Abstract
Immune-checkpoint inhibitors (ICI) are monoclonal antibodies which target molecules to enhance antitumor response. Several adverse events have been described and the major ICI-related endocrinopathies are thyroid dysfunction and hypophysitis. Its occurrence has been associated with improved outcomes, but it is still to be proven. We performed a retrospective study of patients treated with ICI between 2014 and 2019 at an oncologic center to characterize thyroid function test abnormalities (TFTA) and to evaluate clinical outcomes. We excluded patients without regular monitoring of thyroid function, with previous thyroid or pituitary disease, previous head/neck radiotherapy and who performed only one ICI cycle. We included 161 of 205 patients treated with pembrolizumab, nivolumab or ipilimumab for several neoplasms, with a median duration of 18.9 weeks (9.1–42.6) of ICI treatment and 49.4 weeks (26.5–75.8) of follow-up. New-onset TFTA was diagnosed in 18% of patients (n = 29), in median at 10.6 weeks (6.1–31.1) of ICI therapy. On the whole, 8.7% had primary hypothyroidism, 4.3% central hypothyroidism, 2.5% biphasic thyroiditis and 2.5% thyrotoxicosis. Patients who experienced primary or central thyroid dysfunction had a significantly improved overall response rate (58.6% vs 34.2%, p = 0.015) and overall survival (3.27 vs 1.76 years, p = 0.030), compared to the control group. The risk of mortality was two times higher for control group (adjusted HR = 2.43, 95% CI 1.13–5.23, p = 0.023). This study recognizes that primary and central thyroid dysfunction can be a predictive clinical biomarker of a better response to ICI across several neoplasms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
PRÉCIS
Adverse events caused by immune-checkpoint inhibitors (ICI) have been associated with increased survival. This is the first study showing that thyroid dysfunction can be a predictive biomarker of a better response to ICI across several neoplasms.
Despite the known role of the immune system in cancer treatment for several decades, the discovery and development of immune checkpoint inhibitors (ICI) in recent years have been the landmark of the success of cancer immunotherapy [1]. ICI are monoclonal antibodies (Abs) which target molecules (immune checkpoint receptors) and inhibit T-cell activation, enhancing endogenous antitumor response of a broad spectrum of cancers [1,2,3]. Several ICI have been approved in the last few years: cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors as ipilimumab, programmed cell death protein 1 (PD-1) inhibitors as pembrolizumab and nivolumab, and programmed cell death ligand 1 (PD-L1) inhibitors as atezolizumab, avelumab and durvalumab. To date, anti-PD-1 and anti-PD-L1 Abs are approved by Food and Drug Administration (FDA) for treatment of melanoma, non-small-cell lung cancer (NSCLC), renal cell carcinoma, urothelial carcinoma, head and neck squamous cell carcinoma, classical Hodgkin’s lymphoma, primary mediastinal large B-cell lymphoma, microsatellite instability-high (MSI-H) tumours, esophageal and gastric cancer and others [4]. Ipilimumab is approved for melanoma (as monotherapy and combined with nivolumab) and for renal cell carcinoma in combination with nivolumab [4].
Although ICI therapy is usually well tolerated, it can induce immune-related adverse events (irAEs), which vary from mild to fatal [5,6,7]. As immunotherapy has been increasingly used, irAEs are becoming more common. The most prevalent toxicities are dermatological (25.6–44.0%) and gastrointestinal (30.0–40.0%) [8,9,10,11,12]. Endocrine irAEs are reported in 12.0–30.0% of patients [5, 7, 13, 14] and they are usually mild to moderate [14, 15]. The most common endocrine irAE are thyroid dysfunction and hypophysitis, especially in patients treated with anti-PD1 and anti-CTLA-4 agents, respectively [5, 14, 16]. A wide range of incidence rates of primary thyroid dysfunction has been published, probably given the variable assessment methods, ranging from 9.0 to 39.1% in retrospective studies [6, 7, 12, 17,18,19,20] and from 3.2 to 18.9% in prospective studies [13, 14, 21]. Primary thyroid dysfunction specifically due to anti-PD1 agents has been reported to occur in 14.3–39.1% of patients in retrospective studies [6, 7, 17, 20] and 7.1–9.0% in prospective studies [13, 14]. Hypophysitis occurs in 11.0–18.0% of patients treated with ipilimumab [22,23,24,25]. Primary adrenal insufficiency and autoimmune type 1 diabetes mellitus are rare endocrine irAE [25, 26].
Considering the variable and unpredictable response rates of ICI, some effort has been made to determine which patients would most likely benefit from immunotherapy [27]. The occurrence of an irAE has been associated with improvement of prognosis. To date, two studies have reported increased survival in patients who developed endocrine irAEs [15, 22]. This concept needs to be validated by further studies since the association between occurrence of irAEs and better outcomes is still to be proven.
Materials and methods
Patients
We performed a retrospective analysis of adult patients treated with ICI between March 2014 and September 2019 at a tertiary oncologic center: Portuguese Institute of Oncology of Porto, Portugal. Treatment with anti-PD-1 (pembrolizumab and nivolumab) and anti-CTLA-4 (ipilimumab) Abs was initiated until April 30, 2019.
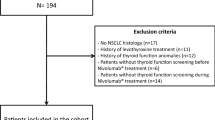
Exclusion criteria were absence of regular monitoring of thyroid function since the initiation of ICI, only one ICI cycle, known thyroid or pituitary disease or surgery, history of head/neck radiation therapy or treatment with levothyroxine, antithyroid drugs or amiodarone.
Two groups of patients were considered: patients with thyroid function test abnormalities (TFTA) and patients with no TFTA abnormalities (considered as the control group, due to the similar baseline sample characteristics between the two groups). The TFTA group included patients with one of the following abnormalities: primary hypothyroidism, thyrotoxicosis, biphasic thyroiditis and central hypothyroidism.
Previous and current clinical data were examined independently by two Endocrinologists and the imaging exams were revised by two Nuclear Medicine Physicians. The clinical severity of TFTA was graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Delayed immune-related event (DIRE) was considered when an irAE manifested later than 90 days after discontinuation of immunotherapy.
Objectives
The aim of this study was to assess the incidence of TFTA in patients treated with ICI with no previous thyroid or pituitary disease. We analyzed the time of onset, clinical course and severity of TFTA and evaluated the impact of TFTA in these patients’ survival.
Endocrine assessments
Data on thyroid stimulating hormone (TSH) and free thyroxine (fT4) levels were retrieved from laboratory records at baseline and during ICI therapy (every treatment visit or commonly at most every four weeks). Data on free triiodothyronine (fT3) levels and the absence or presence of anti‐thyroid peroxidase (anti-TPO) Abs, anti‐thyroglobulin (anti-Tg) Abs and thyrotropin receptor Abs (TRAbs) were collected when available. All measurements were performed in the same laboratory. Measurement of TSH, fT4, fT3 and thyroid Abs (anti‐Tg, anti-TPO and TRAbs) were performed using electrochemiluminescent bridging immunoassay (ECLIA) (Cobas 8000 e801, Hitachi).
Considering the laboratory cut-offs, primary hypothyroidism was defined as isolated increased TSH levels (> 4.2 mUI/L) with normal (subclinical form) or decreased (overt form) free fraction of thyroid hormones (normal range: fT4 0.93–1.7 ng/dL, fT3 2.0–4.4 pg/mL). Thyrotoxicosis was defined as TSH < 0.10 mUI/L with normal (subclinical form) or increased (overt form) fT4 and/or fT3. Cases of a transient thyrotoxicosis followed by hypothyroidism were defined as biphasic thyroiditis and they were not included in the events of primary hypothyroidism or thyrotoxicosis. Central hypothyroidism was defined as decreased free thyroid fractions with decreased or inappropriate normal TSH levels. Concomitant endocrine irAEs were also collected. Central hypocortisolism was defined as decreased cortisol levels with decreased or inappropriate normal ACTH levels in patients with no history of glucocorticoid therapy. Diagnoses were based on integrated clinical and biochemical data.
Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR: quartile 1–quartile 3) and categorical variables as frequencies and percentages. Mann–Whitney U test was performed for non‐parametric continuous variables to compare two groups. We used the Kruskal–Wallis one‐way test to compare more than two groups, and Fisher’s or Chi-square test was used to study differences between categorical variables.
Follow-up period was defined as the time between the date of first ICI cycle and September 30, 2019 or date of death. The event for overall survival was defined as death by all causes. Patients alive at the end of the study were classified as censored. Survival analysis was performed using the Kaplan–Meier method. Log-rank test was used to identify significant survival curves disparities. Cox proportional hazards regression modelling was used to evaluate associations between the occurrence of TFTA and the outcome of all-cause death. It was adjusted for the following clinical covariates: sex (male vs female), age (< 40 vs 40–65 vs 65–85 years old), primary neoplasm (melanoma vs lung cancer vs head and neck cancer vs classical Hodgkin’s lymphoma vs urothelial cancer), stage of disease (III vs IV) and ICI agent (pembrolizumab vs nivolumab vs ipilimumab).
All analyses were performed using IBM-SPSS version 25 and, for all, a level of significance α = 0.05 was noted.
Results
Patient characteristics
We studied the records of 205 patients, from which 161 were included (Fig. 1). The median age was 65.0 years old (IQR 57.5–72.0) at the first cycle of ICI and 67.1% were male. Most patients had melanoma (51.6%, n = 83) and lung cancer (42.9%, n = 69). Other cancer sites included classical Hodgkin’s lymphoma (n = 3), urothelial carcinoma (n = 3) and head and neck cancer (n = 3). The majority of patients (95.0%, n = 153) were on monotherapy, while eight patients had sequential scheme with anti-PD-1/CTLA-4 agents: four with pembrolizumab and ipilimumab and four with nivolumab and ipilimumab. On the whole, 86 patients were treated with pembrolizumab, 59 with nivolumab and 25 with ipilimumab. The doses administered were according to current oncology protocols and indication to continue treatment was evaluated based on imaging response and adverse events. Median total duration of ICI treatment was 18.9 weeks (9.1–42.6) and total follow‐up time was 49.4 weeks (26.5–75.8).
Thyroid function test abnormalities
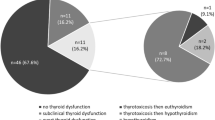
New-onset TFTA was diagnosed in 18.0% (n = 29) of patients on ICI therapy, at median age of 65.0 years old (53.5–73.5) and 55.2% were male. TFTA occurred after a median of 10.6 weeks (6.1–31.1) from onset of treatment and it occurred most frequently after the fourth cycle.
Detailed characterization of patients who developed TFTA is noted in Supplementary Table 1. Almost half of patients (n = 14) had primary hypothyroidism (ten with overt and four with subclinical hypothyroidism) and most of them were asymptomatic at presentation. Four patients spontaneously recovered with no need for therapy and eight started treatment with levothyroxine. Central hypothyroidism due to hypophysitis occurred in seven patients and four were symptomatic. Due to grade 3 of clinical severity of this event, ICI treatment was discontinued in patient 17. Three patients [15,16,17] had concomitant central hypocortisolism. The most severe event needed high-dose pulse intravenous steroids for one week followed by switch to oral hydrocortisone (patient 17). The other two (patients 15 and 16) began immediately treatment with oral hydrocortisone, before replacement with levothyroxine. No patient has recovered from hypopituitarism at the end of the follow-up period. Patient 15, who presented with both central hypothyroidism and hypocortisolism, is considered a DIRE.
Thyrotoxicosis and biphasic thyroiditis occurred in the same proportion (13.8%, n = 4, each). In most patients, anti-TPO and anti-Tg positivity was confirmed and none had positive levels of TRAbs. From patients with thyrotoxicosis, one spontaneously resolved (patient 22) and only one patient was symptomatic (patient 25). Two patients began treatment with beta-blocker (BB) and methimazole. Regarding four cases of biphasic thyroiditis, in which thyrotoxicosis was followed by permanent hypothyroidism, all patients remained asymptomatic but needed to be treated with levothyroxine.
TFTA events were most frequently classified as grade 2 according to the CTCAE and the most severe was 3 in a patient treated with ipilimumab. In two patients (5 and 19), diagnosed with primary and central hypothyroidism, their clinical presentation and TFTA severity are unknown and treatment with levothyroxine was not started due to death from progressive disease shortly after their hospitalization.
Regarding patients with primary thyroid dysfunction, five performed neck ultrasound and pattern of thyroiditis was observed in two cases of biphasic thyroiditis and one case of thyrotoxicosis (patients 23, 28 and 29). 18Fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET/CT) scan, performed for treatment response evaluation, showed bilateral elevated thyroid gland uptake in one of three patients with primary hypothyroidism (patient 1, 11 weeks after diagnosis). Differently, the two patients without increased thyroid uptake (11 and 14) performed 18FDG-PET/CT more than 1 year after the diagnosis. The only patient with thyrotoxicosis who underwent 18FDG-PET/CT (patient 23) did not show any uptake in the thyroid 9 weeks after the diagnosis. Concerning central hypothyroidism, five patients performed pituitary magnetic resonance imaging (MRI) and three patients underwent 18FDG-PET/CT (including those who did not perform MRI). No patient presented mild or moderate diffuse pituitary enlargement in MRI or new focally elevated uptake in the pituitary gland in 18FDG-PET/CT.
Characterization of TFTA events is shown in Table 1. All patients who developed TFTA were on monotherapy. The majority of these (79.0%, n = 23) occurred in patients treated with anti-PD-1 agents, in which pembrolizumab was responsible for 13 and nivolumab for 11 events. Ipilimumab only induced central hypothyroidism (all with concomitant hypocortisolism) and primary hypothyroidism, since thyrotoxicosis and biphasic thyroiditis were caused only by anti-PD1 agents. There were no differences regarding incidence of TFTA events between the three ICI agents (pembrolizumab 15.3% vs nivolumab 18.6% vs ipilimumab 20.0%). Comparing anti-PD-1 with anti-CTLA-4 agents, there were also no differences regarding incidence of all TFTA (16.5% vs 20.0%, p = 0.668) and incidence of primary thyroid dysfunction (13.8% vs 8.0%, p = 0.426). Patients treated with ipilimumab had a higher incidence of central hypothyroidism than the ones treated with anti-PD-1 agents (12.0% vs 2.8%, p = 0.034).
Outcomes
The demographic characteristics of TFTA and control groups are reported in Table 2. There were no differences regarding the baseline clinical characteristics (patients’ age and gender, ICI, primary cancer and its stage) between the two groups. However, patients that experienced TFTA underwent a significant higher number of ICI cycles (p = 0.017) and had a significantly higher response rate (58.6% vs 34.2%, p = 0.015), compared to the control group.
During the follow-up period, 77 deaths occurred. Mean overall survival after the initiation of ICI treatment was 2.42 years. A significantly longer mean overall survival was found to be higher in TFTA group, compared to the control (3.26 years vs 1.76 years, p = 0.030)—Kaplan–Meier analysis is displayed on Fig. 2. Age, sex, primary neoplasm and cancer staging at ICI initiation were not associated with overall survival. Control group had a risk of death approximately two times higher, compared to TFTA group [adjusted hazard ratio (HR) = 2.43, 95% confidence interval (CI) 1.13–5.23, p = 0.023]. This analysis also showed that treatment with nivolumab was associated with a 64% decrease of risk of death, compared to pembrolizumab (adjusted HR = 0.36, 95% CI 0.15–0.85, p = 0.019). Cox proportional hazards regression modelling details are listed on Table 3.
Discussion
Thyroid function test abnormalities
Our study included 161 patients treated with anti-PD1 or anti-CTLA-4 agents for several neoplasms, in which new onset TFTA occurred in 18% of the cases. There are few studies on endocrine irAEs with similar design study, as the majority focuses on a specific ICI agent or class [6, 7, 15, 17] or on a specific primary neoplasm [13, 14, 19, 20]. Patel et al. reported an incidence of new onset TFTA of 35%, concerning patients treated with pembrolizumab, nivolumab or ipilimumab for several malignancies [18]. Despite this disparity of TFTA incidence, its incidence rate could be overestimated due to different baseline sample characteristics, namely inclusion of patients under combined ICI therapy and absence of other exclusion criteria besides history of total thyroidectomy. In fact, 64.5% patients on combined nivolumab and ipilimumab therapy developed TFTA. Although both sequential and combination ICI therapy were reported as independent risk factors for hypophysitis and thyroid dysfunction compared to monotherapy (HR = 2.27, 95% CI 1.09–4.70, p = 0.028) [13], our study included just eight patients (2.5%) who performed sequential scheme with anti-PD-1/CTLA-4 agents and none on combined ICI therapy. On the other hand, Scott et al., on a recent prospective study, analyzed patients treated with pembrolizumab, nivolumab or ipilimumab for melanoma and reported similar incidence rates of endocrine irAEs compared to our study: global irAEs incidence of 18%, thyroid dysfunction incidence of 14% and hypophysitis incidence of 6% [14].
However, our study shows an incidence of anti-PD-1-induced primary thyroid dysfunction of 13.8%, which is lower than most recent retrospective studies [6, 7, 17, 20], approaching the incidences reported on prospective studies [13, 14]. Central hypothyroidism occurred in 12.0% of patients treated with ipilimumab, which is a significantly higher incidence than those treated with anti-PD-1 Abs, and in accordance with other studies [22,23,24,25].
The most prevalent TFTA was primary hypothyroidism (8.7%), followed by central hypothyroidism (4.3%). Patel et al. reported incidences of 18.4%, 13.5% and 5% for primary hypothyroidism, thyrotoxicosis and central hypothyroidism, respectively [18]. If we consider our cases of biphasic thyroiditis as primary hypothyroidism and thyrotoxicosis events separately, the incidence of each type of TFTA is similar between both studies.
Thyroid dysfunction in cancer patients is difficult to diagnose if we only consider the clinical picture, as symptoms can be confused with the underlying disease or its treatment. Most of hypothyroid patients were regarded as asymptomatic. The time of onset of primary hypothyroidism is variable, usually occurring in 21–39 weeks after ICI initiation, but may develop even later [20]. Our cases of primary hypothyroidism manifested between 3 and 44.9 weeks of treatment with ICI. We report four cases with spontaneous recovery of thyroid function, in which three were subclinical, despite it being reported as uncommon [25]. Although this study is retrospective, these cases were detected because of the strict inclusion criteria with monitoring of thyroid function on a regular basis. This highlights the importance of routine screening of thyroid function during ICI treatment in order to perform an early diagnosis of overt cases and proceed to its correct management.
Regarding thyrotoxicosis, its clinical picture is usually mild and transient [25]. Given the pathogenic mechanism of ICI-induced thyrotoxicosis, treatment with antithyroid drugs does not appear to be adequate, unless GD is the proven etiology [25]. However, it was decided to start treatment with methimazole in two cases due to the maintenance of the symptoms and the chronicity of thyrotoxicosis, and curiously it allowed a clinical and analytical improvement. The four cases of biphasic thyroiditis culminated in permanent hypothyroidism, which is frequent in this condition [26]. Thyrotoxicosis and biphasic thyroiditis are more common with the anti-PD1/PD-L1 agents than with anti-CTLA-4 Abs [26, 28]. All our cases were caused only by anti-PD1 agents.
Endocrine irAEs are considered a late adverse event, as the median time of onset is about 11 weeks [28]. In our study, TFTA events manifested after a median of 10.6 weeks from ICI treatment initiation and most of them within 18 weeks of treatment. The average time of onset specifically of primary thyroid dysfunction events is not completely reported in the studies, often being only described individually. Scott et al. reported a similar median time of onset of primary thyroid dysfunction and hypophysitis [14].
Grades 1 and 2 of severity were similarly distributed between the three ICI. The most severe TFTA event was a grade 3 hypophysitis (patient 17), admitted due to hyponatremia and mental confusion 8.9 weeks after the first cycle of ipilimumab for treatment of melanoma. Hypocortisolism and hypothyroidism were diagnosed and hormonal replacement was promptly given. In literature, ipilimumab-induced hypophysitis is the most frequent grade 3 or 4 endocrine irAE [5]. Primary thyroid dysfunction did not preclude the continuation of immunotherapy, while one case of ipilimumab-induced hypophysitis with central hypothyroidism led to its cessation. Contrary to non-endocrine irAEs that require discontinuation of ICI and usually resolve with immunosuppressive therapy [29], endocrine irAEs frequently do not require ICI cessation, if managed appropriately [16]. All cases of central hypothyroidism were irreversible and required long term management.
Early screening for thyroid dysfunction should assess TSH and fT4 at each treatment visit for at least the first five cycles and thereafter every three months [30, 31]. The authors consider that thyroid axis should be checked during and even after ICI treatment, since TFTA can develop several months to years after ICI initiation. Screening for hypophysis during CTLA-4 or PD-1/PDL-1 inhibitors is not consensual. Monitoring of morning cortisol levels seems to be appropriate if performed every month for the first six months, every three months for the next six months and then annually [32].
Outcomes
This study highlights the significant improved response rate and the longer overall survival of patients who experienced TFTA secondary to ICI treatment across several neoplasms. In recent years, correlation between ICI toxicity and clinical outcomes has been suggested. Das S. and Johnson D. B. reviewed several studies investigating the impact of systemic irAEs in prognosis [27]. The studies included patients treated with a specific ICI for a particular neoplasm. The vast majority identified endocrine events as one of the most common irAEs and showed improved response rate, overall survival and/or progression-free survival in patients who developed irAEs [27]. A systematic meta-analysis by Gomes-Lima et al. reviewed 12 randomized controlled trials comprising a total of 7060 patients with head and neck cancer and lung cancer, of whom 3815 were treated with ICI. Both survival rate and progression-free survival of the treatment arm were enhanced (HR = 0.75, 95% CI 0.70–0.80 and HR = 0.77, 95% CI 0.72–0.81, respectively) and a positive correlation between endocrine irAEs and OS was reported (p = 0.019) [33]. Regarding specifically primary thyroid dysfunction, Peiró et al. found it to be associated with improved overall survival among 55 patients with NSCLC treated with nivolumab (HR = 0.4, 95% CI 0.17–0.94, p = 0.035) [15]. Kim et al. included 58 patients and showed longer overall survival (adjusted HR = 0.11, 95% CI 0.01–0.92) and increased progression-free survival (adjusted HR = 0.38, 95% CI 0.17–0.85) in the thyroid dysfunction group and its severity was associated with durable control rate [34]. Osorio et al. showed that the occurrence of primary thyroid dysfunction in 10 patients with NSCLC treated with pembrolizumab was associated with longer overall survival (unadjusted HR = 0.29, 95% CI 0.09–0.94, p = 0.029) and revealed a numerically but not significantly longer progression-free survival [6]. Concerning hypophysitis, Faje et al. included 154 patients with melanoma treated with ipilimumab and showed a borderline significant increased median survival in patients diagnosed with hypophysitis (p = 0.05) [22]. Several other studies have tried to confirm the hypothesis of improved survival related to the occurrence of an irAE, but failed to reach statistically significant results [17, 19], as these studies imply more numerous samples and higher follow-up periods.
Mechanisms involved in endocrine irAEs have not been fully established [27, 30]. Primary thyroid dysfunction most commonly manifests as a painless thyroiditis that develops within weeks to months after ICI initiation. The presentation may be thyrotoxicosis, which spontaneously resolves to euthyroidism (isolated thyrotoxicosis) or evolves into hypothyroidism (biphasic thyroiditis), or overt or subclinical hypothyroidism, which may be transient or permanent. Acute inflammation followed by destruction of the thyroid gland has been advocated as the typical underlying autoimmune process [30]. Although it has been speculated that PD-1 molecules may regulate not only T lymphocytes (cell mediated immunity) but also B lymphocytes (humoral immune response), evidence suggests that anti-PD-1-induced thyroiditis is essentially a T cell-mediated process with endogenous predominance of CD8+ and CD4–CD8− T lymphocytes in the thyroid gland [35, 36]. Intra-thyroid immune cells produce cytokines, which play a role in the pathogenesis of autoimmune thyroid disease [37]. Alternative mechanisms have been advocated for other irAEs. Pre-existing organ specific antigen expression has been described in ipilimumab-induced hypophysitis, considering the evidence of endogenous CTLA-4 expression on the pituitary gland, predominantly in lactotrophic and thyrotrophic cells, which increases the risk of toxicity [27].
To the authors’ knowledge, this is the first study on this subject that includes patients treated with several ICI types and with several primary neoplasms that demonstrates a higher response rate and an increased overall survival in patients who developed primary or central thyroid dysfunction, adjusted for important covariates. A strength of this study was the inclusion of a reasonable number of patients (greater than similar studies), who have been rigorously selected given the importance of exclusion of conditions that might increase the risk of developing a TFTA. In fact, selection criteria have often been undervalued in similar studies. Another important aspect is the multivariate and adjusted analysis of overall survival which has taken into account the eventual contribution of other factors, allowing to achieve reliable data.
One limitation of this study is its retrospective nature, which did not enable the collection of all desirable data and strict follow-up of the patients. Even so, missing data were irrelevant except for previous antithyroid Abs analysis and therefore it was not possible to correctly assess the incidence of pre-existing or new onset thyroiditis. Nevertheless, authors made an effort to reduce biases through restrictive criteria. In the seven cases of hypophysitis, metastases were not seen in the pituitary MRI performed by five patients, nor in the brain CT scan of the two remaining patients who performed 18PET/CT. Supposedly, no patient had metastatic disease of the hypothalamic-pituitary area, but it was not absolutely excluded in two patients due to the low sensitivity of this CT scan.
Some other biases have been suggested about the results of other studies, but their true significance is debatable, namely time on therapy, severity of adverse events, site of primary neoplasm and management of cancer. Although patients who developed TFTA underwent a higher number of ICI cycles, the early onset of TFTA in our study (10.6 weeks in median) reveals the early efficacy of ICI and excludes the possibility that TFTA occurrence was time-dependent and due to accumulative effect of ICI. In fact, irAEs occurrence has been associated with a better efficacy and response to ICI therapy [27, 38,39,40,41]. Furthermore, it has been advocated that time on ICI does not interfere with the outcomes [42]. In the future, the correlation between time on ICI and patients’ survival, adjusted to TFTA occurrence, should be analyzed in order to further confirm our hypothesis. In this case, it would be interesting to evaluate the relationship between the severity of TFTA and the efficacy of ICI in larger sample sizes. In our study, it is not statistically feasible due to a limited number of deaths if we segregate TFTA group by severity grade. Despite the supposition that a more severe event may reflect increased T-cell activity, most studies have not shown any association between irAEs severity and ICI efficacy [27]. Concerning the primary neoplasm, we did not find a direct impact in the survival results. Regarding cancer management, we did not collect data on previous medical and surgical treatments.
In conclusion, the development of primary or central thyroid dysfunction is associated with improved clinical outcomes, namely response rate and survival. This study supports the assumption that irAEs are intimately associated with antitumor effect, recognizing thyroid dysfunction as a clinical biomarker for ICI response across several neoplasms.
Data availability
Data are protected in accordance with indications of Local Ethical Committee.
Abbreviations
- Abs:
-
Antibodies
- BB:
-
Beta-blocker
- CI:
-
Confidence interval
- CTCAE:
-
Common terminology criteria for adverse events
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DIRE:
-
Delayed immune-related events
- 18FDG-PET/CT:
-
18Fluorodeoxyglucose positron emission tomography/computed tomography
- fT3:
-
Free triiodothyronine
- fT4:
-
Free thyroxine
- HR:
-
Hazard ratio
- ICI:
-
Immune checkpoint inhibitors
- IQR:
-
Interquartile range
- irAEs:
-
Immune-related adverse events
- MSI-H:
-
Microsatellite instability-high
- NSCLC:
-
Non-small-cell lung cancer
- PD-L1:
-
Programmed cell death ligand 1
- PD-1:
-
Programmed cell death protein 1
- TFTA:
-
Thyroid function tests abnormalities
- Tg:
-
Thyroglobulin
- TPO:
-
Thyroid peroxidase
- TRAbs:
-
Thyrotropin receptor antibodies
- TSH:
-
Thyroid-stimulating hormone
- vs:
-
Versus
References
Chalan P, Di Dalmazi G, Pani F, De Remigis A, Corsello A, Caturegli P (2018) Thyroid dysfunctions secondary to cancer immunotherapy. J Endocrinol Invest 41:625–638. https://doi.org/10.1007/s40618-017-0778-8
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. https://doi.org/10.1038/nrc3239
Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD (2014) Immune modulation in cancer with antibodies. Annu Rev Med 65:185–202. https://doi.org/10.1146/annurev-med-092012-112807
Hargadon KM, Johnson CE, Williams CJ (2018) Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 62:29–39. https://doi.org/10.1016/j.intimp.2018.06.001(S1567-5769(18)30252-2 [pii])
Ferrari SM, Fallahi P, Galetta F, Citi E, Benvenga S, Antonelli A (2018) Thyroid disorders induced by checkpoint inhibitors. Rev Endocr Metab Disord 19:325–333. https://doi.org/10.1007/s11154-018-9463-2
Osorio JC, Ni A, Chaft JE et al (2017) Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 28:583–589. https://doi.org/10.1093/annonc/mdw640
Presotto EM, Rastrelli G, Desideri I et al (2019) Endocrine toxicity in cancer patients treated with nivolumab or pembrolizumab: results of a large multicentre study. J Endocrinol Invest. https://doi.org/10.1007/s40618-019-01112-8
Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP (2016) Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol 28:254–263. https://doi.org/10.1097/CCO.0000000000000290
Abdel-Wahab N, Shah M, Suarez-Almazor ME (2016) Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS ONE 11:e0160221. https://doi.org/10.1371/journal.pone.0160221
Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A (2014) Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol 71:161–169. https://doi.org/10.1016/j.jaad.2014.02.035(S0190-9622(14)01152-9[pii])
Weber JS, Gibney G, Sullivan RJ et al (2016) Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 17:943–955. https://doi.org/10.1016/S1470-2045(16)30126-7(S1470-2045(16)30126-7[pii])
Bajwa R, Cheema A, Khan T et al (2019) Adverse effects of immune checkpoint inhibitors (programmed death-1 inhibitors and cytotoxic T-lymphocyte-associated protein-4 inhibitors): results of a retrospective study. J Clin Med Res 11:225–236. https://doi.org/10.14740/jocmr3750
Kassi E, Angelousi A, Asonitis N et al (2019) Endocrine-related adverse events associated with immune-checkpoint inhibitors in patients with melanoma. Cancer Med. https://doi.org/10.1002/cam4.2533
Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH (2018) The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur J Endocrinol 178:173–180. https://doi.org/10.1530/EJE-17-0810
Peiro I, Palmero R, Iglesias P et al (2019) Thyroid dysfunction induced by nivolumab: searching for disease patterns and outcomes. Endocrine 64:605–613. https://doi.org/10.1007/s12020-019-01871-7
Illouz F, Drui D, Caron P, Do Cao C (2018) Expert opinion on thyroid complications in immunotherapy. Ann Endocrinol (Paris) 79:555–561. https://doi.org/10.1016/j.ando.2018.07.007(S0003-4266(18)31185-5[pii])
Campredon P, Mouly C, Lusque A, Bigay-Game L, Bousquet E, Mazieres J, Caron P (2019) Incidence of thyroid dysfunctions during treatment with nivolumab for non-small cell lung cancer: retrospective study of 105 patients. Presse Med 48:e199–e207. https://doi.org/10.1016/j.lpm.2018.10.019(S0755-4982(19)30088-0[pii])
Patel NS, Oury A, Daniels GA, Bazhenova L, Patel SP (2018) Incidence of thyroid function test abnormalities in patients receiving immune-checkpoint inhibitors for cancer treatment. Oncologist 23:1236–1241. https://doi.org/10.1634/theoncologist.2017-0375
Al Mushref M, Guido PA, Collichio FA, Moore DT, Clemmons DR (2019) Thyroid dysfunction, recovery, and prognosis in melanoma patients treated with immune checkpoint inhibitors: a retrospective review. Endocr Pract. https://doi.org/10.4158/EP-2019-0244
Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, Gore M, Larkin J (2017) Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 86:614–620. https://doi.org/10.1111/cen.13297
Guaraldi F, La Selva R, Sama MT, D'Angelo V, Gori D, Fava P, Fierro MT, Savoia P, Arvat E (2018) Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice: a long-term prospective study from a referral institution. J Endocrinol Invest 41:549–556. https://doi.org/10.1007/s40618-017-0772-1
Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, Nachtigall L (2014) Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab 99:4078–4085. https://doi.org/10.1210/jc.2014-2306
Albarel F, Gaudy C, Castinetti F, Carre T, Morange I, Conte-Devolx B, Grob JJ, Brue T (2015) Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol 172:195–204. https://doi.org/10.1530/EJE-14-0845
Weber J, Mandala M, Del Vecchio M et al (2017) Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377:1824–1835. https://doi.org/10.1056/NEJMoa1709030
Iglesias P (2018) Cancer immunotherapy-induced endocrinopathies: clinical behavior and therapeutic approach. Eur J Intern Med 47:6–13. https://doi.org/10.1016/j.ejim.2017.08.019(S0953-6205(17)30321-7[pii])
Hryniewicki AT, Wang C, Shatsky RA, Coyne CJ (2018) Management of immune checkpoint inhibitor toxicities: a review and clinical guideline for emergency physicians. J Emerg Med 55:489–502. https://doi.org/10.1016/j.jemermed.2018.07.005(S0736-4679(18)30660-7[pii])
Das S, Johnson DB (2019) Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 7:306. https://doi.org/10.1186/s40425-019-0805-8
Barquin-Garcia A, Molina-Cerrillo J, Garrido P, Garcia-Palos D, Carrato A, Alonso-Gordoa T (2019) New oncologic emergencies: What is there to know about inmunotherapy and its potential side effects? Eur J Intern Med 66:1–8. https://doi.org/10.1016/j.ejim.2019.05.020(S0953-6205(19)30178-5[pii])
Tan MH, Iyengar R, Mizokami-Stout K, Yentz S, MacEachern MP, Shen LY, Redman B, Gianchandani R (2019) Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin Diabetes Endocrinol 5:1. https://doi.org/10.1186/s40842-018-0073-4
Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L (2019) Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev 40:17–65. https://doi.org/10.1210/er.2018-00006
Barroso-Sousa R, Ott PA, Hodi FS, Kaiser UB, Tolaney SM, Min L (2018) Endocrine dysfunction induced by immune checkpoint inhibitors: practical recommendations for diagnosis and clinical management. Cancer 124:1111–1121. https://doi.org/10.1002/cncr.31200
Girotra M, Hansen A, Farooki A et al (2018) The current understanding of the endocrine effects from immune checkpoint inhibitors and recommendations for management. JNCI Cancer Spectr 2:pky021. https://doi.org/10.1093/jncics/pky021
Gomes-Lima C, Kwagyan J, King F, Fernandez S, Burman K, Veytsman I (2019) A comprehensive meta-analysis of endocrine immune-related adverse events of immune checkpoint inhibitors and outcomes in head and neck cancer and lung cancer. J Clin Oncol 37:e14096. https://doi.org/10.1200/JCO.2019.37.15_suppl.e14096
Kim HI, Kim M, Lee SH et al (2017) Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology 7:e1375642. https://doi.org/10.1080/2162402X.2017.1375642.1375642[pii]
Velu V, Titanji K, Zhu B et al (2009) Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458:206–210. https://doi.org/10.1038/nature07662
Kotwal A, Gustafson MP, Bornschlegl S, Kottschade L, Delivanis DA, Dietz AB, Gandhi M, Ryder M (2020) Immune checkpoint inhibitor-induced thyroiditis is associated with increased intrathyroidal T lymphocyte subpopulations. Thyroid. https://doi.org/10.1089/thy.2020.0075
Varricchi G, Loffredo S, Marone G, Modestino L, Fallahi P, Ferrari SM, de Paulis A, Antonelli A, Galdiero MR (2019) The immune landscape of thyroid cancer in the context of immune checkpoint inhibition. Int J Mol Sci 20:3934. https://doi.org/10.3390/ijms20163934
Downey SG, Klapper JA, Smith FO et al (2007) Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 13:6681–6688. https://doi.org/10.1158/1078-0432.CCR-07-0187
Bronstein Y, Ng CS, Hwu P, Hwu WJ (2011) Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol 197:W992–W1000. https://doi.org/10.2214/AJR.10.6198
Sarnaik AA, Yu B, Yu D et al (2011) Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res 17:896–906. https://doi.org/10.1158/1078-0432.CCR-10-2463
Weber J, Thompson JA, Hamid O et al (2009) A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 15:5591–5598. https://doi.org/10.1158/1078-0432.CCR-09-1024
Schadendorf D, Wolchok JD, Hodi FS et al (2017) Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol 35:3807–3814. https://doi.org/10.1200/JCO.2017.73.2289
Acknowledgements
The authors thank Darlene Rodrigues (Gerontology and Geriatrics PhD student and Radiation Oncology Resident of Department of Radiotherapy, Centro Hospitalar e Universitário de São João, E.P.E., Porto, Portugal) for support in survival data analysis (orcid number 0000-0001-8170-3952).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. No writing assistance was utilized in the production of this manuscript.
Author information
Authors and Affiliations
Contributions
JLF, ILS, CC and BM were involved in the study concept and design; JLF, CC, SC, MV and ILS were involved in data acquisition. JLF and BM were involved in the analysis and interpretation of the data. JLF drafted the manuscript. APM, BM and CC were involved in critical revision of an early draft of the manuscript. All authors have reviewed the manuscript and approved its final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest, namely relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. This study was approved by the Local Ethical Committee of the Instituto Português de Oncologia do Porto (CES.180/020) and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not obtained from each patient, because this retrospective analysis of existing data did not require any interaction with patients and did not intervene in their treatment.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lima Ferreira, J., Costa, C., Marques, B. et al. Improved survival in patients with thyroid function test abnormalities secondary to immune-checkpoint inhibitors. Cancer Immunol Immunother 70, 299–309 (2021). https://doi.org/10.1007/s00262-020-02664-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02664-y