Abstract
Though therapy that promotes anti-tumor response about CD8+ tumor-infiltrating lymphocytes (TILs) has shown great potential, clinical responses to CD8+ TILs immunotherapy vary considerably, largely because of different subpopulation of CD8+ TILs exhibiting different biological characters. To define the relationship between subpopulation of CD8+ TILs and the outcome of antitumor reaction, the phenotype and function of CD103+ CD8+ TILs in esophageal squamous cell carcinoma (ESCC) were investigated. CD103+ CD8+ TILs were presented in ESCC, which displayed phenotype of tissue-resident memory T cells and exhibited high expression of immune checkpoints (PD-1, TIM-3). CD103+ CD8+ TILs were positively associated with the overall survivals of ESCC patients. This population of cells elicited potent proliferation and cytotoxic cytokine secretion potential. In addition, CD103+ CD8+ TILs were elicited potent anti-tumor immunity after anti-PD-1 blockade and were not affected by chemotherapy. This study emphasized the feature of CD103+ CD8+ TILs in immune response and identified potentially new targets in ESCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer (EC) is one of the fastest developing malignancies, and the mortality is the sixth in the world [1]. About 90% of EC cases are esophageal squamous cell carcinoma (ESCC), which mainly occur in Asian countries [2]. China accounts for about 50% of new EC cases in the world, and more than 90% of them are ESCC. Although there are multidisciplinary treatments, such as surgery, chemotherapy, and radiotherapy for ESCC [3,4,5], the current 5-year global survival is still poor of around 30–40% [6]. Therefore, to identify potential therapeutic targets and developing new therapeutic strategies that can improve the clinical benefits of ESCC patients is demanding.
Immunotherapy is one of the most effective methods for tumor elimination, especially adoptive transfer of tumor-killing immune cells. CD8+ tumor-infiltrating lymphocytes (TILs) immunotherapy has been rapidly developed as a therapy for tumors [7, 8], containing ESCC [9,10,11], and it has also been the most important element for patient survival by various clinical and histopathologic criteria [12, 13]. However, it remains unclear whether there are different subpopulation in CD8+ TILs that might contribute to the superior outcome, which leads to a variety of clinical responses among ESCC patients [14]. Rosenberg et al. demonstrated that PD-1hi CD8+ TILs were tumor-reactive cells [15]. These observations suggested the better understanding of the subpopulation of CD8+ TILs, which could help us in discovering new biomarkers to stratify patients and identifying more effective therapeutic cell subtypes.
Recently, tissue-resident memory (TRM) T cells have attracted great attention, which occupy tissues without recirculating, with high expression level of CD103 and/or CD69 and convey low level of CCR7 and CD62L [16,17,18,19]. TRM cells can elicit direct cytotoxicity to infected host cells by effector cytokine production and are involved in regulating anti-tumor immune response [20,21,22,23]. CD103+ CD8+ TRM cells play an important role against virus infection in defending human epithelial tissue and express PD-1, CTLA-4 inhibitory receptors [16, 24]. It was reported that CD103+ CD8+ TILs are closely related to survival rate of patients, especially in cancers of epithelial origin [25,26,27,28,29], for example pancreatic cancer, colorectal cancer, ovarian cancer, and bladder carcinoma. However, there is not enough available data to show the presence of CD103+ CD8+ TRM cells in human ESCC tissues, and their roles in the anti-tumor immune reaction.
To investigate whether CD103+ CD8+ TRM cells are presented in ESCC, TCGA data of RNA-based next-generation sequencing (RNA-Seq) were analyzed, and fresh surgical samples were confirmed via flow cytometry. The presence of CD103+ CD8+ TILs in ESCC, with high expression of CD69 and very low CD62L/CCR7, was observed. Next, samples of untreated ESCC were selected to comprehend the relationship between CD103+ CD8+ TILs and clinical prognosis through immunohistochemistry (IHC). The function of this population of CD103+ CD8+ TILs was revealed by using ESCC fresh tissue samples. CD103+ CD8+ TILs had a potent tendency to proliferate and be activated after anti-PD-1 (αPD-1) blockade. Furthermore, by using samples from patients with neoadjuvant chemotherapy, it was found that the ratio and phenotype of these CD103+ CD8+ TILs were not affected by chemotherapy. These results proposed that CD103 has the potential to mark tumor-reactive CD8+ T cells in human ESCC.
Methods
TCGA RNA-seq data analysis
Standardized RNA-seq and pathological data of esophageal cancer with 19 datasets were downloaded from TCGA (https://xena.ucsc.edu/). Gene expression features linked to tumor immune response about tissue-resident cell were analyzed in 95 patients with ESCC, such as CD8A, ITGAE (CD103), PD-1, TIM-3, CTLA-4, TIGIT, LAG-3, IFN-γ, GZMB, CD69, RUNX3, CXCR6, and so on. The data downloaded from TCGA were in compliance with applicable laws and any necessary informed consent rules.
Flow cytometry analysis of TIL, PIL and PBMC from ESCC patients
Peripheral blood, fresh tumor tissue, and matched paracancerous tissue of ESCC patients in Taihang Mountain area of China were obtained from the Affiliated Cancer Hospital of Zhengzhou University. The Ethics Committee of Zhengzhou University approved the sample collection and experimental procedures. Among about 200 recruited tissue samples, only about 65% were infiltrated enough T-cells to be studied in vitro. T cells were analyzed by flow cytometry or separated by microbeads for the sake of further studies. TILs or paracancerous infiltrating lymphocytes (PILs) were isolated with mechanical and enzymatic hydrolysis. Briefly, tumor or paracancerous tissues were cut into small pieces and placed in an orbital shaker for incubation with 2-mL Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, USA) including 1 mg/mL collagenase I (Gibco, USA), 1 mg/mL collagenase II (Gibco, USA), and 1 mg/mL collagenase IV (Gibco, USA) at 37 °C for 30 min. Subsequently, the dispersed cells were filtered through a 70-µm filter, centrifuged at 300 g, and were analyzed directly by flow cytometry or magnetic activated cell sorting (Miltenyi Biotec GmbH, Germany) after resuspension with autoMACS Running Buffer (Miltenyi Biotec GmbH, Germany).
The cells for direct analysis were harvested and flushed in cold phosphate buffer saline (PBS) and re-suspended in 100-µL cold PBS with 5% serum at a concentration of 1 × 106 cells. The following fluorescent-coupled antibodies were stained for the cells: 7-AAD (BioLegend), anti-CD3-PE-Cy7 (clone SK7, BioLegend), anti-CD8-APC-Cy7 (clone HIT8a, BioLegend), anti-CD103-FITC (clone Ber-ACT8, BioLegend), anti-PD-1-APC (clone EH12.2H7, BioLegend), anti-TIM-3-PE (clone F38-2E2, BioLegend), anti-CD69-PE (clone FN50, BioLegend), anti-CD62L-PE (clone DREG-56, BD Biosciences), anti-CCR7-APC (clone G043H7, BioLegend), anti-CTLA-4-PE (clone D4E9I, Cell Signaling Technology), or anti-4-1BB-PE (clone 4B4-1, BioLegend) for 20 min on ice. Peripheral blood samples were treated with pyrolysis liquid after dyeing for peripheral blood lymphocytes (PBLs). FACS analysis was performed using a FACSCanto II flow cytometry and CellQuest software (BD Biosciences).
Cytotoxic molecules and Ki67 proliferation were analyzed by flow cytometry by the following antibodies: anti-CD8-PerCP-Cy5.5 (clone RPA-T8, BioLegend), anti-CD103-FITC (clone Ber-ACT8, BioLegend), anti-IL-2-PE (clone MQ1-17H12, BioLegend), anti-IFN-γ-APC (clone 4S.B3, Invitrogen), anti-CD107a (clone H4A3, BioLegend), and anti-Ki67-APC (clone Ki67, BioLegend). The intracellular staining of Ki67 was carried out by True-Nuclear FIX and True-Nuclear Perm (BioLegend). To comprehend CD107a and cytokine, CD3+ TILs were activated ex vivo with 2 µg/mL anti-CD3 (clone OKT3, Takara, Japan) and 2 µg/mL anti-CD28 (clone CD28.2, eBioscience) for 4–6 h, and Brefeldin A (dilution, 1:1000, BioLegend) was added in the last 2 h of activation. For PD-1 blocking experiment, CD3+ TILs were co-cultured with 1 mg/mL αPD-1 (nivolumab). CD3+ T cells were sorted with CD3 beads (MiltenyiBiotec GmbH, Germany) before intracellular assessment of Ki67 and cytotoxic molecules. At least five patients were tested and representative plots were presented.
4-Nitroquinoline 1-oxide (4-NQO)-induced mouse model of ESCC and αPD-1 therapy
Carcinogenesis was induced by 4-nitroquinoline 1-oxide (4-NQO) in drinking water in mouse esophageal epithelia [1, 2]. Briefly, C57BL/6 mice (6-week-old female) purchased from Vital River Laboratory (Beijing, China) were maintained in a specific pathogen-free facility. The mice were supplied with drinking water of diluted 100 μg/mL 4-NQO stock solution and free access to the drinking water at all times during the treatment. After a 16-week carcinogen treatment, it was changed to normal sterilized drinking water and continued spontaneous induction. Examination of tissue sections from 22- to 24-week treatment mice revealed pathological evidence of carcinogenesis. Mice of 26 weeks were treated with αPD-1 antibody (clone RMP1-14, catalog BE0146) or isotype-matched control mAb (rat IgG). αPD-1 antibody was given i.p. (200 μg/mouse) every 3 days for 5 times. Whole esophagi were opened longitudinally, and tumors of 1–2 mm diameter were counted, on day 15 after treatment with αPD-1 antibody.
For CD8 and CD103 immunohistochemical staining, slides were dewaxed and rehydrated, followed by incubation with primary antibodies of rabbit anti-CD103 (clone EPR22590-27, Abcam, USA, 1/1000) and anti-CD8 (GB11068, Servicebio, China, 1/500), according to the instructions of the manufacturer. The dyeing intensity was divided into missing (0), weak (1+), medium (2+), and strong (3+). The H-score was calculated by multiplying the percentage (P) of positive cells by the intensity (I), according to the formula: H-score = P1 × I1 + P2 × I2 + ··· + Pn× In.
Case cohort, IHC and immunofluorescence
The tumor samples were surgically removed and paraffin-embedded, which were obtained from ESCC patients between September 2012 and May 2013. The study included 76 tumor samples and analyzed the expression of CD8 and CD103 on TILs in the epithelial or stromal area, in association with patient survival outcomes. Of these, the proportion of patients in pathological stage I, II, and III were of 20%, 38%, and 42%, respectively, and all of them had not received neoadjuvant therapy during surgery. The average age at diagnosis was 61 (range 34–79) years old; 70% of the patients were males. Clinicopathologic features of patients were provided (Supplementary Table 1).
Primary tumor samples for tissue microarrays (TMAs) were derived from the Pathology Department of the Affiliated Cancer Hospital of Zhengzhou University. To make TMA, all cases from the database were first selected, then reconfirmed each part, and selected the coring area of the corresponding block. Tissue cores of 1.0 mm diameter were taken from the corresponding area of tumor samples by tissue array instrument (Beecher Instruments, Silver Spring, USA).
CD8 and CD103 staining was performed on sections of TMAs using Ventana BenchMarks XT autostainer (Roche Inc., USA), with reagents from Biocare (Concord, USA). Slides were dewaxed and rehydrated, followed by incubation with primary antibodies of rabbit anti-CD103 (clone EPR4166 (2), Abcam, USA, 1/1000), and anti-CD8 (clone EP334, rabbit IgG, China, 1/1), according to the instructions of manufacturer. The standard H-score was same as that of the experimental part of mice.
For CD8/CD103/DAPI triple staining, preparation and antigen extraction of tumor slides were carried out as described above. Slides were stained with rabbit anti-CD103 (clone EPR4166 (2), Abcam, US, 1/1000) and rabbit anti-CD8 (clone EP334, rabbit IgG, China, 1/1) according to the instructions of manufacturer. Sections were scanned by a TissueFAXS imaging system (TissueGnostics, Austria).
Statistical analysis
The relationship between CD103 expression and characteristics of clinical pathology were assessed by Chi-square test and Fisher’s exact test. Kruskal–Wallis test was used for comparing IHC scores of CD8/CD103 in epithelial and stromal region. Survival rate was calculated using the Kaplan–Meier method, and curves were compared by the log-rank test. Cox regression was carried out with multivariate survival analysis. Graphpad Prism 6.0 or SPSS statistics 17.0 was performed for statistical analysis. All statistical tests were two sided with significance established at p < 0.05.
Results
CD8Ahi tumor samples show enrichment of ITGAE+ cells in ESCC
To identify whether CD103+ CD8+ TRM cells exist in ESCC, RNA-seq data from TCGA database were analyzed by using a CD8A and ITGAE (CD103) transcriptome in ESCC tumor samples of stage I-IV. The expression of CD8 had a strong positive correlation with that of ITGAE gene (Fig. 1a). ITGAE gene showed higher conveyance in CD8Ahi tumors than those in CD8Alo tumor (p < 0.05) (Fig. 1a). TRM cell-associated molecules RUNX3, CD69, CXCR6 in CD8Ahi tumors were considerably higher than those in CD8Alo tumors (p < 0.05) (Supplemental Fig. 1a, b), which also had a high correlation with ITGAE, and in ITGAEhi tumors were remarkably higher than those in ITGAElo tumors (p < 0.05) (Supplemental Fig. 1c, d). Besides that, there was a strong positive correlation between ITGAE and several other immune checkpoint molecules, such as PD-1, TIM-3, TIGIT, and LAG-3. Compared with ITGAElo tumors, ITGAEhi tumors showed higher expression level of immune checkpoints PD-1, TIM-3, LAG-3, and TIGIT and conveyed granzyme B, IFN-γ cytotoxic molecules (Fig. 1b, c). Together, the above findings showed a significant enrichment in TRM gene signature among CD8+ TILs in ESCC. However, no data have directly showed the relationship between the enrichment of CD8A or ITGAE transcripts and clinical features, for example, sex of the patients, invasive depth, lymph metastasis, stage of disease, tumor site, differentiation, or radiotherapy (Supplementary Fig. 1e).
CD8Ahi tumor samples in ESCC show enrichment for ITGAE+ T cells. a Through RNA-based next-generation sequencing (RNA-Seq) from TCGA database, analysis of the correlation between ITGAE transcripts and CD8A transcripts (log2 normalized counts) in tumor tissues of ESCC (left); ITGAE genes expressed differentially in ESCC from CD8Alo tumors vs CD8Ahi tumors. b RNA-Seq analysis of genes linked to tumor immune response from ITGAEhi or ITGAElo tumors. c Correlation between conveyance of ITGAE transcripts and immune checkpoint transcripts (log2 normalized counts) in ESCC. a–c An individual patient was represented by each symbol. Data analyzed from TCGA (n = 98). NS no statistical differences; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
CD103+ CD8+ T cells were presented in ESCC, displayed a TRM phenotype, expressed PD-1, TIM-3, and as a relatively active cell subpopulation
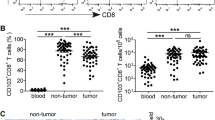
Investigating the existence of CD103+ CD8+ cells in treatment-naive ESCC patients by flow cytometry (Supplementary Table 2), CD103+ CD8+ T cells in TILs were found considerable enrichment compared with PILs (p < 0.0001) and PBLs (p < 0.0001) (Fig. 2a), and the conveyance of PD-1, TIM-3, and co-expression in CD103+ CD8+ were significantly higher than that in PILs (p < 0.0001) (Fig. 2b and Supplementary Fig. 2a). These cells were hardly observed in peripheral blood, indicating they have tissue-resident characteristics. In addition, high expression of CD69 and 4-1BB, but low conveyance of CD62L, CCR7, and CTLA-4 on CD103+ CD8+ T cells in PILs and TILs, was observed. The results conveyed a kind of T cell subpopulation with a CD69+ CCR7lo CD62Llo phenotype characteristic in TRM cells (Supplementary Fig. 2b). Importantly, PD-1, TIM-3, and their co-expression had higher conveyance on CD103+ CD8+ TILs compared with CD8+ CD103− TILs (Fig. 2c and Supplementary Fig. 2a). These results indicated that CD103 identified a special population in CD8+ TILs of ESCC microenvironment, which was important for the diagnosis and evaluation prognosis of the tumors. Additionally, this type of cells may be a kind of expanded, tumor-specific T cells, which is of great significance to study the CD8+ TILs.
CD103+ CD8+ T cells in treatment-naive ESCC. a Dot plots of the phenotype of CD103+ CD8+ T cells from the same patient (left); surface expression of CD103+ CD8+ on peripheral blood lymphocyte (PBL), paracancer infiltrating lymphocyte (PIL) and tumor-infiltrating lymphocyte (TIL) (right), n = 28. b Surface presentation of PD-1+, TIM-3+ on CD103+ CD8+ of PIL and TIL, n = 28. c Surface presentation of PD-1 and TIM-3 among CD103− and CD103+ TIL, n = 28. a–c ****p < 0.0001
CD103+ CD8+ TIL density is predictive to the survival of ESCC patients
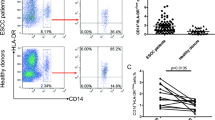
Since CD103+ CD8+ TILs were found in ESCC and showed TRM properties, we investigated subsequently the infiltration of CD8+ and CD103+ TILs in epithelial tumor and stromal region of 76 tumor tissues from stage I-III ESCC patients. Anti-CD8 and anti-CD103 mAb were stained for continuous tumor sections by hematoxylin–eosin. Positive cell in tumoral and stromal region was then analyzed (Fig. 3a). The results showed that the majority of CD8+ TILs were located mostly within the stroma, and CD103+ TILs were particularly observed in tumor nest regions with a high density (Fig. 3b). In addition, multi-color immunofluorescence was used for full tumor slides by florescence-labeled anti-CD8 and anti-CD103 antibodies, to comprehend whether CD103 was majorly located on CD8+ T cells in epithelium. The results indicated that the majority of CD103+ cells coexpressed CD8, revealing CD103+ CD8+ T cell subtype, while most of the CD103+ cells did not coexpressed CD8 in the paracancerous tissue, suggesting a non-T cell origin (Fig. 3c).
Distribution and prognostic impact of CD8+ and CD103+ T cells in tumor tissue (intraepithelial tumoral and stromal regions) of ESCC with immunohistochemical staining. a Tumor and paracancer sections from 76 I-III stage ESCC patients were stained with anti-CD8, or anti-CD103 mAb. Representative images of CD8, and CD103 immunostaining of the same tumor area and paracancer (magnification 400) are shown. Scale bars, 50 µm. b CD8, CD103 cell localization of intratumoral and stromal were quantified. c Representative image of tumor tissue and paracancer tissue from a ESCC patient stained by DAPI (DNA, blue), anti-CD8 (green), and anti-CD103 (red) antibodies (×400). Scale bars, 20 µm. *p < 0.05; ****p < 0.0001. d Prognostic impact of CD8, CD103 in ESCC. Overall survival was represented by Kaplan–Meier plots according to the expression status of CD8 and CD103. The log-rank test was used to compare curves and p < 0.05 were considered significant
Furthermore, the association between the survival rate and clinical-pathological features (age, gender, tumor length, TNM stage, lymph metastasis, differentiation, tumor site) was investigated (Table 1). Univariate analysis confirmed TNM stage, and lymph metastasis was the significant prognostic factor for OS (HR 2.24, 95% CI, p = 0.003 and HR 2.40, 95% CI, p = 0.013, respectively). TNM stage was prognostic and important for OS (HR 4.49, 95% CI, p = 0.002), in multivariate analysis of CD103 and CD8 with clinical prognostic factors about ESCC patients.
The relationship between the density of CD103+ CD8+ TILs and the favorable clinical outcome has also been investigated, using a cutoff value (3.32 H-score with CD8; 18.41 H-score with CD103) to delineate low or high density. Results revealed that CD103+ CD8+ TILs in tumor region yielded the strongest association with overall survival rate (OS) [HR 2.65, (1.03–6.79), p = 0.043], with a 5-year OS of 81.82% in those with higher density compared with 44.20% in those with CD8lo CD103lo tumors. Although the density of CD103+ TILs or CD8+ TILs was not notably correlated with OS in this cohort of patients [HR 2.12, (0.92–4.88), p = 0.077 and HR 1.95, (0.98–3.89), p = 0.059], patients with a higher density of CD103+ TILs or CD8+ TILs have a better survival tendency in tumor region (Fig. 3d). Thereby, these results implied that patients would had better long-term survival effects with a notable intratumoral CD103+ CD8+ TIL cells response.
CD103+ CD8+ TILs associated with tumor immune responses
The function about these CD103+ CD8+ TILs was studied, considering that it was important to elucidate which subpopulation devoted to the anti-tumor immune response in tumor infiltrated CD8+ T cells. The conveyance of Ki67 on CD103+ TILs was higher than on PILs (p < 0.05) and on PBLs (p < 0.001), through analysis of the conveyance of Ki67 in CD103+ T cells obtained from ESCC PBLs (n = 7), PILs (n = 7), TILs (n = 13) (Fig. 4a). In addition, the expression of Ki67 in CD103+ cells was higher than that in CD103− cells obtained from ESCC TILs (n = 13, p < 0.05) (Fig. 4b). The above findings suggested CD103+ CD8+ TILs in ESCC had a tendency to proliferate and be activated. It was proved that CD103+ CD8+ TILs expressed activating and cytotoxic molecules, for example IFN-γ, IL-2, and CD107a, which indicated that CD103+ CD8+ TILs were the major generators (Fig. 4c, d). These findings showed that CD103+ CD8+ T cells were rich in tumor tissue and expressed more cytotoxicity, which would contribute to the greater anti-tumor immune effects.
Functional features of CD103+ CD8+ T cells in ESCC. a Presentation of Ki67 and CD103 in gated CD3+ CD8+ T cells in PBL, PIL, and TIL from the same ESCC patient (left); Ki67 conveyance in CD103+ T cells obtained from PBL (n = 7), PTL (n = 7), TIL (n = 13) was analyzed by flow cytometry (right). b Presentation of Ki67 in CD103− cells and CD103+ cells obtained from ESCC TIL (n = 13). c Dot plots display IFN-γ, CD107a, IL-2 induction on CD3+ CD8+ TIL expressing (or not) CD103 obtained from a ESCC patient. d IFN-γ (n = 9), CD107a (n = 5), IL-2 (n = 7) induction on CD3+ CD8+ TIL expressing (or not) CD103 were analyzed by flow cytometry. e IFN-γ (n = 9), CD107a (n = 6), IL-2 (n = 9) induction on CD103+ CD8+ TIL after αPD-1 blockade. f Experimental protocol of ESCC model: mice were given injection of isotype-matched control mAb (rat IgG) or αPD-1 antibody intraperitoneally (i.p.) at various times after drinking 4-NQO water on week 0-16. g Images of esophageal change in ESCC mice after challenge (n = 7) (left); the number of tumors in esophagus: dot plots display the number of tumor (right). h Representative images of showing CD103, CD8 T cell infiltration in tumor microenvironment as detected by IHC (left), original magnification 400, scale bars, 50 µm; CD103, CD8 cell localization of intratumoral were quantified (right). a, b, d, eNS no statistical differences; *p < 0.05; ***p < 0.001
CD103+ CD8+ TILs exhibited a group of exhausted and tumor-reactive cells that can be reactivated after αPD-1 antibodies blockade in a view of high exhibition of PD-1 in CD103+ cells. Thus, we evaluated the function of CD103+ CD8+ TILs in vitro with human fresh tumor tissue samples and found that this population of TILs secreted higher IFN-γ and IL-2 after αPD-1 blockade (Fig. 4e). In order to better understand the effect of CD103+ cells by αPD-1 therapy, we studied the immune reaction of CD103+ CD8+ TILs in the 4-NQO-induced mouse model of ESCC. In line with those findings, treatment with αPD-1 antibody prevented tumor progression with αPD-1 blockade during tumorigenesis course in the ESCC mouse model (Fig. 4f). Compared with αPD-1 therapy group, the wall was more swollen and the number of tumors was higher than in control group (Fig. 4g). Further study showed that there was an increase in the number of CD8+ T cell and CD103+ T cell infiltration in the tumor of αPD-1 antibody therapy group, compared with mice received IgG as measured by IHC (Fig. 4h). These results indicated that CD103+ CD8+ TILs were exhausted tumor-reactive T lymphocytes and could be resurrected by immune checkpoint blockade therapeutics in order to inhibit tumor progression.
CD103+ CD8+ TILs were not affected by chemotherapy
It is highly controversial about the roles of chemotherapy for CD8+ T cells. In order to elucidate the effects about chemotherapy on CD103+ CD8+ T cells, we further analyzed their ratios in ESCC patients after neoadjuvant chemotherapy and found the ratios of CD103+ CD8+ T cells in neoadjuvant patients were similar to that in treatment-naive ESCC patients (Fig. 5a–c). Further, the function characteristics of CD103+ TILs were similar among neoadjuvant and treatment-naive patients (p > 0.05) (Fig. 5d–e). The findings suggested that CD103+ CD8+ TILs were not affected by chemotherapy in ESCC patients, revealing that the combination of CD103+ CD8+ T cell adoptive therapy and chemotherapy can be used to treat ESCC patients.
CD103+ CD8+ T cells in ESCC with neoadjuvant chemotherapy. a CD103+ CD8+ T cells ratios in PBL (n = 18), PIL (n = 18), and TIL (n = 18) after neoadjuvant chemotherapy. b Surface presentation of PD-1 (n = 18), TIM-3 (n = 16) on CD103+ CD8+ of PIL and TIL after neoadjuvant chemotherapy. c Surface presentation of PD-1+ (n = 18), TIM-3+ (n = 16), and PD-1+ TIM-3+ (n = 16) among CD103− and CD103+ TIL after neoadjuvant chemotherapy. d Surface presentation of CD103+ CD8+ on TILs of treatment-naive compared with neoadjuvant chemotherapy in ESCC. There was no significant statistical significance between neoadjuvant chemotherapy (n = 18) and treatment-naive patients (n = 28) about CD103+ CD8+ cells (p > 0.05). TN: treatment-naive, NCT: neoadjuvant chemotherapy. e Surface presentation of PD-1+, TIM-3+ on CD103+ TILs of treatment-naive (n = 28) compared with neoadjuvant (n = 18) in ESCC. There was no significant statistical significance between neoadjuvant and treatment-naive patients (p > 0.05). Among which, the expression of TIM3+, PD1+ TIM3+ in neoadjuvant was 16 cases because small specimens were got and lack of enough cells. TN: treatment-naive, NCT: neoadjuvant chemotherapy. a–eNS no statistical differences; ****p < 0.0001
Discussion
The therapeutic strategy of ESCC has not been improved significantly over the last decades, which is nothing more than surgery combined with chemo-radiotherapy [30, 31]. The Food and Drug Administration has approved to use pembrolizumab (PD-1 antibody) as a treatment of ESCC in July 2019. Many cases of clinical trials, such as CD8+ TILs, αPD-1 antibody combined with chemo-radiotherapy or indoleamine-2, 3-dioxygenase inhibitor have failed in other malignant tumors. One major reason may be that the mechanism has not been clarified, and the rationale for the combined treatment needs to be uncovered.
In recent years, some studies have reported CD8+ T cells could be divided into many cell subpopulations, especially the group of CD103+ CD8+ TRM cells. In this research, CD8+ T cells in ESCC can be clearly split into two populations by CD103 staining, and the CD103+ tissue-resident T cells presented in ESCC were observed. These cells, with a TRM phenotype, have high levels of exhaustion markers, and the increased frequencies of CD103+ CD8+ TILs significantly correlate with the overall survival of ESCC patients. Moreover, these cells have higher proliferative capacities and expressed molecules linked to cytotoxicity, indicating the presence of a highly activated tumor-active T cell subset in CD8+ TILs. Furthermore, CD103+ TILs were able to be rescued after αPD-1 blockade, and their ratios were not affected by adjuvant chemotherapy. This provided a theoretical platform for the combination therapy strategy of adoptive treatment combined with αPD-1 therapy or chemotherapy for ESCC.
It was reported that CD103 expression was upregulated in response to antigen recognition in CD103+ CD8+ TRM cells [32]. It can be deduced that CD103+ CD8+ tissue-resident T cells were presented at high conveyance in ESCC. In addition, surface marker characters linked to central memory T cells [16,17,18,19], such as high conveyance of CD69 and low expression of CCR7 and CD62L, were consistent with CD103 expression, indicating that the CD103+ CD8+ TILs represented TRM cells. It has been reported recently that CD103+ T cells conveyed high levels of PD-1, which were used as a biomarker of tumor-reactive T cells [33, 34]. Results from this research indicated that CD103+ TILs in ESCC frequently expressed PD-1 and TIM-3, and CD103+ CD8+ TILs had significant conveyance than that of CD103− TILs. Based on the above findings, it can be indicated that CD103 is a marker of tumor-reactive CD8+ TILs educated by antigen; thus, it may be a meaningful subgroup of cells in the anti-tumor immune reaction [35, 36].
Another study also showed that CD103 provided part of the evidence that there was a connection between the high density of CD103+ TILs and I-III stage ESCC patient survival. Similar findings were acquired in endometrial adenocarcinoma and ovarian cancer; the CD103 expression on TILs has influence on improving patient survival [36, 37]. Additionally, endometrial adenocarcinoma patients with remarkable conveyance of CD8+ cytotoxic TIL (CTL) favor in survival, while stromal abundance of these cells was found to have no prognostic benefit [38,39,40]. These findings suggested that it could be an important factor contributing to immune response for the specific accumulation and retention of T-cells in the epithelial compartment. Therefore, CD103 could accurately predict patient outcome by identifying a subpopulation of CD8+ T cells.
This study demonstrated that CD103+ CD8+ TILs displayed cell proliferation marker Ki67. Additionally, CD103+ CD8+ TILs generated more IFN-γ, IL-2, and CD107a linked to cytotoxicity compared with CD103− CD8+ TILs. These findings indicated that CD103+ CD8+ T cells were rich in tumor tissue and expressed more cytotoxicity, which would contribute to the anti-tumor immune effects. Moreover, cytotoxicity of CD103+ CD8+ TILs was improved in vitro and had better tumor control in vivo by αPD-1 antibody blockade. The recent success for αPD-1 and anti-PD-L1-based cancer immunotherapy was supported by blockade of the PD-1/PD-L1 pathway to enhance antitumor immunity with various types of advanced solid tumors, including ESCC [8, 10, 41, 42]. These results further emphasized that CD103+ CD8+ TILs may be tumor-reactive T lymphocytes and could be rescued as a result of blocking PD-1 signals.
Interestingly, the ratios of CD103+ CD8+ TILs remained unchanged after neoadjuvant chemotherapy in ESCC patients. There is increasing evidence on cytotoxic drugs that damage DNA can alter the inflammatory tumor microenvironment and stimulate the production of tumor antigens, thereby activating anti-tumor immunity [43, 44]. Chemotherapy is not only limited to cytotoxicity, but also depends on the reaction of anti-tumor immune response [45]. On the other hand, effector T cells in tumor microenvironment can enhance the function of chemotherapeutic drugs by weakening basal cell-mediated resistance to chemotherapy [46]. These observations suggested that adoptive transfer of CD103+ CD8+ cells combined with chemotherapy could be a promising therapeutic strategy for ESCC patients.
Collectively, this study identified CD103 as a biomarker of tumor-reactive CD8+ TILs and demonstrated that CD103+ CD8+ TIL represented as tumor resident memory T cell subpopulation in ESCC. It can elicit potent anti-tumor immunity after αPD-1 blockade and is not affected by neoadjuvant chemotherapy. In conclusion, we suggested that CD103 is a suitable marker to evaluate the antitumor immune response about CD8 T cell infiltration of ESCC through freshly isolated patient TIL and 4-NQO-induced mouse model of ESCC, which might be a notable biomarker for immunotherapy alone or in combination with either αPD-1 blockade or chemotherapy.
Abbreviations
- EC:
-
Esophageal cancer
- ESCC:
-
Esophageal squamous cell carcinoma
- IHC:
-
Immunohistochemistry
- PIL:
-
Paracancerous infiltrating lymphocyte
- RNA-Seq:
-
RNA-based next-generation sequencing
- TILs:
-
Tumor-infiltrating lymphocytes
- TMAs:
-
Tissue microarrays
- TRM:
-
Tissue-resident memory
References
Rustgi AK, El-Serag HB (2014) Esophageal carcinoma. N Engl J Med 371(26):2499–2509. https://doi.org/10.1056/NEJMra1314530
Stoner GD, Gupta A (2001) Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis 22(11):1737–1746. https://doi.org/10.1093/carcin/22.11.1737
Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI, Cunningham D, Blazeby J, Roy R, Maughan T, Griffiths G (2013) Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 14(7):627–637. https://doi.org/10.1016/s1470-2045(13)70136-0
van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, Henegouwen MIV, Wijnhoven BPL, Richel DJ, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, ten Kate FJW, Creemers GJ, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A, Grp C (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366(22):2074–2084. https://doi.org/10.1056/Nejmoa1112088
Mariette C, Piessen G, Triboulet JP (2007) Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 8(6):545–553. https://doi.org/10.1016/s1470-2045(07)70172-9
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–386. https://doi.org/10.1002/ijc.29210
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. https://doi.org/10.1038/nrc3239
Drake CG, Lipson EJ, Brahmer JR (2014) Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 11(1):24–37. https://doi.org/10.1038/nrclinonc.2013.208
Kono K, Mimura K, Yamada R, Ujiie D, Hayase S, Tada T, Hanayama H, Min AKT, Shibata M, Momma T, Saze Z, Ohki S (2018) Current status of cancer immunotherapy for esophageal squamous cell carcinoma. Esophagus 15(1):1–9. https://doi.org/10.1007/s10388-017-0596-2
Kojima T, Doi T (2017) Immunotherapy for esophageal squamous cell carcinoma. Curr Oncol Rep 19(5):33. https://doi.org/10.1007/s11912-017-0590-9
Hirano H, Kato K (2019) Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J Clin Oncol 49(5):412–420. https://doi.org/10.1093/jjco/hyz034
Atreya I, Neurath MF (2008) Immune cells in colorectal cancer: prognostic relevance and therapeutic strategies. Expert Rev Anticancer Ther 8(4):561–572. https://doi.org/10.1586/14737140.8.4.561
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795):1960–1964. https://doi.org/10.1126/science.1129139
Ganesan AP, Clarke J, Wood O, Garrido-Martin EM (2017) Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol 18(8):940–950. https://doi.org/10.1038/ni.3775
Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA (2014) PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Investig 124(5):2246–2259. https://doi.org/10.1172/JCI73639
Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T (2013) The developmental pathway for CD103(+)CD8(+) tissue-resident memory T cells of skin. Nat Immunol 14(12):1294–1301. https://doi.org/10.1038/ni.2744
Mueller SN, Gebhardt T, Carbone FR, Heath WR (2013) Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 31:137–161. https://doi.org/10.1146/annurev-immunol-032712-095954
Gebhardt T, Mueller SN, Heath WR, Carbone FR (2013) Peripheral tissue surveillance and residency by memory T cells. Trends Immunol 34(1):27–32. https://doi.org/10.1016/j.it.2012.08.008
Carbone FR, Mackay LK, Heath WR, Gebhardt T (2013) Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Curr Opin Immunol 25(3):329–333. https://doi.org/10.1016/j.coi.2013.05.007
Beura LK, Masopust D (2014) SnapShot: resident memory T cells. Cell 157(6):1488–1488.e1481. https://doi.org/10.1016/j.cell.2014.05.026
Mueller SN, Mackay LK (2016) Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 16(2):79–89. https://doi.org/10.1038/nri.2015.3
Schenkel JM, Masopust D (2014) Tissue-resident memory T cells. Immunity 41(6):886–897. https://doi.org/10.1016/j.immuni.2014.12.007
Kaech SM, Wherry EJ, Ahmed R (2002) Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol 2(4):251–262. https://doi.org/10.1038/nri778
Wakim LM, Woodward-Davis A, Liu R, Hu YF, Villadangos J, Smyth G, Bevan MJ (2012) The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol 189(7):3462–3471. https://doi.org/10.4049/jimmunol.1201305
Quinn E, Hawkins N, Yip YL, Suter C, Ward R (2003) CD103(+) intraepithelial lymphocytes—a unique population in microsatellite unstable sporadic colorectal cancer. Eur J Cancer 39(4):469–475. https://doi.org/10.1016/s0959-8049(02)00633-0
Cresswell J, Robertson H, Neal DE, Griffiths TRL, Kirby JA (2001) Distribution of lymphocytes of the alpha(E)beta(7) phenotype and E-cadherin in normal human urothelium and bladder carcinomas. Clin Exp Immunol 126(3):397–402. https://doi.org/10.1046/j.1365-2249.2001.01652.x
Ling KL, Dulphy N, Bahl P, Salio M, Maskell K, Piris J, Warren BF, George BD, Mortensen NJ, Cerundolo V (2007) Modulation of CD103 expression on human colon carcinoma-specific CTL. J Immunol 178(5):2908–2915. https://doi.org/10.4049/jimmunol.178.5.2908
Webb JR, Wick DA, Nielsen JS, Tran E, Milne K, McMurtrie E, Nelson BH (2010) Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (alphaE/beta7 Integrin) in high-grade serous ovarian cancer. Gynecol Oncol 118(3):228–236. https://doi.org/10.1016/j.ygyno.2010.05.016
French JJ, Cresswell J, Wong WK, Seymour K, Charnley RM, Kirby JA (2002) T cell adhesion and cytolysis of pancreatic cancer cells: a role for E-cadherin in immunotherapy? Br J Cancer 87(9):1034–1041. https://doi.org/10.1038/sj.bjc.6600597
Reichenbach ZW, Murray MG, Saxena R, Farkas D, Karassik EG, Klochkova A, Patel K, Tice C, Hall TM, Gang J, Parkman HP, Ward SJ, Tetreault MP, Whelan KA (2019) Clinical and translational advances in esophageal squamous cell carcinoma. Adv Cancer Res 144:95–135. https://doi.org/10.1016/bs.acr.2019.05.004
Kelly RJ (2019) The emerging role of immunotherapy for esophageal cancer. Curr Opin Gastroenterol. https://doi.org/10.1097/MOG.0000000000000542
Mokrani M, Klibi J, Bluteau D, Bismuth G, Mami-Chouaib F (2014) Smad and NFAT pathways cooperate to induce CD103 expression in human CD8 T lymphocytes. J Immunol 192(5):2471–2479. https://doi.org/10.4049/jimmunol.1302192
Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ (2009) Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10(1):29–37. https://doi.org/10.1038/ni.1679
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330. https://doi.org/10.1056/NEJMoa1412082
Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, Chang SC, Grunkemeier G, Leidner R, Bell RB, Weinberg AD (2018) Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. https://doi.org/10.1038/s41467-018-05072-0
Webb JR, Milne K, Nelson BH (2014) Location, location, location CD103 demarcates intraepithelial, prognostically favorable CD8(+) tumor-infiltrating lymphocytes in ovarian cancer. Oncoimmunology. https://doi.org/10.4161/onci.27668
Workel HH, Komdeur FL, Wouters MCA, Plat A, Klip HG, Eggink FA, Wisman GBA, Arts HJG, Oonk MHM, Mourits MJE, Yigit R, Versluis M, Duiker EW, Hollema H, de Bruyn M, Nijman HW (2016) CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur J Cancer 60:1–11. https://doi.org/10.1016/j.ejca.2016.02.026
Chang WC, Li CH, Huang SC, Chang DY, Chou LY, Sheu BC (2010) Clinical significance of regulatory T cells and CD8(+) effector populations in patients with human endometrial carcinoma. Cancer 116(24):5777–5788. https://doi.org/10.1002/cncr.25371
Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB (2004) Intratumoral CD8(+) T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res 10(13):4450–4456. https://doi.org/10.1158/1078-0432.CCR-0732-3
de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AGJ, Hollema H, Nijman HW (2009) Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol 114(1):105–110. https://doi.org/10.1016/j.ygyno.2009.03.022
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, Hironaka S, Hara H, Satoh T, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Doki Y, Kitagawa Y (2017) Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 18(5):631–639. https://doi.org/10.1016/S1470-2045(17)30181-X
Cook AM, Lesterhuis WJ, Nowak AK, Lake RA (2016) Chemotherapy and immunotherapy: mapping the road ahead. Curr Opin Immunol 39:23–29. https://doi.org/10.1016/j.coi.2015.12.003
Brown JS, Sundar R, Lopez J (2018) Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br J Cancer 118(3):312–324. https://doi.org/10.1038/bjc.2017.376
Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G (2015) Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28(6):690–714. https://doi.org/10.1016/j.ccell.2015.10.012
Wang W, Kryczek I, Dostal L, Lin H, Tan L, Zhao L, Lu F, Wei S, Maj T, Peng D, He G, Vatan L, Szeliga W, Kuick R, Kotarski J, Tarkowski R, Dou Y, Rattan R, Munkarah A, Liu JR, Zou W (2016) Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell 165(5):1092–1105. https://doi.org/10.1016/j.cell.2016.04.009
Acknowledgements
We thank the Department of Thoracic Surgery, Department of Pathology, and Center of Biobank of the Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital.
Funding
This work was supported by grants from National Science Foundation of China (U1604286, 81822043).
Author information
Authors and Affiliations
Contributions
Y-FG and Y-MQ designed the study; LH, Q-LG, X-MZ coordinated and drafted of the manuscript; CS, G-YC, Y-PS, Y-JY, Y-MZ, X-YW, S-LL edited and finalized the drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standard
All study protocols and donor consent documents were approved by the Ethics Committee of Zhengzhou University.
Informed consent
Written informed consent was obtained from all individual donors of biological samples for research use included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, L., Gao, QL., Zhou, XM. et al. Characterization of CD103+ CD8+ tissue-resident T cells in esophageal squamous cell carcinoma: may be tumor reactive and resurrected by anti-PD-1 blockade. Cancer Immunol Immunother 69, 1493–1504 (2020). https://doi.org/10.1007/s00262-020-02562-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02562-3