Abstract
At present, significant experimental and clinical data confirm the active involvement of the peripheral nervous system (PNS) in different phases of cancer development and progression. Most of the research effort focuses on the impact of distinct neuronal types, e.g., adrenergic, cholinergic, dopaminergic, etc. in carcinogenesis, generally ignoring neuroglia. The very fact that these cells far outnumber the other cellular types may also play an important role worthy of study in this context. The most prevalent neuroglia within the PNS consists of Schwann cells (SCs). These cells play a substantial role in maintaining homeostasis within the nervous system. They possess distinct immunomodulatory, inflammatory and regenerative capacities—also, one should consider their broad distribution throughout the body; this makes them a perfect target for malignant cells during the initial stages of cancer development and the very formation of the tumor microenvironment itself. We show that SCs in the tumor milieu attract different subsets of immune regulators and augment their ability to suppress effector T cells. SCs may also up-regulate invasiveness of tumor cells and support metastatic disease. We outline the interactive potential of SCs juxtaposed with cancerous cells, referring to data from various external sources alongside data of our own.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The major public health issue of cancer currently stands as the second leading cause of death in the United States. Its generalized national approximation of 1,735,350 new cases (not including skin cancers) corresponds to an equivalent of over 4700 new cancer diagnoses daily [1]. By 2020, it is expected that the number of new cancer cases in the United States to go up to almost 2 million cases per year. Therefore, it is really important to improve our understanding of the biology of cancer, including its initiation, development and progression, as well as the interplay among the individual factors, cells, tissues, organs and internal and external environmental systems influencing cancer biology at different levels. Although over the past decades significant progress has been made in our ability to precisely and effectively prevent and treat the complex group of more than 200 diseases we call cancer [2], our current knowledge of cancer-causing and controlling intrinsic systems and their dysregulation by extrinsic multi-environmental factors is unconditionally incomplete. New scientific concepts are needed to reveal crucial cellular and molecular changes that lead to cancer development, survival and spreading.
Solid tumors act as ectopic ‘rogue organs’ that depend upon the surrounding host tissue for development and dissemination—the formation of the tumor microenvironment (TµE) occurs between the mutationally disrupted cancerous cells, non-transformed normal tissue, bone marrow-derived cells and other stromal elements in the local environs. The TµE demonstrates that tumor cells do not manifest the disease by themselves, but rather conscript the surrounding normal cells and tissues to serve as members in the rebel cellular mass [3]. Communication between native cell types and the tumor cells occurs through an intricate system of chemical messengers, e.g., cytokines, adipokines, chemokines, growth factors, and inflammatory agents, enzymes, hormones, neuromediators that occurs alongside significant disruption to the normal tissue [4].
Historically, the central nervous system (CNS) has revealed a direct involvement in the development and growth of cancer [5, 6]. Animal model studies clearly confirmed that chronic stressors could promote tumor growth, cancer cell dissemination and decreased survival of tumor-bearing hosts [7, 8]. Both the “fight-or-flight” stress responses of the sympathetic-adrenal-medullary axis and the “defeat/withdraw” responses of the hypothalamic–pituitary–adrenal axis influence multiple aspects of tumorigenesis and cancer progression [9, 10]. In fact, the activation of both axes initiates molecular signaling pathways involved in DNA repair, angiogenesis, cell survival, inflammation, immunity, invasion, metastasis and resistance to therapy [9, 11, 12].
While studies of the CNS have brought deeper insights into cancer-initiating pathways, investigations of the peripheral nervous system (PNS), particularly the autonomic nervous system, have provided robust data on the mechanisms underlying stress-related tumorigenesis and invasiveness. The PNS consists of the nerves and ganglia outside the CNS (brain and spinal cord) connecting the CNS to all parts of the body including skin, muscles and organs [13]. The PNS is divided into two parts: (i) the somatic nervous system responsible for transmitting sensory information via sensory (afferent) neurons to the CNS and motor information from the CNS via motor neurons to muscle fibers and (ii) the autonomic nervous system, which is further divided into the sympathetic and parasympathetic divisions regulating involuntary body functions by transmitting efferent signals from the CNS to different tissues. Catecholamines from the sympathetic part of the autonomic nervous system along with acetylcholine from the parasympathetic part can modulate the associated cells and the factors implicated in the processes of angiogenesis and metastasis [14,15,16]. Studies have also shown that the progression of cancer requires autonomic nerve sprouting in solid tumors. Furthermore, research has implicated both sympathetic and parasympathetic nerves as active participants throughout all phases of cancer development in the mouse models [17], thus confirming the role of the PNS in tumorigenesis. However, numerous questions have been left behind, including the role of sensory neurons, involvement of neuroglial elements, neuroimmune axis in the TµE, neurodegenerative pathways, centralized and adjunct tumor innervation and others.
Peripheral innervation of solid tumors
Initial studies focusing on the role of the PNS in cancer arose from the series of observations which identified nerve bundles within various solid tumors. Although histopathological data indicates the presence and prognostic value of intratumoral nerve fibers in various types of cancer [17,18,19,20], alternative studies report the presence of nerve bundles only within the peritumoral area, while tumor lesions contain few if any nerve filaments [21,22,23]. Inconsistancies in those research were explained by the use of different cancer models, tumor staging, methodologies and data analysis. Additionally, one should note that the widely used nerve-bundle staining agents (e.g., anti-PGP 9.5 antibody) may not be the best and most discrete markers for the verification of specific nerve fibers and nerve filaments within specific tumor sites [24]. It appears overall that the ‘traditional’ context of the ‘PNS in cancer’ studies focuses in general on the autonomic nervous system and its role in carcinogenesis. However, research consistently ignores an overwhelming presence of peripheral nerve bundles in this context—namely the free nerve ends of sensory nerves within the PNS. For instance, in spite of the facts that (i) in the skin, cutaneous nerve fibers are principally sensory, (ii) sensory nerves innervate the epidermis and dermis and (iii) autonomic nerves never innervate the epidermis in mammals, data assessing the role of the PNS nerves in skin cancer development are very limited. Functional in vivo models are required to determine the role of abundant skin innervation in the appearance, survival, proliferation and spreading of different types of skin cancer.
Schwann cells within the peripheral nervous system
The supportive tissue of the nervous system includes the network of branched cells in the CNS (astrocytes, microglia and oligodendrocytes) and the supporting cells of the PNS (Schwann cells and satellite glial cells). Glial (neuroglial) cells are far more numerous than neurons and, unlike neurons, do not conduct nerve impulses, but, instead, support, nourish and protect the neurons. In the PNS, Schwann cells (SCs) represent the most prevalent glial cell type [25, 26]. Initially, they were recognized for ensheathing the nerve fibers, producing myelin, providing trophic support for neurons, constructing the nerve extracellular matrix, supporting nerve survival, perineuronal organization and modulating neuromuscular synaptic activity [27]. SCs also play a seminal role in the response to neuronal damage and repair and an increasingly recognized active role in pain syndromes. Specifically, during peripheral nerve injury SCs facilitate endogenous axonal regrowth due to their ability to dedifferentiate, proliferate, migrate, produce promoting growth factors and myelinate regenerating axons. When a nerve fiber is cut or crushed, an active process of degeneration called Wallerian degeneration is initiated, and SCs after sensing of axonal injury take the major role in myelin and neurofilament debris cleaning during the first hours. Then by release of cytokines and chemokines, SCs recruit macrophages that help improve the clearing rate. Morphological and biochemical reprogramming of SCs during Wallerian degeneration, which results in the establishment of a microenvironment supportive of axonal regeneration, includes SC dedifferentiation, proliferation and detachment and moving from an axon (denervation) and results in accumulation of so-called repair SCs [28,29,30].
Chronic denervation proves lethal for SCs [31]; yet, studies note that during Wallerian degeneration the SCs become both a cue and a substratum for the growth cone of regenerating axons. This means that they lose the capability of maintaining myelin and dedifferentiate—along with gaining the ability to both survive without axonal interactions and promote immune cell infiltration [31]. Jessen et al. reported that the dedifferentiation of SCs from the myelinating to the non-myelinating/immature state becomes triggered by an upregulation of c-JUN protein [32]. After the loss of contact with the axon, these dedifferentiated denervated SCs called ‘repair SCs’ [33] upregulate synthesis and secretion of tumor necrosis factor α (TNF-α), interleukin-1α (IL-1α) and IL-1β [34] that contribute to macrophage recruitment [35] and SC proliferation [36]. Interestingly enough, Napoli et al. reported that myelinating SCs expressing an inducible Raf-kinase in themselves sufficiently drive dedifferentiation and cause demyelination, breakdown of the blood–nerve barrier and influx of immune cells in the absence of injury signals derived from axon degeneration [37]. During this stage, repair SCs proliferate and migrate to form bands of Büngner, which provide a pathway for regenerating axons; then SCs differentiate again for myelinating regenerating axons [38]. The SCs in the injured nerves share some features with immature SCs in that both cell types possess an autocrine function to survive and regain the ability to interact with axons [25].
The dynamic interaction between SCs, immune and other somatic cells plays a major role in local nerve tissue homeostasis. During the first day after the injury, neutrophils briefly crowd the site, after which the activated macrophages accumulate by day three. Initially, they contribute to the inflammatory state by their production of TNF-α and IL-1β. However, once the myelin degrades, alternatively-activated M2 macrophages become dominant during Wallerian degeneration—upregulating the anti-inflammatory cytokine IL-10, which then results in down-regulation of pro-inflammatory cytokines [39]. Moreover, SC-produced cytokines stimulate production of IL-6 and granulocyte–macrophage colony-stimulating factor (GM-CSF) by fibroblasts within 4 h after axotomy [40]. Interestingly, SCs associate rather closely with fibroblasts. Human postnatal fibroblasts may evidently transdifferentiate into functional SCs via a transient progenitor step and a conversion procedure that is uniquely based on chemical treatment and does not involve an overexpression of ectopic genes [41]. The resultant induced SCs or iSCs can be characterized by expression of SC-specific proteins and neuron supportive and myelination properties in vitro.
Additionally, SCs secrete several potent regulators of angiogenesis [42, 43] and produce neurotrophic factors—e.g., nerve growth factor, brain-derived neurotrophic factor and ciliary neurotrophic factor [44, 45].
Based on this fact that SCs are the key regulators of peripheral nerve degeneration and repair and on recent data showing that SCs may also directly affect non-neural wound healing [46, 47], we developed and proved a new concept stating that functionally-modified repair-like SCs should also appear during the destruction of neurons as the tumor expands (Shurin et al., submitted). In other words, chronic cancer-induced reprogramming of SCs in the tumor milieu is characterized by a non-resolving neurodegenerative process, unlike the resolving culmination of normal tissue regeneration in the tumor-free environment. Observed denervation of the foremost tumor mass can be explained by a related course of dying back or retrograde degeneration known as ‘Wallerian-like degeneration’ [48] which occurs in various neurodegenerative diseases, especially those where axonal transport is impaired. Using both in vivo and in vitro models, we demonstrated that tumor-induced repair-like SCs could markedly alter the local environs by changing attraction and function of immune cells and altering the extracellular matrix, which resulted in tumor growth and progression in vivo. Importantly, the local inhibition of nerve injury-induced ‘classic’ repair SCs or tumor-induced repair-like SCs significantly decreased the rate of tumor growth suggesting that SCs may present a novel target for cancer therapy (Shurin et al., submitted).
Thus, SCs may play different roles in the TµE both stimulating and inhibiting tumor growth, which results from either (i) direct effect on malignant cell survival, motility and differentiation or (ii) indirect modulation of the tumor environs via immune cells, fibroblasts and angiogenesis. Furthermore, the role of SCs in cancer is not limited to direct and indirect effects on malignant cells—SCs also play an important role in cancer pain symptom and probably in paraneoplastic pathways.
Malignant Schwann cells
SCs, thus, are unique in their ability to dedifferentiate and reprogram the local environmental patterns. SC precursors act as an ontogenic source for various cell types: fibroblasts, melanocytes, neurons, parasympathetic ganglia and SCs themselves [49]. Due to their plasticity and wide dispersion, SCs are considered as a multipotent cellular pool for PNS regeneration and development [50]. Also, one should take into account the fact that a variety of tumors arise from SCs, e.g. malignant peripheral nerve sheath tumors, schwannomas, neurofibromas and the Devil Facial Tumor Disease (DFTD)—the latter comprising one of the rarely-seen contagious malignancies [51, 52]. The rare and transmissible Devil Facial Tumor Disease affects the Tasmanian devil (Sarcophilus harrisii) and supposedly relates to their population collapse [53]. Loh et al. describe it as a soft tissue neoplasm consisting of undifferentiated round/spindle-shaped cells with few defining ultrastructural features [54]. Surprisingly, this cytologically undifferentiated tumor expresses markers of highly differentiated Schwann cells [55]. Murchison has proposed that this peripheral nerve sheath tumor derives from an SCs or SC precursors; the miRNA profile of DFTD supports this claim [56]. Multivariate expression of vimentin, S100, neuron-specific enolase, chromogranin A and synaptophysin markers suggests the possibility of neuroendocrine origin for DFTD [54].
SC development and maturation involves several factors crucially linked to cancer. Autocrine stimulation by neuregulin Nrg1 Type I in lung and ovarian cancer cells that also express ErbB2/ErbB3 neuregulin receptors, and similarly in the neoplastic growth of SCs has been reported [57, 58]. For instance, high-level ErbB2 expression in human lung cancers carries prognostic information [58], while blocking ErbB2 on human lung tumor cell lines expressing ErbB2 inhibits cell line proliferation [59]. Moreover, Schwannian differentiation is frequently observed in benign intradermal nevi (‘neurotization’) and malignant melanocytic tumors [60].
Schwann cells and neuroblastoma
Most of the available experimental data demonstrate a protumor role for SCs in vitro and in vivo: altering the extracellular matrix, chemoattraction of malignant cells, modulating the tumor immunoenvironment and supporting perineural invasion (PNI) of tumor cells [61]. However, SCs play a much different role in neuroblastoma (NB) development and growth. This childhood cancer, which mostly affects children under 15 years old, has a complex pathogenesis with various factors involved in its development [62]. NB tumors exhibit a broad spectrum of clinical behavior reflective of their biologic heterogeneity [63]. These tumors consist of two primary cell populations (neuroblastic/ganglionic cells and SCs) and the quantity of Schwannian stroma directly correlates with tumor maturation [64]. NB tumors with abundant Schwannian stroma have a differentiated phenotype, reduced vascularity and come associated with a favorable prognosis [64, 65] since infiltrating SCs reliably promote neuroblast differentiation, induce apoptosis, inhibit angiogenesis and proliferate in NB xenografts [65].
Thus, SCs get involved in NB tumorigenesis and development through several pathways: inhibiting angiogenesis, impairing NB growth and promoting NB differentiation [66, 67]. SCs can affect neuroblastoma phenotype, as SC-conditioned medium or co-cultured SCs increase neuroblast differentiation [66, 68]. The inflammatory factor high mobility group box 1 (HMGB1) stimulates autophagy in SCs through the TLR4-mediated pathway, affecting the local TµE, which then contributes to NB cell proliferation [69]. However, due to a secretion of specific factors, SC-derived factors may lessen angiogenesis in vivo and in vitro which results in antitumor activity against NB [43, 67]. Several compelling factors have been identified, with Secreted Protein Acidic and Rich in Cysteine (SPARC) as among the most potent [42].
Schwann cells and cancer pain syndrome
Cancer-related pain represents an agonizing problem in clinical oncology as the prevalence of pain in patients with cancer remains high, which constitutes one major reason for a poor quality of life. Approximately one-third of patients, including children, who are receiving treatment for cancer and 75–90% of those with advanced malignant disease experience significant, life-altering cancer-induced pain [70]. An estimated 30 to 40% of people who undergo chemotherapy develop peripheral neuropathy, which is the leading reason why cancer patients stop chemotherapy early. More than one-third of the suffering patients grade their pain as moderate or severe [71]. Despite a significant effort put into investigating the neuron-related mechanisms underlying this phenomenon [70, 72,73,74], glia only recently gained any attention and became a focus of extensive research. Currently, factors responsible for cancer-related pain are poorly understood; however, tumor-induced pathologic sprouting of sensory nerve fibers [75] and SC abnormality [76, 77] have been suggested as possible reasons.
Watkins et al. established the notion that glia act as key drivers of pathological pain [78], although the role of microglia in cancer-related pain remains highly controversial: a broad variety of data exists reporting different levels of spinal microglial activation due to differences in sex, species/strains and the origin of tumor cells [79,80,81]. For instance, in the pancreatic cancer model, tumor cells demonstrably affect SCs, which downregulated the activation of peripheral neurons in cancer and suppressed cancer-associated pain in cases where a prolonged asymptomatic phase and potentially delayed diagnosis took place [82]. However, one should note that a demand emerges for further investigations to reveal the mechanisms underlying interactions between cancer cells and SCs. This will ensure development of feasible approaches to the efficient therapy to overcome the significant clinical problem of cancer-associated pain syndrome.
Perineural invasion and mutual cell tropism
A growing body of evidence reveals that cancerous cells not only grow near the nerve fibers but also respond to the PNS signals by accelerated proliferation, longevity and dissemination [83,84,85]. For instance, we recently reported that dorsal root ganglia (DRG) neurons, i.e., isolated and cultured sensory neurons, can be stimulated by melanoma and, in turn, can significantly enhance tumor growth in vivo [86]. Accepting that the in vivo microenvironment of peripheral nerves is formed and preserved by nerve ensheathing SCs, we presented evidence that SCs could aid tumor growth by indorsing tumor-favorable conditions [61]. Perineural invasion or perineural spread [87], i.e. cancer cell dissemination in and along nerve bundles well beyond the extent of any local invasion, is an excellent example of the protumor activity of SCs.
PNI is associated with a variety of malignancies, including pancreatic, prostate, head and neck, stomach and colon cancers [88,89,90]. The presence of cancer cells in the perineurium is mostly associated with poor prognosis and high recurrence in pancreatic, cervical, colon, esophageal, colorectal and gastric cancers, but not in invasive breast carcinoma [90,91,92,93]. Axonogenesis evidently acts as an initial factor that predisposes and ultimately leads to PNI and cancer spreading, and therefore strong interactions between cancer cells and nerves resulted in greater PNI diameter and enhanced tumor growth [94]. Neoplastic sites identifiably contain SCs before the onset of cancer invasion; for instance, Demir et al. reported a unique attraction of pancreatic cancer cells to the neuronal components of peripheral nerves—albeit primary SCs [95].
The emergence of SCs in the premalignant phase of pancreatic and colon cancer implies that SCs may initiate PNI in contrast to the established view that malignant cells migrate toward the nerves first [95]. This has, in fact, been proven through elegant in vitro and in vivo experiments showing that SCs directly regulate cancer cells in PNI [96]. Co-culturing of tumor cells with DRG neurons revealed that SCs direct malignant cells to migrate toward nerves by protruding and intercalating between the tumor cells and promoting PNI [96]. Interestingly enough, SC death as induced by radiotherapy reportedly acts as a key factor in the impairment of PNI. This preclinical data may suggest that the therapy itself targets nerves and their supporting cells in the case of proven or expected PNI, since these cells directly facilitate PNI through paracrine signaling [97].
Schwann cells augment metastasis formation
Although the main function of SCs is to maintain the integrity of the axons, SCs have been shown to increase the integrin-dependent tumor invasion on laminin in the pancreatic and prostate cancer microenvironment [98]. SCs may also direct pancreatic cancer cell migration toward the nerves, promote PNI and contribute to the malignant cell colonization of the nerves by activating the mesenchymal-epithelial transition (MET) and reducing cell motility [96, 99]. They may also enhance the invasiveness of the salivary adenoid cystic carcinoma cells, as has been shown using rat SCs and human tumor cell line co-cultures [100]. However, the molecular mechanisms and factors utilized by SCs to promote distant metastases are generally unknown.
We have recently reported that adult SCs may directly stimulate lung cancer cell motility and invasiveness by secreting chemokines and inducing signaling from chemokine-specific receptors expressed on tumor cells [101]. SC-dependent activation of the tumor cells was associated with the promotion of the epithelial-mesenchymal transition (EMT). The effect was mediated by increased expression of the EMT transcription factors Snail and Twist since their block eliminated SC-induced motility of malignant cells. Both recombinant and SC-derived chemokine CXCL5 amplified tumor cell motility and transmigration by inducing EMT via CXCR2-mediated PI3K/AKT/GSK-3β/Snail/Twist signaling. Lastly, SC conditioning of lung cancer cells prior to their inoculation into syngeneic mice significantly augmented the establishment of the metastases in the regional lymph nodes [101]. These results thus reveal a new role of the PNS and SCs in the organization and functioning of the TµE.
Schwann cells attract myeloid regulators to the tumor environs
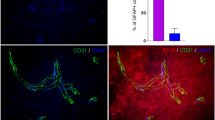
The ability to increase invasiveness of malignant cells and stimulate formation of distant metastases does not cover all of the protumor activities of SCs during carcinogenesis. In fact, we observed an increased expression of various factors like IL-1Ra, TNF-α, CCL3 (MIP-1 α), CCL4 (MIP-1β), CXCL2 (MIP-2), CXCL12 (SDF-1) and CXCL13 (BCA-1) in SCs treated with different tumor cell lines in vitro or obtained from tumor-bearing animals (manuscript in preparation). This suggests that SCs may participate in chemoattraction of immune cells in the tumor milieu, in particular, myeloid regulatory cells, and thus control the immunosuppressive and tolerogenic potential of the tumor immunoenvironment. For example, Fig. 1 shows that both control and tumor-activated SCs are strong chemoattractants of bone marrow-derived MDSC in vitro. Importantly, tumor-conditioned SCs attract MDSC significantly stronger than control cells suggesting that SC-induced attraction of myeloid regulators may be markedly stronger in the TµE than in normal tissues.
Melanoma-activated Schwann cells chemoattract MDSC in vitro. Adult Schwann cells were isolated from sciatic nerve of C57BL/6 mice, purified and cultured as described [101]. Cells were then co-cultured with cell culture medium (see [101]) (Schwann/cntr) and B16 melanoma cells (Schwann/B16) in inserts (2:1 cell ratio) for 48 h and washed. Then, control and B16-pretreated Schwann cells were co-cultured with membrane-separated (5 µm pore size) bone marrow-derived MDSC (upper chamber) for 6 h, and the number of transmigrated CD11b + Gr-1 + MDSC in the bottom chamber was determined by flow cytometry for 60 s (n = 3; *p < 0.05 versus control Schwann cells, ANOVA). Results are shown as the mean ± SEM. SDF-1α (5 ng/ml) was used as a positive control. MDSC, myeloid-derived suppressor cells; SDF-1α, Stromal cell-derived factor 1 (CXCL12)
Together with our data demonstrating the ability of SCs to up-regulate tumor growth in vivo, these results may favor a new concept stating that SCs can act as one of key systemic regulators of cancerogenesis—particularly during the onset or early stages of tumor development and growth.
Schwann cells augment the immunosuppressive activity of myeloid regulators
Interestingly, SCs are not only active chemoattractants of myeloid regulatory cells in the TµE as shown above, but they are also potent modulators of immune cell activity. Actually, melanoma-treated SCs, but not control SCs, significantly enhance MDSC ability to suppress T cell proliferation in vitro. In the search of the mechanism responsible for this phenomenon, we discovered that tumor-treated SCs increase the expression of myelin-associated glycoprotein (MAG) on both the mRNA and protein levels (Fig. 2). MAG, a major inhibitor of axonal growth, is a type I transmembrane glycoprotein that is selectively localized in SCs and oligodendroglial cells functioning in glia-axon interactions [102, 103].
Melanoma up-regulates expression of MAG in Schwann cells. Primary mouse adult Schwann cells were prepared as described in Fig. 1 legend and co-cultured with control medium (Schwann/cntr) and B16 melanoma cells (Schwann/B16) in inserts (48 h). Cells were then isolated, washed and expression of MAG mRNA was determined by qRT-PCR (left) and MAG protein by flow cytometry (right). RPLP0 (Ribosomal Protein Lateral Stalk Subunit P0) served as a housekeeping control (*p < 0.01, Student t test, n = 3). Results are shown as the mean ± SEM (right). The flow cytometry results of a representative experiment are shown (right). MAG, myelin-associated glycoprotein
We have also revealed that MAG increases the immunosuppressive activity of MDSC in T cell inhibitory assay (Fig. 3, right bars) in a way similar to that one of tumor-treated SCs (Fig. 3, right bars). In other words, both melanoma-activated SCs, which overexpress MAG, and recombinant MAG up-regulate the ability of MDSC to suppress proliferation of T cells in a similar way. Altogether, these results allowed hypothesizing that melanoma-derived factors increase SC expression of MAG, which, in turn, is responsible for the activation of chemoattracted MDSC in the TµE. Identification of tumor-derived factors responsible for alteration of SC activity is in progress in our laboratories.
Both melanoma-treated Schwann cells and MAG increase the immune suppressive potential of MDSC. Isolated splenic T cells were activated with CD3/CD28 beads (T cntr), cultured with differentially treated bone marrow-derived MDSC for 72 h and T cell proliferation was assessed by 3H-thymidine incorporation. MDSC, control MDSC; MDSC/MAG, MDSC pre-treated with 0.5 and 5.0 µg/ml MAG; MDSC/SC and MDSC/SC + B16, MDSC pre-treated with control or B16-treated Schwann cells. Schwann cells and MDSC were isolated and cultured as described in Fig. 1 legend. Results are shown as the mean ± SEM. *p < 0.01 versus a corresponding MDSC control (n = 3). T0 non-activated T cells; cpm, counts per minute, SC Schwann cells, MDSC myeloid-derived suppressor cells, MAG myelin-associated glycoprotein
Conclusions
Data from various sources indicate a potential significance of the PNS in the regulation of tumor development, growth and spreading. While the scientific community still requires a better mechanistical understanding of this complex phenomenon, a new field of research emerges where nerves gain an important ‘more than a spectator’ role in carcinogenesis. Both the high plasticity and the sheer abundance of neuroglial Schwann cells make them an appropriate candidate for further laboratory and clinical investigation. Their phenomenal ability to attract various immune and malignant cells, control the microenvironment and regenerate—along with their extensive dissemination among the different types of tissues and organs makes them a perfect target for exploitation by cancerous cells to form and maintain unique tumor microinteractions. However, the common neuroectodermal ontogenic background may result in a distinct role of SCs in tumors that arise from the neural crest, which makes the understanding of their role in solid tumors even more interesting and excitng. Another critically important outcome of research involving SCs in cancer may include the development of efficient approaches towards managing cancer pain syndrome, which involves SC activity in the TµE.
From the perspective of therapy, although targeting nerve fibers in prostate and gastric cancer has been reported to suppress tumor growth and metastasis [17, 104], inhibiting intratumoral nerve infiltration without inducing neuronal and non-neuronal toxicity is a very difficult task [84]. Schwann cell-targeting approaches may represent an interesting alternative. For instance, radiation-induce elimination of SCs can block perineural invasion of human pancreatic cancer cells in experimental models [105]. Recent successes in the development of new treatment strategies aimed at improving the protective and regenerative properties of SCs in peripheral nerve disorders support our plan to target SCs in the TµE. For instance, advances in identifying the factors and signaling molecules that are expressed by SCs have paved the way for new clinical trials which test neurohormones, and transplantation paradigms that have been moved into late stage preclinical models [106]. Furthermore, in the recent years, several pharmacological agents that target SC-dependent nerve regeneration have been proposed [107].
Finally, identifying signaling targets in SCs that regulate their cross-talk with neurons and immune cells will offer novel therapeutic approaches to a number of demyelinating disorders in which SCs are implicated, such as Charcot-Marie-Tooth disease and Guillain–Barré syndrome, as well as genetic disorders such as neurofibromatosis 1 and 2 and infections such as leprosy.
Abbreviations
- CNS:
-
Central nervous system
- DFTD:
-
Devil facial tumor disease
- DRG:
-
Dorsal root ganglion
- EMT:
-
Epithelial–mesenchymal transition
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- HMGB1:
-
High-mobility group box 1
- MAG:
-
Myelin-associated glycoprotein
- MDSC:
-
Myeloid-derived suppressor cell(s)
- MET:
-
Mesenchymal–epithelial transition
- NB:
-
Neuroblastoma
- Nrg:
-
Neuregulin
- PGP 9.5:
-
Protein gene product 9.5
- PNI:
-
Perineural invasion
- PNS:
-
Peripheral nervous system
- SC:
-
Schwann cell(s)
- SDF-1α:
-
Stromal cell-derived factor 1 (CXCL12)
- SPARC:
-
Secreted protein acidic and rich in cysteine
- TµE:
-
Tumor microenvironment
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Shurin MR (2012) Cancer as an immune-mediated disease. Immunotargets Ther 1:1–6
Witz IP (2009) The tumor microenvironment: the making of a paradigm. Cancer Microenviron 2(Suppl 1):9–17
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125(Pt 23):5591–5596
McDonald PG, Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, Stefanek M, Sood AK (2005) A biobehavioral perspective of tumor biology. Discov Med 5(30):520–526
Bruni JE, Montemurro DG (1971) Effect of hypothalamic lesions on the genesis of spontaneous mammary gland tumors in the mouse. Cancer Res 31(6):854–863
Thaker PH, Lutgendorf SK, Sood AK (2007) The neuroendocrine impact of chronic stress on cancer. Cell Cycle 6(4):430–433
Suhail N, Bilal N, Hasan S, Ahmad A, Ashraf GM, Banu N (2015) Chronic unpredictable stress (CUS) enhances the carcinogenic potential of 7,12-dimethylbenz(a)anthracene (DMBA) and accelerates the onset of tumor development in Swiss albino mice. Cell Stress Chaperones 20(6):1023–1036
Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK (2006) The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6(3):240–248
Glaser R, Kiecolt-Glaser JK (2005) Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5(3):243–251
Cole SW, Sood AK (2012) Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 18(5):1201–1206
Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, Towers AJ, Williams B, Lam CM, Xiao K, Shenoy SK, Gregory SG, Ahn S, Duckett DR, Lefkowitz RJ (2011) A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 477(7364):349–353
Catala M, Kubis N (2013) Gross anatomy and development of the peripheral nervous system. Handb Clin Neurol 115:29–41
Krizanova O, Babula P, Pacak K (2016) Stress, catecholaminergic system and cancer. Stress 19(4):419–428
Dang N, Meng X, Song H (2016) Nicotinic acetylcholine receptors and cancer. Biomed Rep 4(5):515–518
Nie H, Cao Q, Zhu L, Gong Y, Gu J, He Z (2013) Acetylcholine acts on androgen receptor to promote the migration and invasion but inhibit the apoptosis of human hepatocarcinoma. PLoS One 8(4):e61678
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341(6142):1236361
Seifert P, Spitznas M (2002) Axons in human choroidal melanoma suggest the participation of nerves in the control of these tumors. Am J Ophthalmol 133(5):711–713
Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, Rowley D (2008) Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 14(23):7593–7603
Albo D, Akay CL, Marshall CL, Wilks JA, Verstovsek G, Liu H, Agarwal N, Berger DH, Ayala GE (2011) Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 117(21):4834–4845
Tomita T (2012) Localization of nerve fibers in colonic polyps, adenomas, and adenocarcinomas by immunocytochemical staining for PGP 9.5. Dig Dis Sci 57(2):364–370
Terada T, Matsunaga Y (2001) S-100-positive nerve fibers in hepatocellular carcinoma and intrahepatic cholangiocarcinoma: an immunohistochemical study. Pathol Int 51(2):89–93
Zhou M, Patel A, Rubin MA (2001) Prevalence and location of peripheral nerve found on prostate needle biopsy. Am J Clin Pathol 115(1):39–43
Campbell LK, Thomas JR, Lamps LW, Smoller BR, Folpe AL (2003) Protein gene product 9.5 (PGP 9.5) is not a specific marker of neural and nerve sheath tumors: an immunohistochemical study of 95 mesenchymal neoplasms. Mod Pathol 16(10):963–969
Jessen KR, Mirsky R (2005) The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6(9):671–682
Kidd GJ, Ohno N, Trapp BD (2013) Biology of Schwann cells. Handb Clin Neurol 11555–11579
Armati PJ, Mathey EK (2013) An update on Schwann cell biology–immunomodulation, neural regulation and other surprises. J Neurol Sci 333(1–2):68–72
Vargas ME, Barres BA (2007) Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci 30:153–179
Jang SY, Shin YK, Park SY, Park JY, Lee HJ, Yoo YH, Kim JK, Park HT (2016) Autophagic myelin destruction by Schwann cells during Wallerian degeneration and segmental demyelination. Glia 64(5):730–742
Chen P, Piao X, Bonaldo P (2015) Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol 130(5):605–618
Xiao Y, Faucherre A, Pola-Morell L, Heddleston JM, Liu TL, Chew TL, Sato F, Sehara-Fujisawa A, Kawakami K, Lopez-Schier H (2015) High-resolution live imaging reveals axon-glia interactions during peripheral nerve injury and repair in zebrafish. Dis Model Mech 8(6):553–564
Jessen KR, Mirsky R (2008) Negative regulation of myelination: relevance for development, injury, and demyelinating disease. GLIA 56(14):1552–1565
Jessen KR, Mirsky R (2016) The repair Schwann cell and its function in regenerating nerves. J Physiol 594(13):3521–3531
Shamash S, Reichert F, Rotshenker S (2002) The cytokine network of wallerian degeneration: tumor necrosis factor-α, interleukin-1α, and interleukin-1β. J Neurosci 22(8):3052–3060
Perrin FE, Lacroix S, Avilés-Trieueros M, David S (2005) Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α and interleukin-1β Wallerian degeneration. Brain 128(4):854–866
Conti G, De Pol A, Scarpini E, Vaccina F, De Riz M, Baron P, Tiriticco M, Scarlato G (2002) Interleukin-1beta and interferon-gamma induce proliferation and apoptosis in cultured Schwann cells. J Neuroimmunol 124(1–2):29–35
Napoli I, Noon L, Ribeiro S, Kerai A, Parrinello S, Rosenberg L, Collins M, Harrisingh M, White I, Woodhoo A, Lloyd A (2012) A central role for the ERK-signaling pathway in controlling schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 73(4):729–742
Ide C (1996) Peripheral nerve regeneration. Neurosci Res 25(2):101–121
DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE (2015) The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience 302:174–203
Reichert F, Levitzky R, Rotshenker S (1996) Interleukin 6 in intact and injured mouse peripheral nerves. Eur J Neurosci 8(3):530–535
Thoma EC, Merkl C, Heckel T, Haab R, Knoflach F, Nowaczyk C, Flint N, Jagasia R, Jensen Zoffmann S, Truong HH, Petitjean P, Jessberger S, Graf M, Iacone R (2014) Chemical conversion of human fibroblasts into functional Schwann cells. Stem Cell Reports 3(4):539–547
Chlenski A, Liu S, Crawford SE, Volpert OV, DeVries GH, Evangelista A, Yang Q, Salwen HR, Farrer R, Bray J, Cohn SL (2002) SPARC is a key Schwannian-derived inhibitor controlling neuroblastoma tumor angiogenesis. Cancer Res 62(24):7357–7363
Crawford SE, Stellmach V, Ranalli M, Huang X, Huang L, Volpert O, De Vries GH, Abramson LP, Bouck N (2001) Pigment epithelium-derived factor (PEDF) in neuroblastoma: a multifunctional mediator of Schwann cell antitumor activity. J Cell Sci 114(Pt 24):4421–4428
Reynolds ML, Woolf CJ (1993) Reciprocal Schwann cell-axon interactions. Curr Opin Neurobiol 3(5):683–693
Ambros IM, Ambros PF (1995) Schwann cells in neuroblastoma. Eur J Cancer 31(4):429–434
Parfejevs V, Debbache J, Shakhova O, Schaefer SM, Glausch M, Wegner M, Suter U, Riekstina U, Werner S, Sommer L (2018) Injury-activated glial cells promote wound healing of the adult skin in mice. Nat Commun 9(1):236
Clements MP, Byrne E, Guerrero LF, Cattin AL, Zakka L, Ashraf A, Burden JJ, Khadayate S, Lloyd AC, Marguerat S, Parrinello S (2017) The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron 96(1):98–114 e117
Massoll C, Mando W, Chintala SK (2013) Excitotoxicity upregulates SARM1 protein expression and promotes Wallerian-like degeneration of retinal ganglion cells and their axons. Invest Ophthalmol Vis Sci 54(4):2771–2780
Dyachuk V, Furlan A, Shahidi MK, Giovenco M, Kaukua N, Konstantinidou C, Pachnis V, Memic F, Marklund U, Muller T, Birchmeier C, Fried K, Ernfors P, Adameyko I (2014) Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science 345(6192):82–87
Kaucka M, Adameyko I (2014) Non-canonical functions of the peripheral nerve. Exp Cell Res 321(1):17–24
Pye RJ, Woods GM, Kreiss A (2016) Devil facial tumor disease. Vet Pathol 53(4):726–736
Karu N, Wilson R, Hamede R, Jones M, Woods GM, Hilder EF, Shellie RA (2016) Discovery of biomarkers for tasmanian devil cancer (DFTD) by metabolic profiling of serum. J Proteome Res 15(10):3827–3840
Lachish S, Jones M, McCallum H (2007) The impact of disease on the survival and population growth rate of the tasmanian devil. J Anim Ecol 76(5):926–936
Loh R, Hayes D, Mahjoor A, O’Hara A, Pyecroft S, Raidal S (2006) The immunohistochemical characterization of devil facial tumor disease (DFTD) in the tasmanian devil (Sarcophilus harrisii). Vet Pathol 43(6):896–903
Clark HB, Minesky JJ, Agrawal D, Agrawal HC (1985) Myelin basic protein and P2 protein are not immunohistochemical markers for Schwann cell neoplasms. A comparative study using antisera to S-100, P2, and myelin basic proteins. Am J Pathol 121(1):96–101
Murchison EP, Tovar C, Hsu A, Bender HS, Kheradpour P, Rebbeck CA, Obendorf D, Conlan C, Bahlo M, Blizzard CA, Pyecroft S, Kreiss A, Kellis M, Stark A, Harkins TT, Marshall Graves JA, Woods GM, Hannon GJ, Papenfuss AT (2010) The tasmanian devil transcriptome reveals Schwann Cell origins of a clonally transmissible cancer. Science 327(5961):84–87
Gollamudi M, Nethery D, Liu J, Kern JA (2004) Autocrine activation of ErbB2/ErbB3 receptor complex by NRG-1 in non-small cell lung cancer cell lines. Lung Cancer 43(2):135–143
Kern JA, Slebos RJ, Top B, Rodenhuis S, Lager D, Robinson RA, Weiner D, Schwartz DA (1994) C-erbB-2 expression and codon 12 K-ras mutations both predict shortened survival for patients with pulmonary adenocarcinomas. J Clin Invest 93(2):516–520
Kern JA, Torney L, Weiner D, Gazdar A, Shepard HM, Fendly B (1993) Inhibition of human lung cancer cell line growth by an anti-p185HER2 antibody. Am J Respir Cell Mol Biol 9(4):448–454
Pytel P, Karrison T, Can G, Tonsgard JH, Krausz T, Montag AG (2010) Neoplasms with schwannian differentiation express transcription factors known to regulate normal schwann cell development. Int J Surg Pathol 18(6):449–457
Bunimovich YL, Keskinov AA, Shurin GV, Shurin MR (2017) Schwann cells: a new player in the tumor microenvironment. Cancer Immunol Immunother 66(8):959–968
Cheung NK, Dyer MA (2013) Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 13(6):397–411
Maris JM, Hogarty MD, Bagatell R, Cohn SL (2007) Neuroblastoma Lancet 369(9579):2106–2120
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, Castleberry RP (1999) The international neuroblastoma pathology classification (the Shimada system). Cancer 86(2):364–372
Liu S, Tian Y, Chlenski A, Yang Q, Zage P, Salwen HR, Crawford SE, Cohn SL (2005) Cross-talk between Schwann cells and neuroblasts influences the biology of neuroblastoma Xenografts. Am J Pathol 166(3):891–900
Ambros IM, Attarbaschi A, Rumpler S, Luegmayr A, Turkof E, Gadner H, Ambros PF (2001) Neuroblastoma cells provoke Schwann cell proliferation in vitro. Med Pediatr Oncol 36(1):163–168
Huang D, Rutkowski JL, Brodeur GM, Chou PM, Kwiatkowski JL, Babbo A, Cohn SL (2000) Schwann cell-conditioned medium inhibits angiogenesis. Cancer Res 60(21):5966–5971
Kwiatkowski JL, Rutkowski JL, Yamashiro DJ, Tennekoon GI, Brodeur GM (1998) Schwann cell-conditioned medium promotes neuroblastoma survival and differentiation. Cancer Res 58(20):4602–4606
Liu Y, Song L (2015) HMGB1-induced autophagy in Schwann cells promotes neuroblastoma proliferation. Int J Clin Experiment Pathol 8(1):504–510
Mantyh PW (2006) Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 7(10):797–809
Everdingen MH, Rijke JM, Kessels AG, Schouten HC, Kleef M, Patijn J (2007) Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 18(9):1437–1449
Schmidt BL, Hamamoto DT, Simone DA, Wilcox GL (2010) Mechanism of Cancer Pain. Mol Interven 10(3):164–178
Mantyh PW (2014) Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care 8(2):83–90
Vendrell I, Macedo D, Alho I, Dionsio MR, Costa L (2015) Treatment of cancer pain by targeting cytokines. Mediat Inflam 984570:11
Jimenez-Andrade JM, Ghilardi JR, Castaneda-Corral G, Kuskowski MA, Mantyh PW (2011) Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 152(11):2564–2574
Campana WM (2007) Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun 21(5):522–527
Hoke A (2006) Mechanisms of disease: what factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol 2(8):448–454
Watkins LR, Milligan ED, Maier SF (2001) Glial activation: a driving force for pathological pain. Trends Neurosci 24(8):450–455
Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM (2009) Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain 13(2):138–145
Ducourneau VR, Dolique T, Hachem-Delaunay S, Miraucourt LS, Amadio A, Blaszczyk L, Jacquot F, Ly J, Devoize L, Oliet SH, Dallel R, Mothet JP, Nagy F, Fenelon VS, Voisin DL (2014) Cancer pain is not necessarily correlated with spinal overexpression of reactive glia markers. Pain 155(2):275–291
Zhou YQ, Liu Z, Liu HQ, Liu DQ, Chen SP, Ye DW, Tian YK (2016) Targeting glia for bone cancer pain. Expert Opin Ther Targets 20(11):1365–1374
Demir IE, Tieftrunk E, Schorn S, Saricaoglu OC, Pfitzinger PL, Teller S, Wang K, Waldbaur C, Kurkowski MU, Wormann SM, Shaw VE, Kehl T, Laschinger M, Costello E, Algul H, Friess H, Ceyhan GO (2016) Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut 65(6):1001–1014
Magnon C (2015) Role of the autonomic nervous system in tumorigenesis and metastasis. Mol Cell Oncol 2(2):e975643
Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H (2015) Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res 75(9):1777–1781
Li S, Sun Y, Gao D (2013) Role of the nervous system in cancer metastasis. Oncol Lett 5(4):1101–1111
Keskinov AA, Tapias V, Watkins SC, Ma Y, Shurin MR, Shurin GV (2016) Impact of the sensory neurons on melanoma growth in vivo. PLoS One 11(5):e0156095
Batsakis JG (1985) Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol 94(4 Pt 1):426–427
Bapat AA, Hostetter G, Von Hoff DD, Han H (2011) Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer 11(10):695–707
Varsha BK, Radhika MB, Makarla S, Kuriakose MA, Kiran GS, Padmalatha GV (2015) Perineural invasion in oral squamous cell carcinoma: case series and review of literature. J Oral Maxillofac Pathol 19(3):335–341
Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID (2016) Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am J Surg Pathol 40(1):103–112
Kuol N, Stojanovska L, Apostolopoulos V, Nurgali K (2018) Role of the nervous system in cancer metastasis. J Exp Clin Cancer Res 37(1):5–17
Cui L, Shi Y, Zhang GN (2015) Perineural invasion as a prognostic factor for cervical cancer: a systematic review and meta-analysis. Arch Gynecol Obstet 292(1):13–19
Gao A, Wang L, Li J, Li H, Han Y, Ma X, Sun Y (2016) Prognostic value of perineural invasion in esophageal and esophagogastric junction carcinoma: a meta-analysis. Dis Markers 5:7340180
Olar A, He D, Florentin D, Ding Y, Ayala G (2014) Biological correlates of prostate cancer perineural invasion diameter. Human Pathol 45(7):1365–1369
Demir IE, Boldis A, Pfitzinger PL, Teller S, Brunner E, Klose N, Kehl T, Maak M, Lesina M, Laschinger M, Janssen KP, Algul H, Friess H, Ceyhan GO (2014) Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J Natl Cancer Inst 106(8):1
Deborde S, Omelchenko T, Lyubchik A, Zhou Y, He S, McNamara WF, Chernichenko N, Lee SY, Barajas F, Chen CH, Bakst RL, Vakiani E, Hall A, Wong RJ (2016) Schwann cells induce cancer cell dispersion and invasion. J Clin Invest 126(4):1538–1554
Bakst RL, Wong RJ (2016) Mechanisms of Perineural Invasion. J Neurol Surg B Skull Base 77(2):96–106
Sroka IC, Chopra H, Das L, Gard JM, Nagle RB, Cress AE (2016) Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding A6 integrin. J Cell Biochem 117(2):491–499
Fujii-Nishimura Y, Yamazaki K, Masugi Y, Douguchi J, Kurebayashi Y, Kubota N, Ojima H, Kitago M, Shinoda M, Hashiguchi A, Sakamoto M (2018) Mesenchymal-epithelial transition of pancreatic cancer cells at perineural invasion sites is induced by Schwann cells. Pathol Int 68(4):214–223
Shan C, Wei J, Hou R, Wu B, Yang Z, Wang L, Lei D, Yang X (2016) Schwann cells promote EMT and the Schwann-like differentiation of salivary adenoid cystic carcinoma cells via the BDNF/TrkB axis. Oncol Rep 35(1):427–435
Zhou Y, Shurin GV, Zhong H, Bunimovich YL, Han B, Shurin MR (2018) Schwann cells augment cell spreading and metastasis of lung cancer. Cancer Res 78(20):5927–5939
Quarles RH (2007) Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem 100(6):1431–1448
McKerracher L, Rosen KM (2015) MAG, myelin and overcoming growth inhibition in the CNS. Front Mol Neurosci 8:51–56
Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, Sandvik AK, Beisvag V, Tomita H, Hara A, Quante M, Li Z, Gershon MD, Kaneko K, Fox JG, Wang TC, Chen D (2014) Denervation suppresses gastric tumorigenesis. Sci Transl Med 6(250):250ra115
Bakst RL, Barajas F, He S, Chernichenko N, Chen C, He S, McNamara W, Lee S, Deborde S, Wong RJ (2013) Are Schwann cells a target in radiation for perineural invasion? Int J Radiat Oncol Biol Phys 87(25):S629
Lehmann HC, Hoke A (2010) Schwann cells as a therapeutic target for peripheral neuropathies. CNS Neurol Disord Drug Targets 9(6):801–806
Magnaghi V, Procacci P, Tata AM (2009) Chap. 15: novel pharmacological approaches to Schwann cells as neuroprotective agents for peripheral nerve regeneration. Int Rev Neurobiol 87:295–315
Funding
This work was supported by London Foundation Grant (to M. R. Shurin) and University of Pittsburgh Cancer Institute (UPCI) Melanoma and SPORE in Skin Cancer Career Enhancement Program Award NIH P50CA121973 (to Y. L. Bunimovich).
Author information
Authors and Affiliations
Contributions
Conceptualization, MRS, AAK and YLB; Methodology, GVS, and MRS; Investigation, GVS and YLB; Writing —Original Draft, GVM and MRS; Writing—Review and Editing, GVM and MRS; Funding Acquisition, MRS and YLB; Resources, MRS and AAK; Supervision, GVS and MRS.
Corresponding author
Ethics declarations
Conflict of interest
The author reports no conflicts of interest in this work.
Rights and permissions
About this article
Cite this article
Martyn, G.V., Shurin, G.V., Keskinov, A.A. et al. Schwann cells shape the neuro-immune environs and control cancer progression. Cancer Immunol Immunother 68, 1819–1829 (2019). https://doi.org/10.1007/s00262-018-02296-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-02296-3