstruction with the hamstring tendon is a well-documented phenomenon in other reports [8, 9] because of greater distance between the location of the extra-tunnel fixation and the tunnel aperture.
In our previous study, we successfully generated direct insertion from the tendon to the bone by intratendinous injection of recombinant human bone morphogenetic protein 2 (rhBMP-2) into the semitendinosus tendon [10] and performed two-stage ACL reconstruction using an engineered BTB graft in a rabbit model. The experimental group showed histological analysis and biomechanical pull-out test results superior to those of the control group at four or eight weeks postoperatively [11]. We modified this previous two-stage surgery to a one-stage surgery. We hypothesized that one-stage ACL reconstruction using our previously established semitendinosus tendon graft injected with rhBMP-2 may allow the grafted tendon to be replaced by bone in the bone tunnel and regenerate the insertion site of the bone–tendon junction.
Materials and methods
Animals
Fifty-four healthy adult female New Zealand white rabbits weighing 3.20 ± 0.42 kg were purchased from a breeder (Japan SLC, Shizuoka, Japan). The animals were kept in separate cages with free access to food and water in an air-conditioned environment. All animal experiments were performed in strict accordance with the regulations of the Institutional Animal Care and Use Committee, Osaka City University Medical School, Japan.
Preparation of rhBMP-2-induced BTB grafts within the semitendinosus tendon and surgical ACL reconstruction procedure
The rhBMP-2 was produced and donated by Osteopharma Inc. (Osaka, Japan). It was mixed with phosphate-buffered saline (PBS) and 0.02 % indocyanine green (ICG) at a concentration of 1.25 μg/μl. The rhBMP-2, colored green by the ICG, was injected into the semitendinosus tendon of the rabbits in the experimental group. One-stage ACL reconstruction using the semitendinosus tendon graft injected with rhBMP-2 in the rabbit model was performed as previously described. Briefly, each animal was anaethetised with an intramuscular injection of ketamine hydrochloride (60 mg/kg) and xylazine (10 mg/kg). The left hind limb hair was shaved, and the leg was sterilized with ethanol and draped. Using a medial longitudinal skin incision, the semitendinosus tendon was exposed and ligated with 3–0 nylon suture at four points. In the experimental group, rhBMP-2 was injected at 15 μg per 12 μl at the first and third portions (7-mm width) of the semitendinosus tendon using a microsyringe with a 28-G needle (Ito Corporation, Shizuoka, Japan) (Fig. 1). In the control group, 12 μl of PBS alone without rhBMP-2 was injected into each portion of the tendon. We made single bone tunnels in the femur and tibia with the knee in flexion. Twenty minutes after the rhBMP-2 injection, all ligatures were removed and the injected tendon graft was transplanted to reconstruct the ACL by passing the graft through the bone tunnels (2.5 mm in diameter) in the femur and tibia. The graft was anchored to extraosseous post-screws with 3–0 wire sutures, and the skin incision was closed (Fig. 1). Cast immobilization was performed for two weeks postoperatively in each animal. At four, eight, and 12 weeks postoperatively, the animals were sacrificed and evaluated by histological examination and biomechanical testing.
a RhBMP-2 colored with a green dye was injected with a microsyringe and 28-G needle (arrowheads) into two semitendinosus tendon sites (white arrow) in a rabbit model. b ACL reconstruction was performed using a semitendinosus graft passed through the bone tunnel. The graft was fixed with post-screws (black arrows)
Histological examination
Twenty-four knees (12 in each group) were histologically examined. Each harvested left knee was fixed in 4 % neutral formalin, decalcified in 0.5-M ethylenediaminetetraacetic acid, dehydrated in a graded ethanol series, and embedded in paraffin. Specimens were cut in the oblique sagittal direction to observe the femoral and tibial sides of the bone tunnel, cut to 7-μm thickness with a microtome (Leica Microsystems, Inc., Wetzlar, Germany), and stained with toluidine blue. They were then observed under bright and polarized light microscopy (Keyence Corporation, Osaka, Japan).
Semiquantitative analysis of bone and fibrocartilage formation in the bone tunnel
For quantitative analysis of bone formation in the rhBMP-2-injected tendon graft in the bone tunnels, we measured the areas of new bone, bone marrow, tendon, and fibrocartilage within the box area (1.0 × 1.5 mm) of the bone tunnel. The box area was established at the centre of the grafted tendon in the bone tunnel beside the tunnel wall. The percentage of each area and the cancellous bone were measured using computer software (ImageJ ver. 1.48; NIH, Bethesda, MA).
Biomechanical testing
Thirty knees (15 in each group) underwent biomechanical testing. The frozen knee specimens were thawed overnight at 4 °C. After removal of the screws and sutures used to fix the grafts, the femur–tibia–tendon graft complexes were embedded in acrylic resin (Ostron II; GC Corporation, Tokyo, Japan), which was used to fix the specimens to a material testing machine (Shimazu Co., Ltd., Kyoto, Japan). The ultimate grafted-tendon failure load was calculated in both groups. The stiffness was determined from the load–displacement curve.
Statistical analysis
Differences in the biomechanical test results between the experimental and control groups were evaluated using the Wilcoxon signed-rank test with SAS version 9.3 (SAS Institute, Cary, NC). Differences with a p value of <0.05 were considered statistically significant.
Results
Gross appearance and histological examination
On the gross oblique sagittal sections in the experimental group, most of the graft in the bone tunnel had diminished at eight and 12 weeks postoperatively and seemed to be replaced by bony tissue (Fig. 2b). No significant bony tissue was observed in the bone tunnels of the control group (Fig. 2a). No ectopic bone formation was seen in the knee joint tendon in either group.
Gross oblique sagittal section of ACL-reconstructed knee at 12 weeks postoperatively [(a) control group (BMP–), (b) experimental group (BMP+)]. No significant bone formation in the bone tunnels was observed in the control group (arrowheads). Most of the graft at the bone tunnel diminished and seemed to be replaced by bony tissue in the experimental group (arrows)
Histological examination
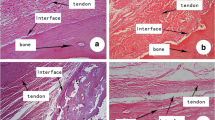
On histological analysis, cartilage tissues in the graft within the bone tunnels were mainly found at four weeks postoperatively in the experimental group (Fig. 3f). More new bone formation in the bone tunnels was found at eight and 12 weeks than at four weeks in the experimental group (Fig. 3g, h). In the experimental group, tidemarks were seen at the interface between the tendon and the rhBMP-2-induced ossicle, which was similar to normal bone–tendon junctions on high-magnification views (Fig. 3i). No cartilage or bone formation was found in the graft tendon within the bone tunnel in the control group (Fig. 3a–d). Polarized light microscopy showed enthesis-like morphology with collagen fibers connecting the bone and tendon in the experimental group (Fig. 3e, j). No ectopic bone formation was seen in the tendon of the knee joint in either group. The results of quantitative analysis of the tendon graft are summarized in Table 1. Postoperatively, the experimental group showed more bone and fibrocartilage formation and less tendon tissue within the box area as time progressed (Fig. 4).
Representative histological findings for oblique sagittal sections of ACL-reconstructed knees at four, eight, and 12 weeks in the control and experimental groups. (a–c) No bone formation was found at four, eight, or 12 weeks in the control group. (f–h) More new bone formation in the bone tunnels was found at eight and 12 weeks than at four weeks in the experimental group. Bar, 1 mm. (d, i) High-magnification views of the boxed area in the columns of (c) and (h), respectively. (i) At 12 weeks, the experimental group showed tidemarks (black arrows) at the interface between the tendon and the rhBMP-2-induced ossicle. T tendon, HB host bone, NB new bone. Bar, 500 μm. (e, j) Polarized light microscopy of the boxed area in (c) and (h), respectively. (e) Tendon grafts were passed through the bone tunnel without direct insertion in the control group. (j) An enthesis-like morphology with collagen fibers connecting bone and tendon was seen in the experimental group
Biomechanical testing to measure the pull-out strength of the reconstructed ACL. a Ultimate failure load at 12 weeks postoperatively was significantly higher than that at four weeks in the experimental group (p < 0.05); however, no significant difference was found between the experimental and control groups at four, eight, or 12 weeks. b Stiffness in the experimental group was significantly greater than that in the control group at 12 weeks (p < 0.05), and stiffness was significantly greater at 12 than at four weeks in the experimental group (p < 0.05)
Biomechanical testing
The mean ultimate grafted-tendon failure loads in the experimental and control groups were 33.5 ± 6.6 and 31.3 ± 13.1 N at four weeks, 38.4 ± 18.3 and 40.9 ± 22.1 N at eight weeks, and 51.1 ± 8.9 and 43.2 ± 15.9 N at 12 weeks, respectively. Although the ultimate failure loads at 12 weeks postoperatively were significantly higher than those at four weeks in the experimental group (p < 0.05), no significant difference was found between the two groups four, eight, and 12 weeks (Fig. 4a). The mean stiffness values in the experimental and control groups were 12.0 ± 4.7 and 13.2 ± 8.1 N at four weeks, 12.2 ± 3.8 and 14.2 ± 9.5 N at eight weeks, and 25.5 ± 8.8 and 10.2 ± 3.9 N at 12 weeks, respectively. Stiffness in the experimental group was significantly greater than that in the control group at 12 weeks (p < 0.05), and stiffness at 12 weeks was significantly greater than that at four weeks in the experimental group (Fig. 4b). Failure in the experimental group specimens occurred predominantly as midsubstance graft rupture or fracture of the graft at the bone–tendon junctions (four of five specimens at four weeks, and all specimens at eight and 12 weeks). However, failure in the control group specimens occurred predominantly as pull-out from the bone tunnels (three of five specimens at four weeks, two of five at eight weeks, and one of five at 12 weeks).
Discussion
In the present study, we showed that one-stage ACL reconstruction using the semitendinosus tendon by direct injection of rhBMP-2 successfully induced bone formation in the tendon and generated a bone–tendon junction similar to that of the normal enthesis at the insertion sites. These findings indicate that tendon grafts can directly generate BTB grafts by injection of rhBMP-2 in one-stage ACL reconstruction. However, the pull-out load was almost the same in both groups.
We previously reported the potential efficacious use of rhBMP-2 to engineer BTB grafting material as a substitute for damaged ACLs in two-stage surgery. In the first stage of the surgery, rhBMP-2 was injected into two semitendinosus tendon sites; in the second stage (six weeks after the first surgery), ACL reconstruction using the semitendinosus tendon with BMP-2-induced ossicles was performed. The experimental group showed histological analysis and biomechanical pull-out test results superior to those of the control group at four and eight weeks postoperatively [11].
For greater clinical applicability, we hypothesized that the current study model would produce the same results as those of our previous study. The experimental results indicate the potential for successful regenerative reconstruction of the ACL with restoration of enthesis-like morphology and stiffness, both of which were significantly greater in the experimental than in the control group at 12 weeks postoperatively (p < 0.05). The current study model may represent an improvement of the weak point of ACL reconstruction in terms of indirect insertion of tendon grafts. However, no significant difference was found in the ultimate failure load between the two groups at four, eight, and 12 weeks.
With respect to the method of ACL reconstruction, tendon grafts reportedly require a greater period of time to re-establish mechanical strength at the graft–tunnel interface than do BTB grafts. Various strategies for accelerating tendon-bone healing with tendon grafts include the use of bone mesenchymal stem cells; synovial mesenchymal stem cells [12, 13]; transforming growth factor-β1 [14]; α2-macroglobulin, which has a blocking effect on synovial matrix metalloproteinase activity [15]; and low-intensity pulsed ultrasound [16]. Previous studies have shown that rhBMP-2 leads to accelerated bone ingrowth into tendon grafts with an associated improvement in tendon attachment strength [17–19]. Ma et al. [20] reported that BMP-2 injected around the periphery of each tunnel surrounding the tendon graft demonstrated a strong effect on osteointegration at the bone–tendon junction in a rabbit model. Although these previous methods reportedly improved the tendon-bone healing of the bone tunnel, they did not replace the tendon with bone at the bone tunnel and regenerate the bone–tendon junction to form a structure similar to the anatomical direct insertion of a normal ACL in the rabbit model. Additionally, enlargement of bone tunnels after ACL reconstruction with the hamstring tendon is a well-documented phenomenon in humans. The rate of enlargement of bone tunnels after ACL reconstruction with tendon grafts ranges from 11.0 to 73.9 % [8, 9]. Tunnel enlargement is reportedly associated with a bungee effect and windshield-wiper motion [21–23] because of the increased working length of the graft. Our method enables reduction of the graft working length and improves its stiffness because of the direct bone-to-bone integration between the rhBMP-2-injected graft and tunnel wall. Therefore, we expect this model to avoid bone tunnel enlargement.
With respect to the mechanical strength at the graft–tunnel interface, Tomita et al. [3] reported that in a dog model, the ultimate failure load of the flexor tendon graft was significantly inferior (45.8 %) to that of the BTB graft at three weeks; however, it was not significantly inferior (85.0 %) to that at six weeks. Mihelic et al. [24] used 25 μg of BMP-7 on an absorbable collagen sponge together with a free tendon graft in bone tunnels. They demonstrated that this low dose of BMP-7 enhanced trabecular bone formation and tendon graft integration in bone tunnels and provided a significantly higher pull-out strength in a sheep model of ACL reconstruction. In the present study, there was a progressive increase in stiffness in the experimental group. Direct bone-to-bone integration between the rhBMP-2-injected graft and tunnel wall might have decreased the graft working length and improved the stiffness in the experimental group [25, 26]. However there was no significant difference in the ultimate load to failure between the two groups. The lack of a significant difference might be explained as follows. First, we suspect that the ectopic bone formation in the tendon graft was fragile because the rupture sites were mainly located at the bone–tendon junctions in the experimental group.
Second, the tendon-bone healing process may differ between BMP-2 and BMP-7, although a previous study [27] on new bone formation of BMP-2 and BMP-7 by ex vivo gene therapy found no significant differences in bone formation between BMP-2 and BMP-7 in a rat model of spinal fusion. Another study [28] reported that BMP-2 induced not only anabolic bone formation, but also catabolic osteoclastic bone resorption. In contrast, Mihelic et al. [24] reported that a low dose of BMP-7 did not induce bone resorption in the bone tunnel. Therefore bisphosphonates should be added to this BMP-2-injected model in future studies.
There are some limitations of this study. First, this study was performed using a rabbit model; therefore, the ACL reconstruction technique differs from that used in humans. The tendon graft was anchored to extraosseous post-screws with 3–0 wire sutures instead of interference screws or Endobuttons. Low initial tension was applied to the tendon graft. Although cast immobilization was performed for two weeks postoperatively in each animal to allow for rest in the early postoperative phase, as in human ACL reconstruction, we could not completely mimic human ACL reconstruction in this study.
Second, the clinical use of BMP is associated with possible side effects and complications. Fortunately, no ectopic or heterotopic bone formation was evident either grossly or histologically in this rabbit model. We could not check for ectopic and heterotopic bone formation after ACL reconstruction with rhBMP-2 on radiographs or micro-computed tomography. Preclinical studies in large animal models are essential to optimize the rhBMP-2 dose for regeneration of the direct insertion without side effects in humans.
In conclusion, we have shown that one-stage ACL reconstruction using the semitendinosus tendon by injection of rhBMP-2 successfully induced the replacement of tendon with bone in the bone tunnel and generated a bone–tendon junction similar to the normal enthesis at the bone–tendon junction. These results indicate the potential usefulness of regenerative ACL reconstruction with restoration of the ACL with regenerated direct insertion morphology while avoiding bone tunnel enlargement. Further studies must be conducted to show the efficacy of rhBMP-2 in large animal models and rhBMP-2 with bisphosphonates in this model.
References
Shino K, Nakata N, Toritsuka Y et al (2005) Anatomically oriented anterior cruciate ligament reconstruction with a bone-patellar tendon-bone graft via rectangular socket and tunnel: a snug-fit and impingement-free grafting technique. Arthroscopy 21(11):1402.e1–1402.e5
Steiner ME, Hecker AT, Brown CH et al (1994) Anterior cruciate ligament graft fixation. Comparison of hamstring and patellar tendon grafts. Am J Sports Med 22(2):240–247
Tomita F, Yasuda K, Mikami S et al (2001) Comparisons of intraosseous graft healing between the double flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy 17:461–476
Beynnon BD, Johnson R, Fleming BC et al (2002) Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. J Bone Joint Surg Am 84:1503–1513
Shaieb MD, Kan DM, Chang SK et al (2002) A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med 30(2):214–220
Feller JA, Webster KE et al (2003) A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med 31:564–573
Kartus J, Magnusson L, Stener S et al (1999) Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc 7:2–8
Clatworthy MG, Annear P, Bulow JU et al (1999) Tunnel widening in anterior cruciate ligament reconstruction. A prospective evaluation of hamstring and patellar tendon grafts. Knee Surg Sports Traumatol Arthrosc 7:138–145
Leonardi ABA, Junior AD, Severino NR (2014) Bone tunnel enlargement on anterior cruciate ligament reconstruction. Acta Orthop Bras 22(5):240–4
Hashimoto Y, Yoshida G, Toyoda H et al (2007) Generation of tendon-to-bone interface “Enthesis” with use of recombinant BMP-2 in a rabbit model. J Orthop Res 25:1415–1424
Hashimoto Y, Naka Y, Fukunaga K, et al. (2011) ACL reconstruction using bone-tendon-bone graft engineered from the semitendinosus tendon by injection of recombinant BMP-2 in a rabbit model. J Orthop Res: 1923–1930
Ju YJ, Muneta T, Yoshimura H et al (2008) Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res 332:469–478
Lui PP-Y, Zhang P, Chan K-M et al (2010) Biology and augmentation of tendon-bone insertion repair. J Orthop Sur Res 5(59):1–14
Yamazaki S, Yasuda K, Tomita F et al (2005) The effect of transforming growth factor-β1 on intraosseous healing of flexor tendon autograft replacement of anterior cruciate ligament in dogs. Arthroscopy 21(9):1034–1041
Demirag B, Sarisozen B, Ozer O et al (2005) Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. J Bone Joint Surg Am 87(11):2401–2410
Walsh WR, Stephens P, Vizesi F et al (2007) Effects of low-intensity pulsed ultrasound on tendon-bone healing in an intra-articular sheep knee model. Arthroscopy 23(2):197–204
Martinek V, Latterman C, Usas A et al (2002) Enhancement of tendon-bone integration of anteriror cruciate ligament grafts with bone morphogenetic protein-2 gene transfer. J Bone Joint Surg Am 84-A:1123–1131
Pecina M, Vukicevic S (2007) Biological aspects of bone, cartilage and tendon regeneration. Int Orthop 31:719–720
Rodeo SA, Suzuki K, Deng X-h et al (1999) Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med 27(4):476–488
Ma CB, Kawamura S, Deng X-H et al (2007) Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing. Am J Sprots Med 35:597–604
Brucker PU, Lorenz S, Imhoff AB (2006) Aperture fixation in arthroscopic anterior cruciate ligament double-bundle reconstruction. Arthroscopy 22(11):1250e, 6
Fu FH, Bennett CH, Ma CB et al (2000) Current trends in anterior cruciate ligament reconstruction. Part II. Operative procedures and clinical correlations. Am J Sports Med 28(1):124–130
L’Insalata JC, Klatt B, Fu FH et al (1997) Tunnel expansion following anterior cruciate ligament reconstruction: a comparison of hamstring and patellar tendon autografts. Knee Surg Sports Traumatol Arthrosc 5:234–238
Mihelic R, Pecina M, Jelic M et al (2004) Bone Morphogenetic protein-7 (Osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sprots Med 32(7):1619–1625
Ishibashi Y, Rudy TW, Livesay GA et al (1997) The effect of anterior cruciate ligament graft fixation site at the tibia on knee stability: evaluation using a robotic testing system. Arthroscopy 13(2):177–182
Woo S-LY, Hollis M, Adams DJ et al (1991) Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med 19(3):217–225
Kaito T, Johnson J, Ellerman J et al (2013) Synergistic effect of bone morphogenetic proteins 2 and 7 by ex vivo gene therapy in a rat spinal fusion model. J Bone Joint Surg Am 95-A:1612–1619
Doi Y, Miyazaki M, Yoshiiwa T et al (2011) Manipulation of the anabolic and catabolic responses with BMP-2 and zoledronic acid in a rat femoral fracture model. Bone 49:777–782
Acknowledgments
We thank Osteopharma Inc. for kindly providing rhBMP. This work was supported by JSPS KAKENHI Grant Number 23791654.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takigami, J., Hashimoto, Y., Yamasaki, S. et al. Direct bone-to-bone integration between recombinant human bone morphogenetic protein-2-injected tendon graft and tunnel wall in an anterior cruciate ligament reconstruction model. International Orthopaedics (SICOT) 39, 1441–1447 (2015). https://doi.org/10.1007/s00264-015-2774-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2774-y