Abstract
Immune checkpoint inhibitors, including ipilimumab (IPI), achieve a clinical benefit in a small proportion of melanoma patients highlighting the need to investigate predictive biomarkers. In this study, we characterized tumor infiltrating lymphocytes (TILs), focusing on the CTLA-4+ subset, and evaluated their possible predictive significance. We characterized TIL density, cell type, and localization in 40 melanoma lesions from 17 patients treated with IPI. Associations of TILs with IPI timing, tissue localization, and response to IPI were estimated using a linear mixed-effects modelling approach. We found that most of TIL subsets increased in situ upon IPI therapy, with particular reference to FoxP3+ cells. TILs and TIL subsets, such as CD3+, CD45RO+, CTLA-4+, CD4+, CD8+ T cells, CD20+ B cells, and NKp46+ NK cells, showed significantly different spatial distributions in the tumor microenvironment being higher at the invasive margin (IM) as compared to the tumor center (TC) (P value < 0.001 for TIL score and P value < 0.05 for all subsets). Remarkably, high TIL score and density of CD3+, CD8+ T cells, and CTLA-4+ immune cells were significantly associated with a better response to IPI (P values = 0.002, 0.023, 0.007, and 0.001, respectively, for responders vs non-responders). In conclusion, we provide a detailed analysis of CTLA-4+ TIL distribution in melanoma tissues taking into account localization, relationship with CD3+/CD8+ TILs, and changes in response to IPI treatment. We identified that CTLA-4+ TILs may represent a marker of IPI response, alone or with CD3+/CD8+ subsets, although this requires confirmation in larger studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human monoclonal antibody ipilimumab (IPI) is the first checkpoint inhibitor (CI) approved by the US FDA for the treatment of metastatic melanoma based on overall survival improvement albeit in a small fraction of patients [1, 2]. The rationale for IPI clinical activity is the targeting of CTLA-4 immune checkpoint molecule on T cells (activated and regulatory), by blocking its inhibitory signaling upon interaction with B7 ligands expressed on the antigen presenting cells (APC). Based on this mechanism, IPI restores T-cell stimulatory pathways (i.e., IFN-γ, IL-2, and TNF-α) [3] leading to activation and expansion of tumor antigen-specific T cells [4, 5], particularly CD8+ CTLs [6], their intratumoral infiltration, and effector functions [4, 5].
Infiltration of the tumor microenvironment (TME) by immune cells is a feature, indicating that the host immune system is promoting a specific anti-tumor response to kill cancer cells and may represent a favorable prognostic factor in tumors including melanoma [7]. The immune infiltrate comprises different lymphoid cell populations (named tumor infiltrating lymphocytes; TILs) of the specific adaptive immunity including not only effector T cells (Teff cells), but also regulatory T cells (Tregs) and B cells, and of the innate immunity such as natural killer (NK) cells, macrophages, dendritic cells (DCs), myeloid-derived suppressor cells (MDCSs), and other immune cell types [8].
Thus, TME infiltration by TILs is likely an important factor for the anti-tumor activity of IPI [9]. Indeed, although IPI therapy has been associated with increased circulating lymphocytes as well as activated CD4+ and CD8+ T cells [10], TIL density and activity in the TME appear to be the major mechanism contributing to tumor regression.
Moreover, the localization of TILs at the tumor invasive margin (IM) or the tumor center (TC) may differently influence clinical response to IPI therapy as shown for the anti-PD-1 therapy [11].
Among the TIL subsets relevant to clinical benefit from IPI therapy, CD3+, CD4+ and CD8+ TILs seem to play a significant role given their higher density detected by immunohistochemistry (IHC) in metastatic lesions of responding patients compared to non-responding patients [7, 12]. The density of CTLA-4+ TIL subset in melanoma TME has not been characterized as well, and results are divergent and not exhaustive. Indeed, a significantly higher level of CTLA-4 expression in pre-treatment tumor samples has emerged from transcriptome data in patients responding to IPI [13] but the distribution of CTLA-4+ TILs was not investigated in that study. In contrast, a high percentage of CTLA-4+ TILs detected by IHC significantly correlated with poor overall survival and progression-free survival [14] but correlation with clinical response was not reported.
We here report a descriptive and exploratory pilot study aimed at characterizing TILs in terms of cell density, subset composition, and localization in melanoma tissues of patients before and after treatment with IPI. In addition, we attempt to correlate the distribution of each considered TIL subset, including the CTLA-4+ one, with patient clinical response to IPI therapy.
Materials and methods
Patients and tissue samples
This retrospective study included 40 tumor samples obtained from 17 patients with stage IV MM, enrolled from July 2010 to November 2013 at the Department of Medical Oncology, IRCCS Ospedale Policlinico San Martino, Genova, Italy, through the Italian Expanded Access Program (EAP) [15].
Patients were treated intravenously with IPI (3 mg/kg) every 3 weeks for four doses as previously described [15]. Clinical response was assessed as the best overall response (BOR), and classified as immune-related complete response (irCR), partial response (irPR), stable disease (irSD), or progressive disease (irPD) according to immune-related Response Criteria [16]. Patients with irCR, irPR, or irSD lasting at least 3 months were considered as responders (R) and patients with irPD as non-responders (NR) to treatment with IPI. The observation period ended by June 30th, 2015.
The tumor tissue samples were collected from the pathology archive of paraffin-embedded diagnostic tissues and were divided into two groups, according to the time of the first IPI cycle, which resulted in 16 pre-treatment (pre-IPI) and 24 post-treatment (post-IPI) tissue samples. All tissues, except three cases of primary tumors in the pre-IPI group, were from different metastatic localizations (MTS). Some patients had more than one sample before and after IPI treatment. Four out of the 40 samples were matched for being pre- and post-IPI tissues.
All tumor samples were excisional biopsies or surgical resection specimens.
Histological staining
Four μm-thick serial sections were freshly microtome cut from each paraffin block for maximum antigenicity [17]. One section was stained with hematoxylin/eosin to assess cell morphology of TILs and tumor cells; other ten sections were used for immunohistochemical staining. Regarding the infiltrate, TIL density and distribution in the entire tissue section were evaluated according to a score based on the grades used by Azimi et al. [18]: TIL grade 0 (absent), grade 1 (mild focal/multifocal or moderate focal), grade 2 (moderate multifocal or marked focal/multifocal or mild diffuse), and grade 3 (moderate or marked diffuse). TIL density and distribution were evaluated at both IM and TC localizations.
Immunohistochemistry for TIL immunological markers
Melanoma tissue sections were mounted on SuperFrost Plus slides (Thermo Scientific, Braunschweig, Germany) for IHC staining to evaluate, in the whole tissue section, the percentage of total T cells (CD3) and T-cell subsets (CD4/CD8/CD45RO/CTLA-4/FoxP3/PD-1), B cells (CD20), NK cells (CD56), and an NK cell subset (NKp46). All these TIL subsets were evaluated at both the IM and TC areas using the marker-specific antibodies described below.
Sections for IHC were dried, deparaffinized, and rehydrated. Endogenous peroxidase was blocked with 5% H2O2 for 10 min. Immunoreactions were performed using the automated BenchMark XT immunostainer® (Ventana Medical Systems, Arizona, USA). Standard heat-based antigen retrieval was performed for 30 min (when needed). Antibody reactivity was detected with AP-conjugated secondary antibody and Fast Red chromogen (red staining). After immunostaining, slides were counterstained with hematoxylin and cover slipped. Slides were evaluated independently by three pathologists (LM, FG, and MG) who were blinded to IPI data, and differences were resolved by consensus.
The following antibodies were used: anti-CD3 (SP7 clone, Ventana, prediluted) as a pan-T-cell marker; anti-CD4 (SP35 clone, Cell Marque, 1:10 dilution) for helper T cells; anti-CD8 (SP47 clone, Cell Marque, prediluted) for cytotoxic T cells; anti-CD45RO (UCHL1 clone, Ventana, prediluted) for memory T cells; anti-CTLA-4 (14D3 clone, eBioscience, 1:50 dilution) for CTLA-4+ immune cells; anti-FoxP3 (236A/E7 clone, Abcam, 1:50 dilution) for Tregs; anti-PD-1 (NAT105 clone, Abcam, 1:50 dilution) for PD-1+ T cells; anti-CD20 (L26 clone, Ventana, prediluted) for B cells; anti-CD56 (NRQ-42 clone, Cell Marque, prediluted) and anti-NKp46/NCR1 (195314 clone, DBA R&D Systems, 1:50 dilution) for evaluation of total NK cells and an NK cell subset, respectively.
For all immunomarkers, the whole tumor area was considered, the number of positive cells in hotspots was counted, and the percentage of positive cells for the single marker was calculated in relation to the comprehensive quantity of inflammatory cells. In particular, the number of immunostained cells was counted in an area at high power (40× magnification) in hot spot areas both at the invasive margin and at the tumor center and divided by the total number of cells (stained and unstained) and expressed as a percentage.
Histology and immunohistochemistry assessments were performed using a Leica Laborlux D Optical microscope (Leica Biosystem, Wetzlar, Germany), field number 18.
Statistical analysis
Patients and disease characteristics were explored using descriptive statistics. Discrete variables (e.g., sex, and ECOG-PS) were expressed as relative frequencies (percentages) and continuous variables (e.g., age, time since diagnosis) were summarized using median and range values.
All main relationships were evaluated using a normal mixed-effects regression modelling applied to log-transformed TIL data. This statistical methodology is appropriate to manage two important data features: first, the random-effects regression allows to take into consideration the within-subject clustering (repeated measurements of cell characteristics on each patient) and the longitudinal structure (pre- and post-IPI assessments) of our observations; second, the log transformation is able to fulfill the normality assumption and, at the same time, allows to compare groups through geometric means (GM) using the geometric mean ratio (GMR) as an index of association; finally, such a method is able to attenuate the statistical drawbacks due to data missingness by borrowing information from the whole sample to compute the GMRs [19].
The first analytical step was to evaluate the correlation of the repeated measurements of TIL and TIL subpopulation density with IPI treatment (post-IPI vs pre-IPI) and TIL localization (IM vs TC). The second step involved the analysis of the longitudinal profiles of TIL data according to BOR (NR vs R) and location within tumor (IM vs TC).
In all regression analyses, GMRs, along with corresponding 95% confidence limits (95% CL), were adjusted for age at first IPI cycle, sex, number of brain metastases, number of pre-IPI therapies, tissue localization, and time of IPI therapy.
A two-tailed P value less than or equal to 0.05 was considered as statistically significant. All analyses were performed using Stata (StataCorp. Stata Statistical Software. Release 13.1. College Station, TX, USA, 2013).
Results
Patient and tumor tissues characteristics
The study was conducted on 40 tumor tissues derived from 17 melanoma patients who underwent treatment with IPI. The pre-IPI and post-IPI median follow-up times were 35.4 and 14.9 months, respectively. The characteristics of melanoma patients analyzed are shown in Table 1.
Among the tissues analyzed, three tissues were primary tumors, while all the others were MTS of different localizations including 6 skin, 4 lymph nodes, 1 lung, 1 liver, and 1 peritoneum in the pre-IPI subgroup and 20 skin, 2 lymph nodes, 1 bone, and 1 ileum in the post-IPI subgroup (Supplementary Table 1). TILs and immunological markers were assessed on the tissues to define type and density of T cells, T-cell subsets, B cells, and NK cells.
We characterized TILs and TIL subsets according to the timing of IPI therapy (pre/post) and tissue localization (IM/TC). Results are expressed as GM values of TIL score and percentage of cell subset and, for comparisons between groups, as the ratio between the GM values (GMR) estimated using a log-normal random-effects regression modelling technique.
Characterization of TILs and TIL subsets according to IPI therapy
TIL grading was defined using a T score which takes into account both the cell density and distribution [18] and varied from 0 to 3 as described in the section “Materials and methods”. TIL subsets were evaluated as GM percentages of infiltrating cells positively stained for the specific immunomarker.
The majority of TILs, in both pre- and post-IPI tissues, were CD3+ or CD45RO+ cells followed by CD8+, CD4+, CTLA-4+, and CD20+ immune cells with the other subsets of NKp46+, FoxP3+, CD56+, and PD-1+ cells, representing a minority of immune cells (representative images in Fig. 1).
Representative immunohistochemical (IHC) stainings of pre-IPI melanoma tissues for different TILs’ immunomarkers. IHC staining was performed on formalin-fixed, paraffin-embedded tissue sections using anti-CD3/CD4/CD8/CD20/CD45RO/CD56/NKp46/FoxP3/PD-1/CTLA-4-specific antibodies. Antibody reactivity was detected with AP-conjugated secondary antibody and Fast Red chromogen (red staining), as described in the section “Materials and methods”. Slides were counterstained with hematoxylin before microscopical evaluation. Panels show the positively stained immune cell subsets distributed along the invasive margin (peritumoral immune cells) at the left of each image. For CTLA-4 staining, the positivity is in both the immune cells (at the left) and tumor cells (at the right of the image). H&E, haematoxylin and eosin. Original magnification: 60×. Scale bar, 100 µm
We did not find any consistent variation in TIL score between pre- and post-IPI tissues (TIL score GMR = 0.96, 95% CL = 0.72–1.29) (Fig. 2a and Supplementary Table 2a); however, we observed some differences, although not statistically significant, in the density of TIL subsets according to IPI therapy. Indeed, in the post-IPI tissues, we observed a mean increasing tendency of about 32% for CD3+ (GMR = 1.25, 95% CL = 0.48–3.27), CD4+ (GMR = 1.20, 95% CL = 0.54–2.67), CD8+ (GMR = 1.18, 95% CL = 0.50–2.80), CD45RO+ (GMR = 1.29, 95% CL = 0.49–3.37), CTLA-4+ (GMR = 1.22, 95% CL = 0.47–3.20), CD56+ (GMR = 1.32, 95% CL = 0.92–1.89), and FoxP3+ (GMR = 1.77, 95% CL = 0.92–3.40) immune cells. In contrast, all other immune cells (PD-1+, CD20+ and NKp46+) showed an average reduction of about 23% (Fig. 2a and Supplementary Table 2a).
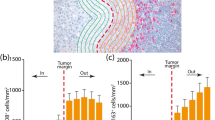
Caterpillar plots of TIL score and TIL subset density according to a IPI treatment (post-IPI vs pre-IPI) and b TIL localization (IM, invasive margin vs TC, tumor center). TIL score and TIL density were evaluated by haematoxylin/eosin and IHC, respectively. The value of the geometric mean ratio (GMR, represented by a dot) and corresponding 95% confidence limits (95% CL, represented by two horizontal “whiskers”) estimated using a linear mixed-effects modelling are reported on the abscissa. Vertical line (GMR = 1) is used as a reference to indicate whether the ratio is in favor of the numerator (GMR > 1) or in favor of the denominator (GMR < 1). When “whiskers” do not cross GMR = 1, the difference in the two GM values is to be considered as statistically significant, namely GMR ≠ 1. All GMRs were adjusted for age at IPI, sex, number of brain metastases, number of pre-IPI therapies, and tissue localization for a and time of IPI therapy for b. a GMR shows the ratio of post-IPI GM to pre-IPI GM, while b GMR shows the ratio of IM GM to TC GM. TIL tumor infiltrating lymphocytes
Characterization of TILs and TIL subsets according to IM and TC localization
We characterized TIL density and TIL subsets in the separate areas of IM and TC in the TME of all melanoma tissues, independently from the time of IPI delivery.
TIL score was found 75% higher in IM with respect to TC (TIL score GMR = 1.75, 95% CL = 1.43–2.04, P value < 0.001) and this remarkable enhancement was accompanied by an increase of almost all the immune cell subsets in the IM area (Fig. 2b and Supplementary Table 2b). An IM density of about fourfold higher was observed for CD3+ (GMR = 4.02, 95% CL = 2.00–8.33, P value < 0.001), CD45RO+ T cells (GMR = 4.17, 95% CL = 2.04–8.31, P value < 0.001), as well as for CTLA4+ immune cells (GMR = 4.00, 95% CL = 2.04–7.69, P value < 0.001), CD4+ (GMR = 3.45, 95% CL = 1.85–6.25, P value < 0.001) and CD8+ (GMR = 3.23, 95% CL = 1.79–5.88, P value < 0.001) T cells. In IM, a lower but significant GM rise in density of about 90% was observed for CD20+ cells (GMR = 2.08, 95% CL = 1.23–3.45, P value = 0.006) and NKp46+ cells (GMR = 1.75, 95% CL = 1.10–2.86, P value = 0.018), whereas a non-significant excess of about 10%, 20%, and 55% was found for PD-1+, CD56+ and FoxP3+ cells, respectively (Fig. 2b and Supplementary Table 2b).
Correlation of TILs and TIL subsets with response to IPI therapy
Correlations between TIL score and density of TIL subsets with BOR were also assessed over the whole period of follow-up (i.e., from diagnosis of primary melanoma). For this purpose, non-responding patients (NR, i.e., irPD) were compared to responding patients (R, i.e., irCR/irPR or irSD). Of the 17 patients, 7 (41.2%) showed irPR or irCR, 3 (17.6%) irSD, and 7 (41.2%) irPD.
Using R patients as a reference category (Table 2), NR patients were found to have a 57% lower TIL score than that computed for patients with a better prognosis (GMR = 0.43, 95% CL = 0.25–0.74, P value = 0.002) (Table 2). The same was observed for CD3+ cells that showed a 74% reduced density (GMR = 0.26, 95% CL = 0.08–0.84, P value = 0.023) and for almost all TIL subsets. In particular, CD8+ and CTLA-4+ cells showed a reduction of 73% and 87%, respectively (GMR = 0.27, 95% CL = 0.10–0.69, P value = 0.007 and GMR = 0.13, 95% CL = 0.04–0.43, P value = 0.001).
CD4+, CD45RO+, FoxP3+, and CD20+ cells altogether presented on average a decrease of about 60% (Table 2). Also for NKp46+ cells, a 44% reduction (GMR = 0.56, 95% CL = 0.21–1.46, P value = 0.234) was pointed out in NR patients with respect to R patients. Only the CD56+ subset showed a higher GMR for the same comparison (GMR = 1.64, 95% CL = 0.76–3.51, P value = 0.207) (Table 2).
Correlation of TILs, CD3+, CD8+, and CTLA-4+ TIL subsets with response to IPI therapy according to IM and TC localization
We further characterized TIL grading and density of CD3+, CD8+, and CTLA-4+ TIL subsets, that showed significant correlation with BOR, in relation to their localization in IM and TC.
Also in this context, the results are represented as GMR of TIL data (Table 3). In particular, using TILs in TC (TILTC) as a reference category, we found that TILs in IM (TILIM) showed a significant increase of almost twofold (GMR = 1.75), fourfold (GMR = 4.02), threefold (GMR = 3.19), and fourfold (GMR = 3.98) for TIL score and percentages of CD3+, CD8+, and CTLA-4+ TILs, respectively, in R patients as compared to NR patients (P value < 0.001, Table 3). An identical relationship between the TIL variables in IM versus TC was found in NR patients (P value < 0.001, Table 3), but with remarkably lower values in respect to R patients.
Interestingly, these results highlight the novel finding of a possible role of the CTLA-4+ TIL subset in response to IPI therapy, regardless of TIL tissue localization (IM or TC). Images of different density of CTLA-4+ TILs in representative cases of responding and non-responding patients are shown (Fig. 3).
Representative immunohistochemical (IHC) stainings of CTLA-4 expressing TILs in melanoma tissues from patients responding (R) and non-responding (NR) to IPI therapy. CTLA-4 IHC staining was performed, with the antibody 14D3, on formalin-fixed, paraffin-embedded tissue sections from melanoma patients before (pre-IPI) and after (post-IPI) treatment with ipilimumab (IPI). Antibody reactivity was detected with AP-conjugated secondary antibody and Fast Red chromogen (red staining), as described in the section “Materials and methods”. Slides were counterstained with hematoxylin before microscopical evaluation. a and e Melanoma tissues from two R patients showing CTLA-4 positively stained TILs (white arrows) distributed along the invasive margin at the left of the tumor cells. b and f Melanoma tissues from two NR patients showing CTLA-4 negative TILs (white arrows) distributed along the invasive margin at the left (b) and at the right (f) of the tumor cells. Tumor cells show a moderate CTLA-4 positivity (a, b) and a strong CTLA-4 positivity (e, f). H&E, haematoxylin and eosin (c, d, g, h). Original magnification: 60×. Scale bar, 100 µm. TIL tumor infiltrating lymphocytes
Discussion
IPI was the first human monoclonal antibody developed for targeting anti-tumor immune response through the blockade of CTLA-4 immune checkpoint (IC). Initially used as a single agent to treat metastatic melanoma [1, 2], IPI is currently used mainly in combinatorial therapies (with anti-PD-1 or other agents) or in second-/third-line setting [20]. Despite its remarkable success in improving patient survival, only a subgroup of patients benefit from IPI therapy [21]. The identification of robust and valid biomarkers predictive of response to IPI is thus needed.
In this line, the role of TILs as predictive biomarkers has been less intensively studied than other peripheral markers (i.e., levels of LDH, CRP, FoxP3/Treg cells, WBC, and ALC, ref [22]) or intratumoral markers (i.e., mutational burden and neoepitope signature, ref [23]).
In the present study, we characterized TIL score and subset distribution before and after IPI therapy and in different TME localizations (IM, invasive margin or TC, tumor center).
Our results did not reveal any consistent variation in TIL score upon IPI therapy, probably because of the limited number of tissues which we had at our disposal. By contrast, TIL score in IM was almost twofold higher than that in TC. Similarly, all the TIL subsets analyzed were found higher in IM than in TC. These findings not only are in agreement with the previous observations in melanoma and other tumors [24, 25], suggesting that the immune response is taking place predominantly in this area, but they can also predict a better response to IC blockers [26].
To deeply characterize TIL composition, we conducted a subset analysis by performing immunostainings for T cell, B cell, and NK cell phenotypic markers. In both pre- and post-IPI tissues, we found a prevalence of total T cells (CD3+) with high density of cytotoxic CD8+ and memory CD45RO+ T cells, findings that have been previously reported in a different clinical setting [5] and associated with a good prognosis, with particular reference to CD8+ T cells [27].
The density of all TIL subsets, except those expressing PD-1, CD20, and NKp46, had a tendency to increase upon IPI therapy and to maintain the same relationship in cellular density (i.e., order of pre- and post-IPI density: CD3 > CD45RO > CD8 > CD4 > CTLA-4 > CD20 > NKp46 > FoxP3 > CD56 > PD-1 expressing cells). These findings are in line with the increase of CD8+ T cells previously reported in a pilot study on tumor biopsies from patients treated with tremelimumab [28].
Interestingly, FoxP3+ T cells showed the highest increase after IPI therapy, although they remained a minority of immune cells. This result is in line with the finding that IPI can increase the density of FoxP3+ cells within melanoma TME [29].
Similar changes in TIL score and TIL subset distribution upon IPI therapy and in IM vs TC localization were also observed when we analyzed separately both the four patients with pre-/post-IPI matched tissues and those with the metastatic tissues (Supplementary Table 3).
We then correlated our results on TILs with patients’ response to IPI. We found that NR patients showed a significantly reduced density of TILs and a strong decrease in CD3+ T cells (by 74%), with particular reference to the cytotoxic CD8+ T cells (by 73%) and activated CTLA-4+ immune cells (by 87%), as compared to R patients. Also CD45RO+ T cells and FoxP3+ cells were quite reduced in NR patients. Thus, a high density of TILs and the specific CD3+, CD8+, and CTLA-4+ TIL subsets resulted significantly associated with a better response to IPI treatment.
The trend towards higher levels of FoxP3+ cells that we observed in responding patients is consistent with the previous findings in advanced melanoma patients treated with IPI [30] and they may suggest that FoxP3 marker does not necessarily identify a CD4+ Treg cell population but other anti-tumor Teff cells with no suppressive function [31].
It is worth noting that CTLA-4+ immune cells were found highly reduced in NR patients as compared to R patients. The tissue localization of CTLA-4+ TILs did not appear to influence the response to IPI as they showed an increased density in IM vs TC in both R and NR patients although with lower values in the latter group.
Thus, an increased density of CTLA-4+ cells in responding patients might indicate a higher density of activated immune cells in respect to non-responding patients. This can be due to the functional role of CTLA-4 that, besides being an inhibitory IC, can be regarded as an activation marker which appears at the primary phase of CD4+ and CD8+ T-cell activation and can be expressed also by activated B cells and other immune cells.
Our findings are consistent with previous observations regarding the correlation between high CTLA-4 mRNA content and favorable outcome of melanoma patients [13], although those studies, performed only at transcriptional level, included also the CTLA-4 transcript most likely derived from the tumor cells that can express CTLA-4 at variable levels as we previously reported [32].
Indeed, we found that CTLA-4 was also expressed, at different levels, on the tumor cells of our tissue samples (Figs. 1, 3), but its possible relevance in IPI-treated melanoma patients remains to be defined.
In conclusion, our results show a positive correlation between high TIL density and distribution of CD3+, CD8+ and CTLA-4+ TIL subsets with the response to IPI in MM patients, independently from their tissue localization.
Moreover, to date, there are no studies on the relationship between the density of CTLA-4+ infiltrating immune cells, evaluated by IHC, and response to IPI therapy in melanoma patients, also in relation to their tissue localization. The results of our pilot study may suggest that the density of CTLA-4+ TILs, alone or in combination with CD8+ and/or CD3+ TILs, may be a useful indicator of a possible response to IPI therapy in melanoma patients. Interestingly, the higher density of CTLA-4+ TILs that we observed in R patients with respect to NR patients may be similar to the one demonstrated, within the tumor infiltrating CD8+ T-cell subset, in melanoma patients responding to anti-PD-1 treatment [11].
Although this study has several limitations, among which the small sample size, the lack of matched pre-/post-IPI tissue cases, the inclusion of primary tumors (n = 3) among the metastatic lesions analyzed (n = 37), and the IHC single-stainings for immune markers, we infer from our findings that CTLA-4+ TILs might be indicative of a better outcome in IPI-treated melanoma patients. Certainly, further experimental evidence obtained in larger and prospective cohorts of melanoma patients is required to corroborate this hypothesis.
Abbreviations
- 95% CL:
-
95% Confidence limits for GMR
- BOR:
-
Best overall response
- CI:
-
Checkpoint inhibitor
- CTLA-4:
-
Cytotoxic T lymphocyte antigen-4
- EAP:
-
Expanded access program
- GM:
-
Geometric mean
- GMR:
-
Geometric mean ratio
- IC:
-
Immune checkpoint
- IHC:
-
Immunohistochemistry
- IM:
-
Invasive margin
- IPI:
-
Ipilimumab
- irCR:
-
Immune-related complete response
- irPR:
-
Immune-related partial response
- irPD:
-
Immune-related progressive disease
- irSD:
-
Immune-related stable disease
- MTS:
-
Metastatic tumors
- NR:
-
Non-responder patients
- R:
-
Responder patients
- TC:
-
Tumor center
- TILs:
-
Tumor infiltrating lymphocytes
- TILIM :
-
TILs at the invasive margin
- TILTC :
-
TILs at the tumor center
- TME:
-
Tumor microenvironment
References
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. https://doi.org/10.1056/NEJMoa1003466
Robert C, Thomas L, Bondarenko I et al (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517–2526. https://doi.org/10.1056/NEJMoa1104621
Ji R-R, Chasalow SD, Wang L et al (2012) An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother CII 61:1019–1031. https://doi.org/10.1007/s00262-011-1172-6
Peggs KS, Quezada SA, Korman AJ, Allison JP (2006) Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol 18:206–213. https://doi.org/10.1016/j.coi.2006.01.011
Tarhini AA, Edington H, Butterfield LH et al (2014) Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE 9:e87705. https://doi.org/10.1371/journal.pone.0087705
Felix J, Lambert J, Roelens M et al (2016) Ipilimumab reshapes T cell memory subsets in melanoma patients with clinical response. Oncoimmunology 5:1136045. https://doi.org/10.1080/2162402X.2015.1136045
Balatoni T, Ladányi A, Fröhlich G et al (2018) Biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Pathol Oncol Res POR. https://doi.org/10.1007/s12253-018-0466-9
Lee N, Zakka LR, Mihm MC, Schatton T (2016) Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology (Phila) 48:177–187. https://doi.org/10.1016/j.pathol.2015.12.006
Hodi FS, Butler M, Oble DA et al (2008) Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA 105:3005–3010. https://doi.org/10.1073/pnas.0712237105
Martens A, Wistuba-Hamprecht K, Yuan J et al (2016) Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab. Clin Cancer Res 22:4848–4858. https://doi.org/10.1158/1078-0432.CCR-16-0249
Daud AI, Loo K, Pauli ML et al (2016) Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest 126:3447–3452. https://doi.org/10.1172/JCI87324
Diem S, Hasan Ali O, Ackermann CJ et al (2018) Tumor infiltrating lymphocytes in lymph node metastases of stage III melanoma correspond to response and survival in nine patients treated with ipilimumab at the time of stage IV disease. Cancer Immunol Immunother CII 67:39–45. https://doi.org/10.1007/s00262-017-2061-4
Van Allen EM, Miao D, Schilling B et al (2015) Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350:207–211. https://doi.org/10.1126/science.aad0095
Chakravarti N, Ivan D, Trinh VA et al (2017) High cytotoxic T-lymphocyte-associated antigen 4 and phospho-Akt expression in tumor samples predicts poor clinical outcomes in ipilimumab-treated melanoma patients. Melanoma Res 27:24–31. https://doi.org/10.1097/CMR.0000000000000305
Queirolo P, Dozin B, Morabito A et al (2017) Association of CTLA-4 gene variants with response to therapy and long-term survival in metastatic melanoma patients treated with ipilimumab: an Italian melanoma intergroup study. Front Immunol. 8:386. https://doi.org/10.3389/fimmu.2017.00386 (Erratum in: Front Immunol. 2018, 9:403. doi: 10.3389/fimmu.2018.00403)
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420. https://doi.org/10.1158/1078-0432.CCR-09-1624
Grillo F, Pigozzi S, Ceriolo P et al (2015) Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol 144:93–99. https://doi.org/10.1007/s00418-015-1316-4
Azimi F, Scolyer RA, Rumcheva P et al (2012) Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 30:2678–2683. https://doi.org/10.1200/JCO.2011.37.8539
Ghisletta P, Renaud O, Jacot N, Courvoisier D (2015) Linear mixed-effects and latent curve models for longitudinal life course analyses, chapter 8. In: Burton-Jeangros C, Cullati S, Sacker A, Blane D (eds) A life course perspective on health trajectories and transitions. Springer, Cham, p 2015
Luke JJ, Flaherty KT, Ribas A, Long GV (2017) Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 14:463–482. https://doi.org/10.1038/nrclinonc.2017.43
Schadendorf D, Hodi FS, Robert C et al (2015) Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33:1889–1894. https://doi.org/10.1200/JCO.2014.56.2736
Simeone E, Gentilcore G, Giannarelli D et al (2014) Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother CII 63:675–683. https://doi.org/10.1007/s00262-014-1545-8
Bassani-Sternberg M, Bräunlein E, Klar R et al (2016) Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun 7:13404. https://doi.org/10.1038/ncomms13404
Steele KE, Tan TH, Korn R et al (2018) Measuring multiple parameters of CD8+ tumor-infiltrating lymphocytes in human cancers by image analysis. J Immunother Cancer 6:20. https://doi.org/10.1186/s40425-018-0326-x
Miyan M, Schmidt-Mende J, Kiessling R et al (2016) Differential tumor infiltration by T-cells characterizes intrinsic molecular subtypes in breast cancer. J Transl Med 14:227. https://doi.org/10.1186/s12967-016-0983-9
Tumeh PC, Harview CL, Yearley JH et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568. https://doi.org/10.1038/nature13954
Piras F, Colombari R, Minerba L et al (2005) The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer 104:1246–1254. https://doi.org/10.1002/cncr.21283
Ribas A, Comin-Anduix B, Economou JS et al (2009) Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res 15:390–399. https://doi.org/10.1158/1078-0432.CCR-08-0783
Sharma A, Subudhi SK, Blando J et al (2019) Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers—response. Clin Cancer Res 25:3469–3470. https://doi.org/10.1158/1078-0432.CCR-19-0402
Hamid O, Schmidt H, Nissan A et al (2011) A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 9:204. https://doi.org/10.1186/1479-5876-9-204
Allan SE, Crome SQ, Crellin NK et al (2007) Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 19:345–354. https://doi.org/10.1093/intimm/dxm014
Laurent S, Queirolo P, Boero S et al (2013) The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med 11:108. https://doi.org/10.1186/1479-5876-11-108
Acknowledgements
We thank Simona Pigozzi for her technical help in the immunohistochemistry laboratory.
Funding
This work was supported by grants awarded by: 5×1000 Italian Ministry of Health 2014 and 2015 (to Maria Pia Pistillo, Luca Mastracci, and Vincenzo Fontana) and Italian Ministry of Health, project Code RF-2016-02362288 (to Paola Queirolo).
Author information
Authors and Affiliations
Contributions
Study concept and design, study supervision: MPP and AP. Recruitment and management of melanoma patients: PQ, FS, ET, and AB. Immunohistochemical stainings and evaluation of results: LM, FG, MG, and SS. Acquisition, analysis, or interpretation of data: VF, AM, LM, BD, and MPP. Drafting of the manuscript: MPP, VF, BD, and AP. Statistical analyses: VF, BD, and MPP. Administrative, technical, or material support: AM, RC, and BB. Critical revision of the manuscript for important intellectual content: MPP, AP, MR, FS, and PQ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Paola Queirolo served on the Advisory Boards of Roche, Novartis, Bristol-Myers Squibb, Merck Sharp and Dohme, Sanofi, and Pierre-Fabre. All other authors have declared no conflict of interest.
Ethical approval
The study was approved by the local Ethics Committee (CE) of the Liguria Region (CE-IST OMA07.024 amended on January 2nd 2011). All procedures performed in the study were in accordance with the Helsinki Declaration
Informed consent
All patients signed an informed consent before enrollment in the study. In the leaflet of the consent, it was clearly explained that the patient would agree that part of the tumor tissue excised would be used for the immunological assessment foreseen in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mastracci, L., Fontana, V., Queirolo, P. et al. Response to ipilimumab therapy in metastatic melanoma patients: potential relevance of CTLA-4+ tumor infiltrating lymphocytes and their in situ localization. Cancer Immunol Immunother 69, 653–662 (2020). https://doi.org/10.1007/s00262-020-02494-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02494-y