Abstract
Purpose
Although there are various new scaffold-based techniques for cartilage regeneration it remains unclear up to which defect size they can be used. The present study reports of a cell-free collagen type I gel matrix for the treatment of large cartilage defects of the knee after a two-year follow-up.
Methods
Twenty-eight patients with a mean cartilage defect size of 3.71 ± 1.93 cm² were treated with a cell-free collagen type I gel matrix (CaReS-1S®, Arthro Kinetics AG, Krems/Donau, Austria) via a mini-arthrotomy. Clinical outcome was assessed preoperatively and six weeks as well as six, 12 and 24 months after surgery using various clinical outcome scores (IKDC, Tegner, KOOS, VAS). Cartilage regeneration was evaluated via MRI using the MOCART score.
Results
Seventeen male and 11 female patients with a mean age of 34.6 years were included in this study. Significant pain reduction (VAS) could be noted after six weeks already. Patient activity (IKDC, Tegner) could be significantly improved from 12 months on and nearly reached reported pre-operative values. All subject categories of the KOOS except for symptom (swelling) showed significant improvements throughout the study. Constant significant improvements of the mean MOCART score were observed from 12 months on. MR images did not yield any signs of infection or synovitis. After 24 months a complete defect filling could be noted in 24 out of 28 cases with a mainly smooth surface, complete integration of the border zone and homogenous structure of the repaired tissue.
Conclusion
Cell-free collagen type I matrices appear to be a safe and suitable treatment option even for large cartilage defects of the knee. Results of this study were comparable to the better-established findings for small cartilage defects. Mid- and long-term results will be needed to see if clinical and MR-tomographic outcome can be maintained beyond 24 months.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Possibilities for surgical cartilage repair have evolved rapidly during the last ten years. Various methods have been described in the literature reaching from bone marrow stimulation via microfracture [30] or full thickness osteochondral transfer (OCT) [22] to classic two-staged autologous chondrocyte transplantation (ACT) [2]. The latter has been refined by the supportive use of various matrices produced from different bioactive materials [21, 31]. Cell-free implants, mainly composed of collagen type I, are the most recent additions in today’s cartilage repair portfolio. These implants are logic advancements based on previously-used cell-seeded matrices that were used for matrix-associated autologous chondrocyte transplantation (MACT). Instead of a carrier system for precultivated autologous cells these new implants should serve as structural scaffolds to attract and differentiate chondrocytes in vivo directly after implantation. No previous cartilage biopsy is needed. As a result treatment times are hence much faster and there is no donor-site morbidity, which is quite common after harvesting a considerable amount of cartilage [19]. Moreover, due to European Union regulations as of November 2007 [38] a sufficient proof of efficacy has to be provided in cases of autologous cell transplantation. This act further promoted the use of cell-free implants instead of classic ACT or MACT in order to avoid inadequate bureaucracy and cost explosion.
Besides limited indications, all mentioned techniques have certain disadvantages. While classic ACT tends to fail because of periosteal flap hypertrophy in the long run [23], cell-free implants may detach from the subchondral bone [5] or fail to be colonised adequately. That is why defect size plays an important role in the process of decision-making prior to surgical intervention. It has been demonstrated that MACT techniques may provide excellent clinical results even for defect sizes beyond 5 cm² [8, 26]. Cell-free implants however were first used for relatively small cartilage defects with sizes less than 1 cm² [7]. Given the fact that clinical mid-term results were good to excellent for small defects [28], the first clinical trials like this one were designed to investigate if defects larger than 1 cm² treated with cell-free implants could yield equivocal results in terms of cartilage restoration, pain reduction and patient activity.
The aim of the present study was to evaluate suitability and efficacy of a previously used cell-free collagen type I matrix in the treatment of large cartilage defects of the knee. It was hypothesized that results of this treatment method would be absolutely comparable to that of the small defects investigated earlier.
Materials and methods
This study represents a prospective case series of patients with large cartilage defects of the knee. Written informed consent was obtained from all patients prior to inclusion into the study cohort. All included patients failed conservative treatment and experienced ongoing knee pain limiting their sportive or recreation abilities as well as their quality of life. Inclusion criteria are shown in Table 1.
Twenty-eight patients (11 females, 17 males) with a mean age of 34.6 years (range 18–47) were included in the study. Cartilage defects were located on the medial femoral condyle (n = 18), the lateral femoral condyle (n = 3) or on the retropatellar surface (n = 7).
Patients were assessed pre-operatively, at six weeks as well as six, 12 and 24 months postoperatively using the subjective survey of the International Knee Documentation Committee (IKDC) score [16], the Knee Injury and Osteoarthritis Outcome Score (KOOS) [24], a visual analogue scale (VAS) for pain [11] and the Tegner activity scale [32]. Magnetic resonance imaging (MRI) was performed using a 1.5-Tesla Magnetom Espree Scanner (Siemens AG, Erlangen, Germany) as previously described [7]. MR sequences recorded for coronal, sagittal and transverse slices were as follows: proton density fat suppressed turbospin-echo (320 9 320; thickness 3 mm; repeat time (TR) 3,000 ms; echo time (TE) 37 ms); T1 (384 9 384; thickness 3 mm; TR 411 ms; TE 13 ms); T1-weighed volume-interpolated breath-hold examination (VIBE) (280 9 320; thickness 1.5 mm, TR 16; TE 7); and T2 (512 9 512; thickness 3 mm; TR 460 ms; TE 15 ms). All MR images were assessed by a senior musculoskeletal radiologist and graded using the MOCART score [20].
An a priori power analysis based upon the short- and mid-term results of small cartilage defects published earlier [6, 28] was conducted using a two-tailed t-test for connected samples with a significance level of P ≤ 0.05. A power of 90 % could be reached with n = 13 after six months and n = 8 after 12 months.
All research procedures were in accordance with the Declaration of Helsinki. Approval for this study was obtained from the institutional review board of the University Hospital Marburg (study no. 10/11).
Surgical procedure
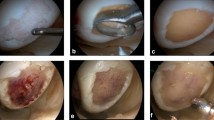
Diagnostic arthroscopy was performed to evaluate the chondral lesion in vivo and to verify that the patient met the inclusion criteria. After verification, a mini-arthrotomy was performed directly over the defect exposing the ulcerated compartment (Fig. 1a). Incisions had to be altered according to exact defect localisation and had to be slightly larger for the retropatellar approach. Defects were punched out using an appropriate cutter and debrided down to the subchondral bone via curettage (Fig. 1b). Damage to the subchondral lamina or bone was carefully avoided in all cases. Five patients were diagnosed with late Osteochondritis dissecans with subsequent ulceration of the subchondral bone. In these cases a thorough debridement of the sclerotic bone had to be performed, and resulting defects were augmented with autologous cancellous bone from the ipsilateral tibial head. Cell-free collagen type I implants were tailored to fit the defect sizes using the same cutting instrument as for defect debridement and measured again prior to implantation (Fig. 1c). After drying the debrided defect with a swab, implants were placed in a press-fit manner without any additional fixation (Fig. 1d). The matrices used (CaReS-1S®, Arthro Kinetics AG, Krems/Donau, Austria), and the surgical procedure have already been described in detail in a previous publication [7]. Femoral condyle defects were treated with 4-mm thick implants whereas retropatellar defects received 6-mm thick implants to address the higher cartilage level in that compartment.
Postoperative rehabilitation included toe-touch weight-bearing for four weeks and limitation to 0° flexion for two days followed by 30° flexion for the next three weeks. Early full weight-bearing and flexion were allowed within the fourth to sixth week. This is in accordance with recently published randomized controlled trials on early versus traditional delayed weight bearing in MACI procedures, suggesting an increased importance of mechanical load for an optimal cartilage regeneration [4, 35].
Statistical analysis
All data were processed with Graphpad Prism 6 (Graphpad Inc., La Jolla, CA). Comparisons between means were made using paired Student’s t tests with a significance level set at P ≤ 0.05 for IKDC, KOOS, VAS and MOCART scores. A nonparametric Wilcoxon test with a significance level set at P ≤ 0.05 was used for the Tegner activity scale. Pearson’s product moment correlation was performed to identify relationships between variables of the MOCART score and IKDC, KOOS, VAS or Tegner activity scale. Graphics were produced using the same software package.
Results
All 28 patients completed clinical and MR-tomographic follow-up at 24 months. Mean defect size was 3.71 ± 1.93 cm² (range 1.20–9.00). No implant-related or surgical complications were recorded.
Clinical outcome
The complete clinical data are listed in detail in Table 2. Consistent pain reduction as measured by a VAS could be recorded starting from six weeks postoperatively. Compared to the pre-operative baseline these significant results could be maintained until the latest follow-up after 24 months (P < 0.0001). The subjective category of the IKDC score showed a slight but significant decrease after six weeks constantly improving until the latest follow-up after 24 months. Significant differences as compared to the pre-operative baseline could be reached and maintained from six months (P = 0.0061) onward until 24 months (P < 0.0001). Activity level as measured by the Tegner score remained unchanged until six weeks postoperatively and then slowly increased again reaching and maintaining significance from six months onward (P = 0.0001). The KOOS score showed global improvements in all except for the symptom category. Significant improvements were reached in the pain and ADL categories from six weeks onward until the latest follow-up at 24 months (P < 0.0001). The sport/rec category showed an initial decrease (P = 0.0075) during the rehabilitation phase, but reached significant improvements as compared to the baseline after six months until the latest follow-up at 24 months (P < 0.0001). The QOL category showed significant improvements from six months onward until the latest follow-up at 24 months (P = 0.0007). The symptom category failed to show significant differences from six months onward until the latest follow-up at 24 months.
Magnetic resonance imaging outcome
The MOCART score showed significant improvements after 12 and 24 months as compared to the first postoperative values right after six months (Fig. 2). After 24 months all defects were filled completely. Only one implant showed a slight hypertrophy. Full integration of the implants into the border zones of the previous defects was recorded in all except four cases. These four implants showed minor skips in at least one slice of the MRI scans. Signal intensity of the repair tissue changed to normal or nearly normal in most cases (Fig. 3). No correlation between the MOCART score and respective clinical scores could be detected.
Discussion
The most important finding of the present study was that results of a repair of large cartilage defects with cell-free implants were perfectly comparable to those of the previously published small defects [7] in terms of MR-tomographic cartilage regeneration, clinical pain reduction and patients’ return to recreational activities after a 24-month follow-up.
Sufficient colonisation of cell-free implants in vivo could be shown in cases of re-arthroscopies with subsequent biopsies, for example, in a study by Schüttler et al. [29]. This case presentation dealt with the same implant type as the present study. Histologic specimens of this collagen type I cell-free scaffold showed an appearance typical of articular cartilage, with neither scaffold leftovers, nor heterotopic intralesional calcification or scar tissue 36 months after implantation. Comparable results have been shown in animal studies using cell-free cartilage scaffolds [25, 27].
It remains unclear up to which defect sizes a treatment with cell-free implants can be successful in clinical practice. Transmigration of vital primary chondrocytes into a collagen scaffold has been successfully demonstrated in an experimental setting already, which showed the bare movement abilities of these cells in a stationary 3D environment [12, 14]. However, an absolute migration distance of chondrocytes kept in a standard culture environment is still unknown. As a consequence there are no standardised critical size cartilage defect models to prove the efficacy of cartilage regenerative procedures. Therefore, it is also difficult to objectively compare different methods with each other or to establish a defect size cut-off for individual methods or implants.
The origin of progenitor cells that make up the repair tissue leading to cartilage regeneration is also not fully understood. These cells may be recruited from the adjacent intact cartilage [15], the subchondral lamina or even out of the synovial fluid [17]. In regards to the surgical technique of the cell-free collagen type I implants it seems unlikely that progenitor cells or MSCs could be recruited from the subchondral lamina, as it ought to be intact during implantation and serves as a barrier to the underlying bone. We reported on five patients with a late Osteochondritis dissecans, which received an augmentation of the ulcerated subchondral bone prior to implantation, but did not notice significant differences in any of the clinical categories as compared to the other patients with intact subchondral laminae. Further studies in an experimental setting would be of use to compare the different cell origins mentioned previously. It will have to be clarified if there is one main source for cell regeneration after implantation of a cell-free scaffold or if there is a complex interaction between the different cell reservoirs.
Additional fixation of gelatinous cell-free implants with fibrin glue, for example, was a major issue during the emergence of this new technique. While first clinical attempts utilized a surgical technique with an additional fibrin glue fixation [1], a biomechanical evaluation by Efe et al. revealed that there is no significant difference between a press-fit technique with or without the addition of fibrin glue for implants with a diameter of 11 mm [5]. Large implants however may be prone to delamination due to more unfavourable mechanical properties (e.g. higher friction) as demonstrated by Vahdati and Wagner in a finite element analysis [33]. In this collective, all implants showed full integration into the defect margins except for four cases in which some small skips could be seen on at least one MRI slice. No relation between defect size and skip formation could be noticed. As there is practically no implant detachment, this strongly fortifies a future fixation-free implantation not least to save costs.
Considerable improvements could be noticed in all clinical scores in the present study. Decreased IKDC scores and Tegner activity levels after six weeks can be explained by the ongoing rehabilitation program with an interdiction of full weight-bearing up to this time point. Close inspection of the KOOS score reveals consistent low results in the symptom category despite improvements in all other categories. This is in accordance with the literature regarding other procedures like the MACT for example [3]. This phenomenon may be explained by a bias, as the KOOS score is a highly subjective interview. A foreign body reaction caused by the collagen type I scaffold seems unlikely, as proceeding implant degradation does not correlate with an improvement in this subject category.
Literature review renders no direct comparison between cell-free cartilage regeneration and other established procedures like microfracturing or OCT for the treatment of large cartilage defects until today. Studies with similar preconditions and defect sizes of the knee using microfracture showed comparable clinical results (IKDC, VAS, Tegner activity scale) after a two-year follow-up in athletes [13]. Microfracture however has long been compared to other procedures. Knutsen et al., for example, compared microfracture against classic ACT with no significant differences after five years. However, a correlation between larger defect sizes and higher failure rates could be seen for microfracture [18]. These results are in accordance with the five-year results of the TIG/ACT/01/2000&EXT Study Group, showing that mid-term results of ACT and microfracture do not differ significantly [34]. Studies investigating OCT also showed comparable clinical results (IKDC, KOOS, VAS and Tegner activity scale) to the present study after a two-year follow-up [9, 10]. Consistency was also noted for the MR-tomographic evaluation although OCT and cell-free implants may not be fully comparable in this category [37].
Clinical (IKDC, KOOS, VAS and Tegner activity scale) two-year results of a comparable collagen-based scaffold colonized with autologous chondrocytes (MACT) surprisingly do not differ as compared to the results of the cell-free scaffold used in the present study. MR-tomographic results (MOCART) also yielded no significant differences [36].
The present study has some limitations. First, two-year results have only been presented until now and the only perspective for this study can be derived from a comparison to the small defects. Mid- and long-term follow-ups are needed to formulate a clear recommendation for the use of cell-free implants in cases of large cartilage defects. The sample size of this study allows significant conclusions but is still very small as compared to large clinical trials. Due to the nature of this study a negative control group is missing. Comparison of the results of cell-free and cell-seeded [26] implants in a matched-pair manner however would be of quite an interest. Unfortunately the study also misses biopsy samples to show the scaffold transformation. For ethical reasons there is no justification to routinely collect biopsies from the regenerative tissue after a certain time. Therefore cases like the one reported by our own workgroup [29] will remain singular examples in the clinical setting.
Conclusion
Cell-free collagen type I matrices appear to be a safe and suitable treatment option even for large cartilage defects of the knee. Results of this study were comparable to the better-established findings for small cartilage defects.
References
Andereya S, Maus U, Gavenis K, Müller-Rath R, Miltner O, Mumme T, Schneider U (2006) First clinical experiences with a novel 3D-collagen gel (CaReS) for the treatment of focal cartilage defects in the knee. Z Orthop Ihre Grenzgeb 144:272–280
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Chiang H, Liao C-J, Hsieh C-H, Shen C-Y, Huang Y-Y, Jiang C-C (2013) Clinical feasibility of a novel biphasic osteochondral composite for matrix-associated autologous chondrocyte implantation. Osteoarthritis Cartilage 21:589–598
Ebert JR, Fallon M, Zheng MH, Wood DJ, Ackland TR (2012) A randomized trial comparing accelerated and traditional approaches to postoperative weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation: findings at 5 years. Am J Sports Med 40:1527–1537
Efe T, Füglein A, Heyse TJ, Stein T, Timmesfeld N, Fuchs-Winkelmann S, Schmitt J, Paletta JRJ, Schofer MD (2012) Fibrin glue does not improve the fixation of press-fitted cell-free collagen gel plugs in an ex vivo cartilage repair model. Knee Surg Sports Traumatol Arthrosc 20:210–215
Efe T, Getgood A, Schofer MD, Fuchs-Winkelmann S, Mann D, Paletta JRJ, Heyse TJ (2012) The safety and short-term efficacy of a novel polyurethane meniscal scaffold for the treatment of segmental medial meniscus deficiency. Knee Surg Sports Traumatol Arthrosc 20:1822–1830
Efe T, Theisen C, Fuchs-Winkelmann S, Stein T, Getgood A, Rominger MB, Paletta JRJ, Schofer MD (2012) Cell-free collagen type I matrix for repair of cartilage defects-clinical and magnetic resonance imaging results. Knee Surg Sports Traumatol Arthrosc 20:1915–1922
Enea D, Cecconi S, Busilacchi A, Manzotti S, Gesuita R, Gigante A (2012) Matrix-induced autologous chondrocyte implantation (MACI) in the knee. Knee Surg Sports Traumatol Arthrosc 20:862–869
Erdil M, Bilsel K, Taser OF, Sen C, Asik M (2013) Osteochondral autologous graft transfer system in the knee; mid-term results. Knee 20:2–8
Filardo G, Kon E, Perdisa F, Balboni F, Marcacci M (2014) Autologous osteochondral transplantation for the treatment of knee lesions: results and limitations at two years’ follow-up. Int Orthop 38:1905–1912
Flandry F, Hunt JP, Terry GC, Hughston JC (1991) Analysis of subjective knee complaints using visual analog scales. Am J Sports Med 19:112–118
Frenkel SR, Clancy RM, Ricci JL, Di Cesare PE, Rediske JJ, Abramson SB (1996) Effects of nitric oxide on chondrocyte migration, adhesion, and cytoskeletal assembly. Arthritis Rheum 39:1905–1912
Gobbi A, Karnatzikos G, Kumar A (2014) Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc 22:1986–1996
Gosiewska A, Rezania A, Dhanaraj S, Vyakarnam M, Zhou J, Burtis D, Brown L, Kong W, Zimmerman M, Geesin JC (2001) Development of a three-dimensional transmigration assay for testing cell-polymer interactions for tissue engineering applications. Tissue Eng 7:267–277
Hunziker EB, Schenk RK (1989) Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol 414:55–71
Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD (2001) Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 29:600–613
Kim YS, Lee HJ, Yeo JE, Kim YI, Choi YJ, Koh YG (2014) Isolation and characterization of human mesenchymal stem cells derived from synovial fluid in patients with osteochondral lesion of the talus. Am J Sports Med 43(2):399–406. doi:10.1177/0363546514559822
Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 89:2105–2112
LaPrade RF, Botker JC (2004) Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy 20:69–73
Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S (2006) Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 57:16–23
Niemeyer P, Salzmann G, Feucht M, Pestka J, Porichis S, Ogon P, Südkamp N, Schmal H (2014) First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop 38:2065–2070
Oztürk A, Ozdemir MR, Ozkan Y (2006) Osteochondral autografting (mosaicplasty) in grade IV cartilage defects in the knee joint: 2- to 7-year results. Int Orthop 30:200–204
Pelissier A, Boyer P, Boussetta Y, Bierry G, Van Hille W, Hamon P, Jaeger JH, Massin P (2014) Satisfactory long-term MRI after autologous chondrocyte implantation at the knee. Knee Surg Sports Traumatol Arthrosc 22:2007–2012
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 28:88–96
Schagemann JC, Erggelet C, Chung H-W, Lahm A, Kurz H, Mrosek EH (2009) Cell-laden and cell-free biopolymer hydrogel for the treatment of osteochondral defects in a sheep model. Tissue Eng A 15:75–82
Schneider U, Rackwitz L, Andereya S, Siebenlist S, Fensky F, Reichert J, Löer I, Barthel T, Rudert M, Nöth U (2011) A prospective multicenter study on the outcome of type I collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med 39:2558–2565
Schneider U, Schmidt-Rohlfing B, Gavenis K, Maus U, Mueller-Rath R, Andereya S (2011) A comparative study of 3 different cartilage repair techniques. Knee Surg Sports Traumatol Arthrosc 19:2145–2152
Schüttler KF, Schenker H, Theisen C, Schofer MD, Getgood A, Roessler PP, Struewer J, Rominger MB, Efe T (2013) Use of cell-free collagen type I matrix implants for the treatment of small cartilage defects in the knee: clinical and magnetic resonance imaging evaluation. Knee Surg Sports Traumatol Arthrosc 22:1270–1276
Schüttler KF, Struewer J, Rominger MB, Rexin P, Efe T (2013) Repair of a chondral defect using a cell free scaffold in a young patient—a case report of successful scaffold transformation and colonisation. BMC Surg 13:11
Steadman JR, Rodkey WG, Briggs KK (2002) Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg 15:170–176
Steinwachs M (2009) New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy 25:208–211
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49
Vahdati A, Wagner DR (2013) Implant size and mechanical properties influence the failure of the adhesive bond between cartilage implants and native tissue in a finite element analysis. J Biomech 46:1554–1560
Vanlauwe J, Saris DBF, Victor J, Almqvist KF, Bellemans J, Luyten FP (2011) Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med 39:2566–2574
Wondrasch B, Risberg M-A, Zak L, Marlovits S, Aldrian S (2015) Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle: a prospective, randomized controlled study presenting MRI-based and clinical outcomes after 5 years. Am J Sports Med 43:146–153
Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, Marlovits S, Aldrian S (2014) Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold: an analysis of clinical and radiological data. Am J Sports Med 42:1618–1627
Zak L, Krusche-Mandl I, Aldrian S, Trattnig S, Marlovits S (2014) Clinical and MRI evaluation of medium- to long-term results after autologous osteochondral transplantation (OCT) in the knee joint. Knee Surg Sports Traumatol Arthrosc 22:1288–1297
Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Off J Eur Union 324:121–137
Conflict of interest
Magnetic resonance imaging was supported by a research fund of Arthro Kinetics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roessler, P.P., Pfister, B., Gesslein, M. et al. Short-term follow up after implantation of a cell-free collagen type I matrix for the treatment of large cartilage defects of the knee. International Orthopaedics (SICOT) 39, 2473–2479 (2015). https://doi.org/10.1007/s00264-015-2695-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2695-9