Abstract
Tissue engineering techniques to enhance tendon-bone healing in anterior cruciate ligament (ACL) reconstruction, including stem cells and growth factors/cytokines, are gaining wide acceptance, and their clinical feasibility has also been recognized. Among them, vascular stem cells at the site of ruptured ACL, which have high proliferation and multi-differentiation potential, accelerate tendon-bone healing by enhancing angiogenesis and osteogenesis in human-rat xenotransplantation and canine autologous transplantation model of ACL reconstruction. A pilot clinical study, which used ruptured tissue for ACL reconstruction, indicated reduction of tunnel enlargement despite no improvement in clinical scores. However, for effective clinical application in future, detailed analysis is required regarding enrolled patient demographic parameters, such as age, sex, surgical timing, and type of ACL injury. This chapter highlights effectiveness of vascular stem cells application for early tendon-bone healing in ACL reconstruction, providing an insight for future strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
When an anterior cruciate ligament (ACL) is ruptured, the healing potential is considered to be extremely poor [1, 2]. Therefore, ACL reconstruction has become fairly standardized, with clinical success rates of 70–95 % [3–5]. Anatomical double-bundle (DB) reconstruction procedures using hamstring grafts have recently become widespread with promising results [6–9]. Whereas most surgical procedures in this area require healing and maturation of tendon grafts in a surgically created bone tunnel, the attachment between the tendon and the bone is the weakest region in the early posttransplantation period [10, 11]. In fact, mechanical properties of the healing ligament did not return to normal 1 year after injury in both rabbit and canine models [12, 13]. Therefore, secure fixation of the tendon graft to the bone is a significant factor in allowing earlier and more aggressive rehabilitation and earlier return to sports and work.
Current treatment with hamstring grafts has achieved satisfactory anteroposterior and rotational stability but can cause significant tunnel enlargement [14, 15]. Tunnel widening is believed to be multifactorial in origin. Some mechanical causes are graft motion [15] and stress deviation inside the tunnel and inappropriate location of the graft and tunnel [16]. From a biological aspect, poor healing potential at the tendon-bone interface also results in tunnel enlargement. Large tunnels often require revision ACL surgery and necessitate staged procedures [17]. Therefore, enhancing tendon-bone healing and preventing bone tunnel enlargement are closely related and, therefore, vital in ACL reconstruction.
Tissue engineering techniques with stem cells or growth factors/cytokines have been explored to achieve early healing and better tendon-bone integration [18]. Several animal studies focused on enhancement of tendon-bone healing in ACL reconstruction used periosteum [19, 20], bone marrow stromal cells [21], bone marrow mesenchymal stem cells (MSCs) [20, 22], injectable tricalcium phosphate [23], and other growth factors [24–28]. Although these biological engineering strategies are currently experimental, they are expected to be used in clinical setting in the near future.
2 Blood Vessels as a Potential Target for Tendon-Bone Healing in ACL Reconstruction
Over the last decade, there have been considerable controversies regarding the ACL’s intrinsic healing potential. Some surgeons are of the view that the ACL does not heal without reconstruction due to the lack of blood clot formation, insufficient vascular supply, deficits in intrinsic cell migration, impaired growth factor ability, and effects of synovial fluid on cell morphology [29, 30]. On the other hand, others have reported that the ACL spontaneously heals without surgery [31–33], or only with primary sutures [34–37]. In fact, during acute and subacute arthroscopic procedures for ACL reconstruction, a tibial stump is often visualized that can have connecting fibers to the femur and the tibia or between the posterior cruciate ligament and tibia, suggesting healing potential in ACL fibers. However, there is no scientific evidence till now.
Stem cells’ qualities of high expansion, self-renewal, and multi-differentiation present a reasonable explanation for the healing potential of the ACL. Although some findings show the existence of MSC-like cells in human ACL tissues [38, 39], their origin and characteristics still remain unclear. Recently, blood vessels have been reported to be a richer supply of stem/progenitor cells with expression of CD34 and CD146 surface cell marker [40–43].
Matsumoto et al. demonstrated the presence in subacutely ruptured ACL tissues of CD34-expressing vascular cells with potential for multi-lineage differentiation that can be recruited to the ACL rupture site to support healing [44]. They confirmed the rich vascularity in the ruptured site and septum region when compared with mid-substance using H&E and immunohistochemical vascular staining. In addition, using immunohistochemistry and flow cytometry analysis, they confirmed recruitment of CD34+ and CD146+ cells with multi-lineage differentiation potential to the ruptured site when compared with the gathered cells as the mid-substance (Fig. 42.1a). These cells demonstrated multi-lineage differentiation potential including osteogenesis, adipogenesis, chondrogenesis, and endotheliogenesis (Fig. 42.1b). Covas et al. recently discovered that MSCs and pericytes are similar cells located in the vasculature wall, and they function as cell sources for repair and tissue maintenance [40, 45]. Findings reveal that CD34+ cells are committed not only to endothelial cells but also mural perivascular cells (i.e., pericytes and smooth muscle cells) [46, 47]. Similarly, vascular pericytes with CD146 expression may arise from CD34+ cells [41]. Furthermore, Zengin et al. reported the existence of endothelial progenitor cells and stem cells in a distinct zone between the smooth muscle and the adventitial layer of human adult vascular wall that are capable to differentiate among mature endothelial cells and hematopoietic and local immune cells, such as macrophages [43]. Based on these findings, CD34+ cells with high expansion and multi-differentiation potential in the ACL ruptured site, which were converted into cell population positive for CD146, CD44, CD90, and CD73 expression [44], may have similar characteristics of MSCs described over the last decade [48] and have a possibility to provide an attractive cell source for tissue repair and regeneration.
In vitro experiments showing vascular stem cells in the ACL ruptured tissue
(a) Tissues showing more positive staining for CD34 in the ruptured site than in the mid-substance
(b) CD34-positive cells from ACL ruptured tissue showing multi-lineage differentiation potential including osteogenesis, adipogenesis, chondrogenesis, and endotheliogenesis
Among multi-lineage differentiation potentials, osteogenic and endothelial differentiations are especially important for ligament or tendon-bone healing. There are some reports concerning osteogenesis and angiogenesis/vasculogenesis for ligament or tendon-bone healing. To accelerate osteogenesis and/or angiogenesis for tendon-bone healing, vascular endothelial growth factor (VEGF), granulocyte colony-stimulating factor (G-CSF), transforming growth factor-β (TGF-β), bone morphogenetic protein 2 (BMP2), and BMP7 have recently received attention for their therapeutic potential [27, 28, 49–51]. However, Tei et al. reported that human G-CSF-mobilized peripheral blood CD34+ cells contribute to ligament healing via their endothelial differentiation (vasculogenesis) and enhanced intrinsic angiogenesis by VEGF secretion in a immunodeficient rat model [52]. In addition, Matsumoto et al. showed that peripheral blood CD34+ cells could be differentiated into osteoblasts and endothelial cells in a fracture model [53, 54]. Ratio of CD34+ cells is only 1 % in the peripheral blood cells [53] compared to 44 % [44] in ACL ruptured tissue cells, suggesting that isolation of CD34+ cells from the ACL tissue is less important than that from peripheral blood.
3 Therapeutic Potential of ACL-Derived Vascular Stem Cells or Ruptured Tissue for Tendon-Bone Healing in ACL Reconstruction
Based on the report showing the existence of CD34+ vascular stem cells in ACL ruptured tissue [44], Mifune Y et al. demonstrated that intracapsular transplantation of human ACL-derived CD34+ cells from the ruptured site contributed to tendon-bone healing via angiogenesis/vasculogenesis and osteogenesis in an immunodeficient rat model of ACL reconstruction [55]. Using a molecular approach, they confirmed enhanced intrinsic angiogenesis/osteogenesis and human-derived vasculogenesis/osteogenesis by intracapsular transplantation of human ACL-derived cells. Histological, radiological (CT), and biomechanical assessment exhibited early tendon-bone healing by cell transplantation. Nonselected as well as CD34+ cells contributed to tendon-bone healing and reduction of tunnel enlargement in a rat model of ACL reconstruction.
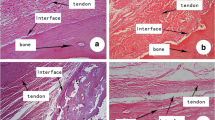
During cell therapy for ACL reconstruction, second-step arthroscopic surgery is unavoidable due to the necessity of cell isolation, cell culture, and cell expansion, thus affecting the clinical feasibility of CD34+ cell transplantation. Based on the rich supply of CD34+ cells in the ACL ruptured site [44] and effectiveness of nonselected cells in a rat ACL reconstruction model [55], Matsumoto et al. explored the effect of ACL ruptured tissue on tendon-bone healing in ACL reconstruction. To explore the feasibility of the use of ruptured tissue in the clinical setting, the study was designed as an autologous transplantation model with a large animal canine [56]. ACL ruptured tissue was harvested 2 days after ACL resection and was sutured to the grafts in the tibial tunnel in ACL reconstruction (Fig. 42.2a). The results in histological, CT, and biomechanical testing showed early tendon-bone healing and reduction of tunnel enlargement compared to control group (no tissue suture) (Fig. 42.2b). These findings may lead to the effectiveness of ruptured tissue in ACL reconstruction in the clinical application.
4 Clinical Application of ACL Ruptured Tissue in ACL Reconstruction
Based on previous findings, Matsumoto and Kuroda et al. compare 2-year clinical outcomes and tunnel enlargement of DB ACL reconstruction with and without suturing of the autologous ruptured tissue to the grafts in patients with subacute ACL injury (Fig. 42.3) [57]. In this study, 10 patients with subacute (within 3 months after injury) ACL rupture were randomly allocated to undergo DB ACL reconstruction with suturing of the ruptured tissue to hamstring grafts or conventional DB ACL reconstruction in two equal control groups (n = 5 each). The results showed significant reduction in tunnel enlargement as assessed with 3D-MDCT in the tissue group, especially at the femoral side. However, the postoperative Lysholm score, anterior stability of the knee measured with the KT-1000 arthrometer, and rate of negative manual pivot shift test did not differ significantly between the two groups. In several animal studies, the use of periosteum [19, 20], bone marrow mesenchymal stem cells [20, 22], injectable tricalcium phosphate [23], and growth factor [26–28] was reported to enhance tendon-bone healing in ACL reconstruction. Among those, application for human ACL reconstruction was only limited to periosteum with promising results [58–60]. Chen CH et al. reported after their 2–7-year clinical follow-up in 312 patients that satisfactory results could be achieved with the periosteum-enveloping hamstring tendon graft in single-bundle ACL reconstruction with minimal tunnel widening (more than 1 mm tunnel widening: 5.4 % of femoral and 6.1 % of tibial side). Considering this comparison, concept for the treatment is similar and successful tunnel reduction was found on radiographs [58]. If the strategy using ruptured tissue has advantages over previous strategies for enhancement of tendon-bone healing, the ruptured ACL tissue can be used with easy clinical settings without any additional incision, procedure, and cell isolation and expansion.
Preservation of the remnant ACL reconstruction has recently received attention focused on the existence of mechanoreceptors in the ACL remnant that contribute to the proprioceptive function of the ACL [61–65]. However, the intrinsic healing potential of ACL remnants after ACL reconstruction has not been fully investigated. In the pilot study based on a previous series [44, 55, 56], the rupture site of the ACL remnant was harvested and transplanted to the grafts to augment healing, especially at the tendon-bone integration site. This technique is reliable, simple, surgeon-friendly, and inexpensive, and thus clinically feasible.
To predict outcomes of ACL reconstruction surgery, the characteristics of patients should be considered. Uefuji et al. recently reported that ruptured ACL remnants have a healing potential with multi-lineage differentiation, including osteogenesis and endotheliogenesis; however, this potential is age dependent and decreases with age, as CD34+ cells were more prevalent in the ACL remnants in younger patients [66]. ACL remnants in younger patients exhibited high proliferation and great multi-lineage differentiation potential, especially in osteogenic and endothelial differentiation. Furthermore, with the use of in vivo rat ACL reconstruction model, Nakano et al. reported that the healing potential of human ACL-derived cells on the maturation of tendon-bone healing is dependent on the patient’s age [67]. Considering these evidences, patient age can be one of the factors that influence postoperative outcomes in healing potential for ACL-derived cells or remnant. In the near future, other demographic factors such as interval between injury and surgery, sex, type of injury, and patient activity level should be assessed to explore other factors that impact ACL remnant-derived cells in the healing potential of reconstructed ACL.
References
Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE (2005) Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med 33(10):1579–1602. doi:10.1177/0363546505279913
Sundar S, Pendegrass CJ, Blunn GW (2009) Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater 88(1):115–122. doi:10.1002/jbm.b.31157
Aglietti P, Giron F, Buzzi R, Biddau F, Sasso F (2004) Anterior cruciate ligament reconstruction: bone-patellar tendon-bone compared with double semitendinosus and gracilis tendon grafts. A prospective, randomized clinical trial. J Bone Joint Surg Am 86-A(10):2143–2155. doi:86/10/2143 [pii]
Bach BR Jr, Tradonsky S, Bojchuk J, Levy ME, Bush-Joseph CA, Khan NH (1998) Arthroscopically assisted anterior cruciate ligament reconstruction using patellar tendon autograft. Five- to nine-year follow-up evaluation. Am J Sports Med 26(1):20–29
Beynnon BD, Johnson RJ, Fleming BC, Kannus P, Kaplan M, Samani J, Renstrom P (2002) Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. A prospective, randomized study. J Bone Joint Surg Am 84-A(9):1503–1513
Fu FH, Shen W, Starman JS, Okeke N, Irrgang JJ (2008) Primary anatomic double-bundle anterior cruciate ligament reconstruction: a preliminary 2-year prospective study. Am J Sports Med 36(7):1263–1274. doi:10.1177/0363546508314428
Fujita N, Kuroda R, Matsumoto T, Yamaguchi M, Yagi M, Matsumoto A, Kubo S, Matsushita T, Hoshino Y, Nishimoto K, Araki D, Kurosaka M (2011) Comparison of the clinical outcome of double-bundle, anteromedial single-bundle, and posterolateral single-bundle anterior cruciate ligament reconstruction using hamstring tendon graft with minimum 2-year follow-up. Arthroscopy 27(7):906–913. doi:10.1016/j.arthro.2011.02.015
Muneta T, Koga H, Mochizuki T, Ju YJ, Hara K, Nimura A, Yagishita K, Sekiya I (2007) A prospective randomized study of 4-strand semitendinosus tendon anterior cruciate ligament reconstruction comparing single-bundle and double-bundle techniques. Arthroscopy 23(6):618–628. doi:10.1016/j.arthro.2007.04.010
Yasuda K, Kondo E, Ichiyama H, Kitamura N, Tanabe Y, Tohyama H, Minami A (2004) Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts. Arthroscopy 20(10):1015–1025. doi:10.1016/j.arthro.2004.08.010
Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH (1989) Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res 7(4):474–485. doi:10.1002/jor.1100070404
Grana WA, Egle DM, Mahnken R, Goodhart CW (1994) An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med 22(3):344–351
Weiss JA, Woo SL, Ohland KJ, Horibe S, Newton PO (1991) Evaluation of a new injury model to study medial collateral ligament healing: primary repair versus nonoperative treatment. J Orthop Res 9(4):516–528
Woo SL, Inoue M, McGurk-Burleson E, Gomez MA (1987) Treatment of the medial collateral ligament injury. II: structure and function of canine knees in response to differing treatment regimens. Am J Sports Med 15(1):22–29
Fauno P, Kaalund S (2005) Tunnel widening after hamstring anterior cruciate ligament reconstruction is influenced by the type of graft fixation used: a prospective randomized study. Arthroscopy 21(11):1337–1341. doi:S0749-8063(05)01186-2 [pii] 10.1016/j.arthro.2005.08.023
L’Insalata JC, Klatt B, Fu FH, Harner CD (1997) Tunnel expansion following anterior cruciate ligament reconstruction: a comparison of hamstring and patellar tendon autografts. Knee Surg Sports Traumatol Arthrosc 5(4):234–238
Ekdahl M, Nozaki M, Ferretti M, Tsai A, Smolinski P, Fu FH (2009) The effect of tunnel placement on bone-tendon healing in anterior cruciate ligament reconstruction in a goat model. Am J Sports Med 37(8):1522–1530. doi:0363546509332503 [pii] 10.1177/0363546509332503
Getelman MH, Friedman MJ (1999) Revision anterior cruciate ligament reconstruction surgery. J Am Acad Orthop Surg 7(3):189–198
Petrigliano FA, McAllister DR, Wu BM (2006) Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy 22(4):441–451. doi:S0749-8063(06)00109-5 [pii] 10.1016/j.arthro.2006.01.017
Chen CH, Chen WJ, Shih CH, Yang CY, Liu SJ, Lin PY (2003) Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: a biomechanical and histologic study in rabbits. Arthroscopy 19(3):290–296. doi:10.1053/jars.2003.50014
Karaoglu S, Celik C, Korkusuz P (2009) The effects of bone marrow or periosteum on tendon-to-bone tunnel healing in a rabbit model. Knee Surg Sports Traumatol Arthrosc 17(2):170–178. doi:10.1007/s00167-008-0646-3
Ouyang HW, Goh JC, Lee EH (2004) Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med 32(2):321–327
Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH (2004) Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy 20(9):899–910. doi:10.1016/j.arthro.2004.06.035
Huangfu X, Zhao J (2007) Tendon-bone healing enhancement using injectable tricalcium phosphate in a dog anterior cruciate ligament reconstruction model. Arthroscopy 23(5):455–462. doi:10.1016/j.arthro.2006.12.031
Anderson K, Seneviratne AM, Izawa K, Atkinson BL, Potter HG, Rodeo SA (2001) Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med 29(6):689–698
Nakase J, Kitaoka K, Matsumoto K, Tomita K (2010) Facilitated tendon-bone healing by local delivery of recombinant hepatocyte growth factor in rabbits. Arthroscopy 26(1):84–90. doi:10.1016/j.arthro.2009.06.029
Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF (1999) Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med 27(4):476–488
Sasaki K, Kuroda R, Ishida K, Kubo S, Matsumoto T, Mifune Y, Kinoshita K, Tei K, Akisue T, Tabata Y, Kurosaka M (2008) Enhancement of tendon-bone osteointegration of anterior cruciate ligament graft using granulocyte colony-stimulating factor. Am J Sports Med 36(8):1519–1527. doi:10.1177/0363546508316282
Yoshikawa T, Tohyama H, Katsura T, Kondo E, Kotani Y, Matsumoto H, Toyama Y, Yasuda K (2006) Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med 34(12):1918–1925
Frank CB, Jackson DW (1997) The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am 79(10):1556–1576
Murray MM, Martin SD, Martin TL, Spector M (2000) Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am 82-A(10):1387–1397
Fujimoto E, Sumen Y, Ochi M, Ikuta Y (2002) Spontaneous healing of acute anterior cruciate ligament (ACL) injuries – conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg 122(4):212–216
Kurosaka M, Yoshiya S, Mizuno T, Mizuno K (1998) Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am 80(8):1200–1203
Sandberg R, Balkfors B, Nilsson B, Westlin N (1987) Operative versus non-operative treatment of recent injuries to the ligaments of the knee. A prospective randomized study. J Bone Joint Surg Am 69(8):1120–1126
Drogset JO, Grontvedt T, Robak OR, Molster A, Viset AT, Engebretsen L (2006) A sixteen-year follow-up of three operative techniques for the treatment of acute ruptures of the anterior cruciate ligament. J Bone Joint Surg Am 88(5):944–952. doi:88/5/944 [pii] 10.2106/JBJS.D.02876
Marshall JL, Warren RF, Wickiewicz TL (1982) Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med 10(2):103–107
Marshall JL, Warren RF, Wickiewicz TL, Reider B (1979) The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res 143:97–106
Strand T, Molster A, Hordvik M, Krukhaug Y (2005) Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15–23 years postoperatively. Arch Orthop Trauma Surg 125(4):217–221. doi:10.1007/s00402-004-0766-2
Lee IC, Wang JH, Lee YT, Young TH (2007) Development of a useful technique to discriminate anterior cruciate ligament cells and mesenchymal stem cells-the application of cell electrophoresis. J Biomed Mater Res A 82(1):230–237. doi:10.1002/jbm.a.31163
Lee SY, Miwa M, Sakai Y, Kuroda R, Matsumoto T, Iwakura T, Fujioka H, Doita M, Kurosaka M (2007) In vitro multipotentiality and characterization of human unfractured traumatic hemarthrosis-derived progenitor cells: a potential cell source for tissue repair. J Cell Physiol 210(3):561–566. doi:10.1002/jcp.20890
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3):301–313. doi:S1934-5909(08)00337-8 [pii] 10.1016/j.stem.2008.07.003
Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF (2005) The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol 289(6):C1396–C1407. doi:00168.2005 [pii] 10.1152/ajpcell.00168.2005
Tavian M, Zheng B, Oberlin E, Crisan M, Sun B, Huard J, Peault B (2005) The vascular wall as a source of stem cells. Ann N Y Acad Sci 1044:41–50
Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S (2006) Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133(8):1543–1551. doi:dev.02315 [pii] 10.1242/dev.02315
Matsumoto T, Ingham SM, Mifune Y, Osawa A, Logar A, Usas A, Kuroda R, Kurosaka M, Fu FH, Huard J (2012) Isolation and characterization of human anterior cruciate ligament-derived vascular stem cells. Stem Cells Dev 21(6):859–872. doi:10.1089/scd.2010.0528
Covas DT, Panepucci RA, Fontes AM, Silva WA Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA (2008) Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 36(5):642–654. doi:S0301-472X(08)00005-2 [pii] 10.1016/j.exphem.2007.12.015
Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T (2006) Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation 113(10):1311–1325
Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z (2003) Transdifferentiation of human peripheral blood CD34 + −enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation 108(17):2070–2073. doi:10.1161/01.CIR.0000099501.52718.70 01.CIR.0000099501.52718.70 [pii]
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, Fu FH, Huard J (2002) Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am 84-A(7):1123–1131
Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, Simic P, Bobinac D, Lah B, Legovic D, Vukicevic S (2004) Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med 32(7):1619–1625
Yasuda K, Tomita F, Yamazaki S, Minami A, Tohyama H (2004) The effect of growth factors on biomechanical properties of the bone-patellar tendon-bone graft after anterior cruciate ligament reconstruction: a canine model study. Am J Sports Med 32(4):870–880
Tei K, Matsumoto T, Mifune Y, Ishida K, Sasaki K, Shoji T, Kubo S, Kawamoto A, Asahara T, Kurosaka M, Kuroda R (2008) Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells 26(3):819–830. doi:10.1634/stemcells.2007-0671
Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, Miwa M, Horii M, Hayashi S, Oyamada A, Nishimura H, Murasawa S, Doita M, Kurosaka M, Asahara T (2006) Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol 169(4):1440–1457. doi:10.2353/ajpath.2006.060064
Mifune Y, Matsumoto T, Kawamoto A, Kuroda R, Shoji T, Iwasaki H, Kwon SM, Miwa M, Kurosaka M, Asahara T (2008) Local delivery of granulocyte colony stimulating factor-mobilized CD34-positive progenitor cells using bioscaffold for modality of unhealing bone fracture. Stem Cells 26(6):1395–1405. doi:10.1634/stemcells.2007-0820
Mifune Y, Matsumoto T, Ota S, Nishimori M, Usas A, Kopf S, Kuroda R, Kurosaka M, Fu FH, Huard J (2012) Therapeutic potential of anterior cruciate ligament-derived stem cells for anterior cruciate ligament reconstruction. Cell Transplant 21(8):1651–1665. doi:10.3727/096368912X647234
Matsumoto T, Kubo S, Sasaki K, Kawakami Y, Oka S, Sasaki H, Takayama K, Tei K, Matsushita T, Mifune Y, Kurosaka M, Kuroda R (2012) Acceleration of tendon-bone healing of anterior cruciate ligament graft using autologous ruptured tissue. Am J Sports Med 40(6):1296–1302. doi:10.1177/0363546512439026
Matsumoto T, Kuroda R, Matsushita T, Araki D, Hoshino Y, Nagamune K, Kurosaka M (2014) Reduction of tunnel enlargement with use of autologous ruptured tissue in anterior cruciate ligament reconstruction: a pilot clinical trial. Arthroscopy 30(4):468–474. doi:10.1016/j.arthro.2013.12.014
Chen CH, Chang CH, Su CI, Wang KC, Liu HT, Yu CM, Wong CB, Wang IC (2010) Arthroscopic single-bundle anterior cruciate ligament reconstruction with periosteum-enveloping hamstring tendon graft: clinical outcome at 2 to 7 years. Arthroscopy 26(7):907–917. doi:10.1016/j.arthro.2009.11.011
Li H, Jiang J, Wu Y, Chen S (2012) Potential mechanisms of a periosteum patch as an effective and favourable approach to enhance tendon-bone healing in the human body. Int Orthop 36(3):665–669. doi:10.1007/s00264-011-1346-z
Robert H, Es-Sayeh J (2004) The role of periosteal flap in the prevention of femoral widening in anterior cruciate ligament reconstruction using hamstring tendons. Knee Surg Sports Traumatol Arthrosc 12(1):30–35. doi:10.1007/s00167-003-0380-9
Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M (2002) Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand 73(3):330–334. doi:10.1080/000164702320155356
Ochi M, Abouheif MM, Kongcharoensombat W, Nakamae A, Adachi N, Deie M (2011) Double bundle arthroscopic anterior cruciate ligament reconstruction with remnant preserving technique using a hamstring autograft. Sports Med Arthrosc Rehabil Ther Technol 3:30. doi:10.1186/1758-2555-3-30
Ochi M, Adachi N, Deie M, Kanaya A (2006) Anterior cruciate ligament augmentation procedure with a 1-incision technique: anteromedial bundle or posterolateral bundle reconstruction. Arthroscopy 22(4):463 e461–463 e465. doi:10.1016/j.arthro.2005.06.034
Ochi M, Adachi N, Uchio Y, Deie M, Kumahashi N, Ishikawa M, Sera S (2009) A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy 25(2):117–122. doi:10.1016/j.arthro.2008.10.011
Yasuda K, Kondo E, Kitamura N, Kawaguchi Y, Kai S, Tanabe Y (2012) A pilot study of anatomic double-bundle anterior cruciate ligament reconstruction with ligament remnant tissue preservation. Arthroscopy 28(3):343–353. doi:10.1016/j.arthro.2011.08.305
Uefuji A, Matsumoto T, Matsushita T, Ueha T, Zhang S, Kurosaka M, Kuroda R (2014) Age-related differences in anterior cruciate ligament remnant vascular-derived cells. Am J Sports Med 42(6):1478–1486. doi:10.1177/0363546514529092
Nakano N, Matsumoto T, Takayama K, Matsushita T, Araki D, Uefuji A, Nagai K, Zhang S, Inokuchi T, Nishida K, Kuroda R, Kurosaka M (2015) Age-dependent healing potential of anterior cruciate ligament remnant-derived cells. Am J Sports Med 43(3):700–708. doi:10.1177/0363546514561436
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Matsumoto, T., Kuroda, R. (2016). Strategies to Enhance Biological Tendon-Bone Healing in Anterior Cruciate Ligament Reconstruction. In: Ochi, M., Shino, K., Yasuda, K., Kurosaka, M. (eds) ACL Injury and Its Treatment. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55858-3_42
Download citation
DOI: https://doi.org/10.1007/978-4-431-55858-3_42
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55856-9
Online ISBN: 978-4-431-55858-3
eBook Packages: MedicineMedicine (R0)