Abstract
Introduction
The immune checkpoint inhibitors (ICPIs) agents anti-T lymphocytes-associated antigen 4 (CTLA-4) and anti-programmed cell death protein-1 (PD-1) and its ligands (PD-L1/PD-L2) have opened a new scenario in the treatment of cancer. These agents can induce immuno-related adverse events (irAEs), which may affect the endocrine system.
Purpose
The aim of this study was to analyze the occurrence and the course of endocrine irAEs in cancer patients treated with anti-PD-1 immunotherapy.
Methods
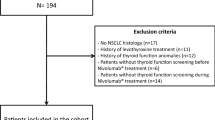
This was a retrospective, multicentre study, involving cancer patients treated with the PD-1 inhibitors nivolumab or pembrolizumab at reference Oncology Centres. One hundred and seventy-nine consecutive patients with different types of cancer (mostly non-small cell lung cancer, melanoma, kidney cancer) were included in the study. Patients had received nivolumab (70.9%) or pembrolizumab (29.1%) for 2–33 months. The study evaluated clinical data records until the established date of July 15, 2018. The primary end point was the assessment of endocrine toxicity and possible predictive factors.
Results
Endocrine toxicity occurred in 54 out of 179 patients (30.2%) and was related to thyroid dysfunction, with the exception of one case of diabetes mellitus. Thyroid toxicity occurred mostly within 2 months from the initiation of immunotherapy (83% of cases). A pre-existing thyroid dysfunction was a significant predictor of disease flare.
Conclusions
Thyroid alterations are frequently associated with anti PD-1 treatment in cancer patients. Regular thyroid assessment should be performed, particularly in the first months of treatment and in patients with a pre-existing thyroid disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of immune checkpoint inhibitors (ICPIs) in recent years has resulted in an effective new tool for the treatment of advanced cancer. ICPIs are monoclonal antibodies that inhibit negative regulatory components of the immune response, thus stimulating T cells to attack cancer cells. Three specific targets of these pharmacological agents are the T lymphocytes-associated antigen 4 (CTLA-4), the programmed cell death protein-1 (PD-1) and its ligands (PD-L1/PD-L2) [1]. The first ICPI that received approval by the Food and Drug Administration in the U.S. for use in advanced-stage melanoma was the CTLA-4 inhibitor ipilimumab. Subsequently, PD-1 inhibitors (e.g. nivolumab, pembrolizumab) and PD-L1 inhibitors (e.g. atezolizumab, avelumab, durvalumab) have been approved and the spectrum of indications has been widely extended, including for instance melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, Hodgkin lymphoma, head and neck squamous cell cancer, hepatocellular carcinoma [2,3,4,5,6,7,8,9,10].
Because immune checkpoints play also a crucial role in maintaining immunological self-tolerance, treatment with ICPIs can induce the onset or the exacerbation of autoimmune adverse effects. Immuno-related adverse events (irAEs) may affect many organs and tissues. Most commonly reported irAEs are represented by respiratory, gastrointestinal, cutaneous and endocrine toxicities [11].
Endocrine irAEs include hypophysitis, which is more commonly observed with ipilimumab, thyroid dysfunction, which is more commonly associated with the use of anti-PD-1 drugs or a combination of anti-PD-1 and anti-CTLA4, insulin-deficient diabetes mellitus, and primary adrenal insufficiency [12, 13]. Thyroid dysfunction may present as hypothyroidism or as thyrotoxicosis, which can be transient and followed by hypothyroidism. Exacerbation of a pre-existing thyropathy may also occur [13]. The relationship between ICPI treatment and irAEs is a clinically relevant issue, particularly because patients with autoimmune disorders, whose prevalence is estimated around 7.5–9.5% in the general population [14], have been typically excluded from immunotherapy clinical trials.
The aim of this multicentre retrospective study was to analyze the occurrence and the course of endocrine irAEs in a series of 179 patients with different cancers treated with the PD-1 inhibitors nivolumab or pembrolizumab.
Patients and methods
Patients
One hundred and seventy-nine patients consecutively referred to reference Centres in the Florence area (e.g. Radiation Oncology Unit, Medical Oncology Unit and Unit of Translational Oncology at the AOU Careggi; Melanoma and Skin Cancer Unit, Tuscany Central District, IOT Hospital) and in the Northeastern Italy (CRO, Aviano) and treated with nivolumab or pembrolizumab for advanced cancer disease were enrolled in this retrospective study. Each patient provided an informed consent for the data collection. Clinical data recorded until the established date of July 15, 2018, for patients with a follow-up of at least 2 months from the initiation of immunotherapy, were analyzed. Nivolumab was administered i.v. (usually 3 mg/kg) every 2 weeks and pembrolizumab was administered i.v. (usually 2 mg/kg) every 3 weeks. The demographic characteristics of the patients are reported in Table 1. Data on thyroid function, cortisol, glycemia, serum electrolytes at baseline and during ICPIs therapy were retrieved from clinical charts. Data on anti-thyroid antibodies (Ab) were collected, when available, from medical records. Hypothyroidism was defined as an elevated TSH (> 4.5 mUI/L) with normal (subclinical form), or decreased (overt form) free thyroid fractions (normal range: fT4 10–19 pM, fT3 3.1–6.8 pM). Thyrotoxicosis was defined as reduced TSH levels (TSH < 0.3 mUI/L) with normal (subclinical form), or increased (overt form) free thyroid fractions. All the hormone and biochemistry measurements were performed at the central lab of the Careggi or Aviano Hospitals, which use comparable methods and reference range. Blood samples were drawn after an overnight fast. All the blood parameters were measured as part of clinical practice as a screening of possible complications of ICPI treatment.
Statistical analysis
Continuous variables with a normal distribution and categorical variables were expressed as mean ± standard deviation or percentages, respectively. Differences between groups in continuous and categorical variables were assessed by Student t and Chi-squared test, respectively. The analysis of the possible predictors of thyroid alteration was performed by a multilevel binary logistic regression for the evaluation of the association between five putative predictors and the occurrence of alterations in thyroid function as outcome, with “no thyroid disease or stable disease” being the referent category. The trend over time of thyroid toxicity was evaluated by a survival analysis using the log-rank test to evaluate the difference in events among the months of observation.
Results
Endocrine toxicity
Endocrine toxicity occurred in 54 out of 179 patients (30.2%), 29 (53.7%) of whom were males and 25 (46.2%) females. Twenty-nine (53.7%) patients had NSCLC, 13 (24%) melanoma, 7 renal cancer, 3 head and neck cancer, 1 breast cancer and 1 bladder cancer. Forty-four (81.4%) of the 54 patients, in which endocrine toxicity occurred, were treated with nivolumab and 10 (18.6%) with pembrolizumab. Almost the totality of patients who experienced endocrine toxicity developed thyroid alterations (n = 53, 29.6%). In one patient the onset of type 1 diabetes mellitus was observed. This was the case of a 43 years old female treated with nivolumab for a melanoma. The patient developed polyuria and polydipsia, weight loss and asthenia 12 months after the initiation of nivolumab. Plasma glucose concentrations > 300 mg/dl were repeatedly found, together with suppressed C-peptide levels, and insulin treatment was prescribed. Nivolumab was continued and the patient rapidly achieved a good glycemic control.
Thyroid alterations
With regard to thyroid dysfunction, in 32 cases (17.9% of the whole group of 179 patients) a new onset thyropathy was observed upon the initiation of nivolumab or pembrolizumab (Table 2A). In the remaining 21 patients, who experienced thyroid toxicity, a pre-existing thyroid dysfunction was present at baseline and a disease flare occurred upon the initiation of ICPI treatment, which required drug regimen adjustment (Table 2B). In other 9 patients, who were receiving treatment for a thyroid dysfunction at baseline, ICPIs treatment was not associated with a thyroid disease flare. Therefore, 21 out of 30 patients (70%) with a pre-existing thyroid function alteration experienced a disease flare upon initiation of ICPIs. The statistical analysis of possible predictors of thyroid alterations during ICPI therapy with nivolumab or pembrolizumab actually revealed that any pre-existing thyroid dysfunction is a significant risk factor for the development of toxicity (p < 0.0001). In this group of patients anti-thyroid Ab had been assessed only in a very few cases and it was not possible to determine whether a pre-existing thyroid autoimmunity is a predictor of thyroid toxicity. No correlation between gender, type of cancer, type of anti PD-1, age and the development of thyroid toxicity was observed (Fig. 1).
As per the type of thyroid dysfunction, in 13 (24.5%) out of the 53 patients with thyroid alterations thyrotoxicosis was observed, whereas in 24 (45.3%) patients a new onset hypothyroidism or the progression of a pre-existing hypothyroidism occurred. Finally, in 16 (30.2%) patients transient thyrotoxicosis was followed by hypothyroidism. Anti-thyroid Ab were assessed in 11 cases and were found positive in six of them.
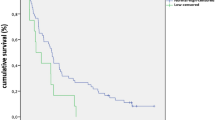
Overall, 40 (75.5%) patients received pharmacological treatment for thyroid dysfunction, whereas 13 (24.5%) patients underwent spontaneous resolution without the need to initiate specific therapy. An example of the biphasic pattern (e.g. thyrotoxicosis followed by hypothyroidism) is shown in Fig. 2. A woman treated with nivolumab for advanced NSCLC developed mild thyrotoxicosis 2 months after the initiation of therapy. A couple of weeks later subclinical hypothyroidism occurred, followed by overt hypothyroidism at month 3. Nivolumab had not been discontinued and l-thyroxine treatment had been initiated at that point, yet at a very low dose, which did not avoid further severe decline of the thyroid function. The dose had been tapered up to 125 mcg/day during the following months and at the time of the most recent follow-up visit TSH had returned close to the normal range.
Example of thyroid toxicity with a biphasic pattern in a woman with NSCLC treated with nivolumab (patient 22 in Table 2A)
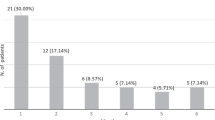
In 33 out of 53 patients (62.3%) thyroid toxicity was observed within the first 2 months after the initiation of ICPI treatment and the risk was significantly higher in the first month, when 24 cases were observed. From month 3–5 thyroid alterations occurred in additional 11 patients (Fig. 3). The remaining 9 patients developed endocrine toxicity between month 6 and 9 (1 at month 6, 2 at month 7, 3 at month 8, 3 at month 9) (not shown).
Discussion
In this study, we have evaluated the occurrence of endocrine toxicity in a series of 133 consecutive cancer patients treated with the anti PD-1 agents nivolumab and pembrolizumab at different oncology centres. Thirty-tree patients experienced a new onset endocrine dysfunction, which involved the thyroid gland, with the exception of one case of insulin-dependent diabetes mellitus. This result is in agreement with current clinical experience indicating that thyroid disorders represent the most common endocrine complication observed in cancer patients treated with anti PD-1 agents (12). In two of the largest studies addressing thyroid toxicity in patients treated with nivolumab or pembrolizumab for melanoma, the percentage of thyroid dysfunction was 39% (18/46 patients) in one case [15] and 9% (9/103 patients) in the other case [16].
A change in the course of disease in patients with a pre-existing thyropathy, who initiate ICPI treatment, has been also described previously [17, 18]. In another recent study, 29 patients with advanced melanoma were first treated with ipilimumab and then shifted to pembrolizumab or nivolumab for disease progression. Among these patients, 14 presented thyroid function abnormalities at the end of treatment with ipilimumab. After anti PD-1 initiation, thyroid function alterations were observed in additional 7 patients and 3 of them had a pre-existing autoimmune thyropathy with euthyroidism [19]. In our series, 21 (70%) of the 30 patients with a pre-existing thyroid dysfunction experienced a disease flare after they received PD-1 inhibitors. Similar results were obtained in a series of patients treated with at least one dose of ipilimumab and/or nivolumab/pembrolizumab. Out of 61 patients, 43 (77%) developed an exacerbation of thyroid alteration, which was more frequent in patients receiving a combined treatment [20].
Noteworthy, statistical analysis revealed that a pre-existing alteration of thyroid function was the unique predictor of thyroid toxicity among the parameters that were analyzed. These data suggest that thyroid function should be regularly assessed in cancer patients undergoing ICPI treatment, particularly in those receiving anti PD-1 agents, which affect thyroid function more frequently than anti CTLA-4 agents, and in those with a pre-existing thyroid alteration.
With regard to the type of thyroid alteration that occurred during anti PD-1 therapy, different scenarios were observed: hypothyroidism was the most frequent alteration (45.3% of cases), but thyrotoxicosis (24.5%) or transient thyrotoxicosis followed by hypothyroidism (30.2%) were also observed. Similar patterns have been observed in other studies and have been extensively reviewed [13]. If we consider the total number of patients (n = 179), our results indicate that 13.4%, 7.3%, 8.9% of them developed hypothyroidism, thyrotoxicosis, or transient thyrotoxicosis followed by hypothyroidism after the initiation of ant PD-1 therapy, respectively. A meta-analysis of 38 randomized controlled trials, which included anti-CTLA-4, anti-PD-1, and anti-PD-L1 therapy, indicated the presence of 472 cases of hypothyroidism among 7551 patients (6.2%), and 194 cases of thyrotoxicosis among 7531 patients (2.6%) [21]. The lower frequency of thyroid dysfunction that resulted from the analysis of these trials may be likely due to the fact that also anti-CTLA-4 therapy, which less frequently causes thyroid toxicity than PD-1 and PD-L1 inhibitors, had been included. In addition, the authors claimed that a number of cases of thyrotoxicosis may have been missed in the studies that were analyzed, because they may occur earlier than hypothyroidism and may be transient and, therefore, not diagnosed [21].
Another finding from our study is that thyroid toxicity may require specific pharmacological treatment (75.5%), but it may be also asymptomatic or mildly symptomatic and with a spontaneous resolution without the need of pharmacological treatment (24.5%).
The majority of thyroid toxicities occurred within 5 months from the initiation of anti-PD-1 treatment. Our results are in agreement with those found in studies involving the largest series of patients, published so far, which indicate a median time from the first dose of nivolumab or pembrolizumab and thyroid function alteration of about 60 days [15, 16, 22]. Therefore, it can be suggested that thyroid function monitoring should be routinely performed and with higher frequency in the first months of anti PD-1 therapy, when the risk to develop endocrine toxicity is higher.
Because ICPI-associated thyrotoxicosis may resolve spontaneously or convert into hypothyroidism, routine measurement of anti-thyroid Ab has not been considered to be of help and has not been recommended for cancer patients treated with ICPI, so far [13]. Accordingly, also in our retrospective study anti-thyroid Ab had been checked, in the oncology centres involved, in only 11 patients and resulted positive in 6 cases. It should be noted that the assessment of anti-thyroid Ab has been rarely performed also in the large afore mentioned studies. In particular, in the study by Morganstein et al. [15], anti-thyroid Ab were assessed in 5 out of 56 patients and were found positive in 4 cases, whereas in the study by Scott et al. [16] they were analyzed in 7 out of 103 patients and were found positive in 5 cases. However, the assessment of anti-thyroid Ab might be reconsidered and be viewed as a possible prognostic biomarker, should the development of thyroid autoimmune response be associated with a better effectiveness of cancer immunotherapy. With regard to this issue, more than 10 years ago a study concluded that the appearance of clinical and laboratory evidence of autoimmunity during therapy with high dose interferon in patients with melanoma had a significant impact on relapse-free survival and overall survival [23]. A more recent study reported that the development of anti Tg Ab in cancer patients treated with GVAX (for vaccine made of a tumor cell type transfected with GM-CSF) and/or ipilimumab was associated with a significant prolonged survival [24]. Skin toxicity has been suggested as a possible biomarker of efficacy in patients treated with EGFR inhibitors [25]. Similarly, vitiligo has been associated with a longer survival in patients receiving immunotherapy for melanoma [26]. Admittedly, this might be an important issue to be further investigated. Unfortunately, exhaustive data are lacking, so far, also because patients with autoimmune diseases have been typically excluded from ICPI trials and immunotherapy in these patients is still considered investigational [27]. Nevertheless, in cancer patients with a pre-existing autoimmune disease, who did not experience a disease flare for 2 years and who are not receiving immunosuppressive treatment, ICPIs are a conceivable option [28]. In case of a new onset thyropathy or of a thyroid disease flare, it is considered safe to maintain ICPI treatment for low-grade disease (1–2) and to temporarily withhold it for advanced grade (≥ 3) [13, 29]. Therefore, considering the clinical relevance of ICPIs as a new tool for cancer treatment, it should be highlighted that immunotherapy has not in principle to be interrupted in the presence of endocrine or metabolic side effects.
The main limitation of our study is represented by its retrospective nature, which did not allow a formal sample size calculation, a strict follow-up program and the availability of anti-thyroid Ab. On the other hand, the assessment of blood parameters during the scheduled hospital admissions for the administration of ICPI therapy allowed a standardized collection of data. Admittedly, the main strength of the study relies on the sample size, which includes a large series of patients outside of a clinical trial protocol, thus representing real-life data.
In summary, in this study we have described the presence of endocrine-related toxicity, almost invariably due to thyroid alterations, in about one-third of cancer patients treated with nivolumab or pembrolizumab. A pre-existing thyroid dysfunction is a predictor of thyroid toxicity, which may be represented by a wide spectrum of alterations (thyrotoxicosis, hypothyroidism, thyrotoxicosis followed by hypothyroidism). In many cases thyroid toxicity raises within a few months from the beginning of immunotherapy. Thyroid alterations may require specific pharmacological intervention and, therefore, thyroid function should be routinely assessed in these patients. Because of the complex and sometimes unpredictable scenarios that may occur, a close monitoring by an endocrinologist should be warranted. To this purpose, practical algorithms have been suggested [29]. More in general, with the expanding use of immunotherapy in cancer, a multidisciplinary approach is needed for the management of side effects and post-marketing surveillance.
References
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O’Day SJ, Lebbé C (2010) Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 11:155–164
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Ribas A, Wolchok JD (2013) Combining cancer immunotherapy and targeted therapy. Curr Opin Immunol 25:291–296
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, KEYNOTE-006 investigators (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974–1982
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387:1837–1846
Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, O’Donnell PH, Drakaki A, Tan W, Kurland JF, Rebelatto MC, Jin X, Blake-Haskins JA, Gupta A, Segal NH (2016) Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 34:3119–3125
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR (2017) OAK Study Group (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265
Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D (2018) Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. https://doi.org/10.1136/bmj.k793
Kumar V, Chaundary N, Garg M, Floudas CS, Soni P, Chandra AB (2017) Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. https://doi.org/10.3389/fphar.2017.0004
Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L (2019) Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev 40:17–65
Cooper GS, Bynum ML, Somers EC (2009) Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun 33:197–207
Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, Gore M, Larkin J (2017) Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 86:614–620
Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH (2018) The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur J Endocrinol 178:175–182
Leonardi GC, Gainor JF, Altan M, Kravets S, Dahlberg SE, Gedmintas L, Azimi R, Rizvi H, Riess JW, Hellmann MD, Awad MM (2018) Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 36:1905–1912
Danlos FX, Voisin AL, Dyevre V, Michot JM, Routier E, Taillade L, Champiat S, Aspeslagh S, Haroche J, Albiges L, Massard C, Girard N, Dalle S, Besse B, Laghouati S, Soria JC, Mateus C, Robert C, Lanoy E, Marabelle A, Lambotte O (2018) Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer 91:21–29
Guaraldi F, La Selva R, Samà MT, D’Angelo V, Gori D, Fava P, Fierro MT, Savoia P, Arvat E (2018) Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice: a long-term prospective study from a referral institution. J Endocrinol Invest 41:549–556
Patel NM, Michelini VV, Snell JM, Balu S, Hoyle AP, Parker JS, Hayward MC, Eberhard DA, Salazar AH, McNeillie P, Xu J, Huettner CS, Koyama T, Utro F, Rhrissorrakrai K, Norel R, Bilal E, Royyuru A, Parida L, Earp HS, Grilley-Olson JE, Hayes DN, Harvey SJ, Sharpless NE, Kim WY (2018) Enhancing next-generation sequencing-guided cancer care through cognitive computing. Oncologist 23:179–185
Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM (2018) Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 4:173–182
Lee H, Hodi FS, Giobbie-Hurder A, Ott PA, Buchbinder EI, Haq R, Tolaney S, Barroso-Sousa R, Zhang K, Donahue H, Davis M, Gargano ME, Kelley KM, Carroll RS, Kaiser UB, Min L (2017) Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunol Res 5:1133–1140
Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos A, Papadopoulos O, Stratigos A, Markopoulos C, Bafaloukos D, Pectasides D, Fountzilas G, Kirkwood JM (2006) Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med 354:709–718
De Remigis A, de Gruijl TD, Uram JN, Tzou SC, Iwama S, Talor MV, Armstrong TD, Santegoets SJ, Slovin SF, Zheng L, Laheru DA, Jaffee EM, Gerritsen WR, van den Eertwegh AJ, Le DT, Caturegli P (2015) Development of thyroglobulin antibodies after GVAX immunotherapy is associated with prolonged survival. Int J Cancer 136:127–137
Shah RR, Shah DR (2019) Safety and tolerability of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in oncology. Drug Saf 42:181–198
Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM (2015) Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 33:773–781
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA, National Comprehensive Cancer Network (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol 36:1714–1768
Califano R, Lal R, Lewanski C, Nicolson MC, Ottensmeier CH, Popat S, Hodgson M, Postmus PE (2018) Patient selection for anti-PD-1/PD-L1 therapy in advanced non-small-cell lung cancer: implications for clinical practice. Future Oncol 14:2415–2431
Ruggeri RM, Campennì A, Giuffrida GP, Trimboli L, Giovanella F, Cannavò TS (2018) Endocrine and metabolic adverse effects of immune checkpoint inhibitors: an overview (what endocrinologists should know). J Endocrinol Invest. https://doi.org/10.1007/s40618-018-0984-z[EPub ahead of print]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have not any conflict of interest.
Ethical approval
For this study formal authorization was not required.
Informed consent
Informed consent was obtained from all subjects included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Presotto, E.M., Rastrelli, G., Desideri, I. et al. Endocrine toxicity in cancer patients treated with nivolumab or pembrolizumab: results of a large multicentre study. J Endocrinol Invest 43, 337–345 (2020). https://doi.org/10.1007/s40618-019-01112-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01112-8