Abstract
Objectives
Lung cancer is the leading cause of cancer-related death in the USA. Regulatory T cells (Tregs) normally function to temper immune responses and decrease inflammation. Previous research has demonstrated different subsets of Tregs with contrasting anti- or pro-inflammatory properties. This study aimed to determine Treg subset distributions and characteristics present in non-small cell lung cancer (NSCLC) patients.
Methods
Peripheral blood was collected from healthy controls (HC) and NSCLC patients preceding surgical resection, and mononuclear cells were isolated, stained, and analyzed by flow cytometry. Tregs were defined by expression of CD4 and CD25 and classified into CD45RA+Foxp3int (naïve, Fr. I) or CD45RA−Foxp3hi (activated Fr. II). Activated conventional T cells were CD4+CD45RA−Foxp3int (Fr. III).
Results
Samples from 23 HC and 26 NSCLC patients were collected. Tregs isolated from patients with NSCLC were found to have enhanced suppressive function on naive T cells. Cancer patients had significantly increased frequencies of activated Tregs (fraction II: FrII), 17.5 versus 3.2 % (P < 0.001). FrII Tregs demonstrated increased RORγt and IL17 expression and decreased IL10 expression compared to Tregs from HC, indicating pro-inflammatory characteristics.
Conclusions
This study demonstrates that a novel subset of Tregs with pro-inflammatory characteristics preferentially expand in NSCLC patients. This Treg subset appears identical to previously reported pro-inflammatory Tregs in human colon cancer patients and in mouse models of polyposis. We expect the pro-inflammatory Tregs in lung cancer to contribute to the immune pathogenesis of disease and propose that targeting this Treg subset may have protective benefits in NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most common solid malignancy and the leading cause of cancer mortality worldwide [1]. The role of the immune system in the development of lung cancer is highly investigated. Pro-inflammatory cells, such as mast cells (MC) and lymphocytes, are important components of the tumor microenvironment.
Regulatory T cells (Tregs) are CD4+/CD25+/Forkhead box P3 (Foxp3)+ cells that maintain self-tolerance and immune homeostasis [2]. Tregs can suppress a variety of innate and adaptive immune responses. Tregs suppress tumor-specific CD8 T cells [3], and recently, it has become evident that Tregs can also potently suppress cancer-associated inflammation [4]. Furthermore, Tregs inhibit the differentiation and degranulation of MC [5]. It has been reported in patients with non-small cell lung cancer (NSCLC) that those with a higher ratio of Tregs to total tumor infiltrating T cells had an increased risk of recurrence [6]. Patients with NSCLC have also been shown to have higher circulating levels of Tregs in their peripheral blood compared to healthy controls (HC), and the proportion increased in a stage-dependent manner [6]. However, the role Tregs play in the pathogenesis of lung cancer is poorly understood.
Tregs subpopulations, with functional delineations, exist in different proportions in patients with inflammatory diseases and colon cancer (CC) compared to HC [7, 8]. Recent studies have demonstrated phenotypic and functional changes in peripheral blood circulating Tregs as well as in tumor infiltrating Tregs in CC patients [8, 9]. Tregs suppress interleukin (IL)-17-producing CD4 helper T cells (TH17) cells in an IL-10-dependent manner [10]. However, in CC Tregs change to a pro-inflammatory phenotype marked by co-expression of Foxp3 and RORγt, a decrease in expression of IL-10, and an increase in expression of IL-17¸ a potent pro-inflammatory cytokine [8]. The expansion of the pro-inflammatory Tregs in CC patients is tumor dependent, and they are no longer detectable after surgical excision of the primary tumor in non-metastatic disease [9]. In this study, we sought to investigate the status of Tregs subpopulations in patients with NSCLC. We show that Tregs also expand in NSCLC and that the expanded cells have the same pro-inflammatory characteristics previously reported in human CC. The similarity between the pro-inflammatory characteristics of these two cancer types suggests similar immune pathologies.
Materials and methods
Patient samples
Informed consent was obtained from all participants. The protocols were approved by the Scientific Review Committee and the Institutional Review Board of Northwestern University. All diagnoses were histologically confirmed by a board-certified pathologist at Northwestern University Hospital.
Isolation of mononuclear cells from peripheral blood for lung cancer patients
Thirty milliliters of peripheral blood was collected in standard blood collection tubes containing potassium EDTA (366643, BD) at the time of surgery, prior to extirpation of the tumor. Total mononuclear cells (MNC) were isolated using Biocoll Separating Solution (density 1.077 g/ml; Cedarlane Laboratories Ltd #L6155) at 2400 rpm for 20 min. The interphase was collected and washed with RPMI, containing 1 % Pen/strep + 1 % HEPES and centrifuged at 1400 rpm for 10 min.
Inhibition of CD4 T cell proliferation assay
A Treg suppression assay was performed with 40,000 naïve CD4 T cells (CD4+CD25−) and Tregs (CD4+CD25+) that were magnetically purified from peripheral blood (Miltenyi). Purity of the subsets (90 % or better) was assessed by FACS. Naïve CD4+CD25− T cells were labeled with 0.1 μM carboxyfluorescein succinimidyl ester (CFSE) for 10 min at 37 °C. CFSE labeled cells were mixed with anti-CD3, CD28 beads and co-cultured with Tregs in 96-well plates for 96 h (37°, 5 % CO2). Dilution of CFSE was measured by FACS.
FACS
For total MNC, dead cells were excluded using LIVE/DEAD Violet Dead cell Stain kit (Invitrogen). Human antibodies included: CD4 PE-Cy5 (MT310) Dako, CD25 PE-Cy7 (M-A251), IL10 APC (JES3-19F1) BD; Foxp3 FITC (PCH101), IL17A PE (eBio64CAP17), RORγt PE (AFKJS-9) (eBioscience); and CD45RO APC (UCH21) and CD45RA (HI100) (Biolegend). CFSE (Life Technologies; C34554) was used to label cells that were proliferating. For intracellular cytokine stainings, GolgiPlug (BD Bioscience), PMA (Sigma) and ionomycin (Sigma) were added 4 h before Fc block. Dead cells were excluded using LIVE/DEAD Violet Dead cell Stain kit (Invitrogen). Acquisition was performed with a BD FACSCanto II instrument to assess frequencies of subpopulations and expression of surface and intra-cellular markers. Data analyses were performed using FlowJo software (Tree Star). For cell sorting, DAPI was used to exclude dead cells and sorting was performed on a Dako MoFlow.
Statistical analysis
Prism 5 (GraphPad Software Inc, La Jolla, CA) was used for statistical analysis. Except were indicated, P values determined with Mann–Whitney test, and <0.05 was considered significant.
Results
Patients and healthy controls
Twenty-six new cases of NSCLC and 23 healthy controls were included in the study. None of the NSCLC patients had received prior treatment for their lung cancer. Patient’s clinicopathologic information is illustrated in Table 1. Sixteen patients (61.5 %) had adenocarcinoma (ADC), and 10 (38.5 %) had squamous cell carcinoma (SCC). The mean age of the NSCLC patients was slightly older than that of the HC (67.2 ± 9.0 vs. 43.3 ± 11.2 years, p < 0.001). However, no difference in age was observed between patients with ADC and SCC. There was no difference in the size of the tumors between ADC and SCC (2.7 ± 1.5 vs. 2.5 ± 1.5, p = 0.7). The most frequent pathologic stage was stage I (17/26, 65 %). P values were calculated using an unpaired t test.
Tregs fraction II is expanded in peripheral blood of patients with NSCLC
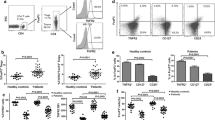
To distinguish Tregs fractions from Foxp3+ non-Tregs CD4+ cells, Foxp3+ lymphocytes were subdivided into three different fractions as defined by Sakaguchi and colleagues: Fr. I CD4+CD45RA+Foxp3int and Fr. II CD4+CD45RA-Foxp3high and Fr. III CD4+CD45RA−Foxp3int [7] (Supplementary Figure 1). As previously reported, Fr. I has naïve characteristics (CD45RA-high, CD45RO-low, CD25-int), Fr. II has activated characteristics (CD45RA-low, CD45RO-high, CD25-high) and Fr. III lacks T cell-suppressive properties despite expressing Foxp3 and is, therefore, activated effector or helper T cells [7, 8]. In NSCLC, Fr. II was expanded (4.8-fold) among peripheral blood mononuclear cells (PBMC) compared to HC (Fig. 1). There was a decrease in Fr. III in NSCLC patients compared to healthy controls and no difference in Fr. I. When analyzed by histologic subtype, Fr. II was expanded in both ADC (5.4-fold) and SCC (4.4-fold) compared to HC (Fig. 2). In Fr. III, there was a decrease in ADC compared to HC although there was no difference in SCC compared to HC. Fr. I was not expanded when ADC or SCC patients were compared separately to HC. There was no significant difference based on pathologic stage between stage I and II patients (Fig. 3). The small sample size precluded statistical analysis of advanced stage patients.
T regulatory cell fraction II is expanded in peripheral blood of NSCLC patients. Frequency of Tregs cell fractions I–III among CD4+ T cells in healthy controls (HC) compared to patients with non-small cell lung cancer (NSCLC). Peripheral blood was collected prior to tumor removal at the time of surgery. Mononuclear cells were isolated and subsequently analyzed by flow cytometry for Tregs-specific markers. Tregs cell fraction II was significantly elevated in NSCLC patients compared with HC (***p < 0.001). There was no significant difference between NSCLC patients and HC in Tregs fraction I, and a slight decrease in fraction III (*p < 0.05)
T regulatory cell fraction II is expanded in peripheral blood in patients with adenocarcinoma and squamous cell carcinoma. Frequency of Tregs cell subpopulations I–III among CD4+ T cells in HC compared to patients with NSCLC divided by histologic type: adenocarcinoma (ADC) and squamous cell carcinoma (SCC). Peripheral blood was collected prior to tumor removal at the time of surgery. Mononuclear cells were isolated and subsequently analyzed by flow cytometry for Tregs-specific markers. Tregs fraction II was significantly elevated in patients with ADC or SCC compared with HC (***p < 0.001). There was no significant difference in Tregs subpopulations I and III between patients with ADC and SCC compared to HC, except for a slight decrease in fraction III for ADC (*p < 0.05). There was no significant difference between ADC and SCC in any of the Tregs fractions
Tregs have increased IL-17 expression and decreased IL-10 expression in peripheral blood in patients with NSCLC
Tregs were assessed for expression of pro-inflammatory cytokine IL-17 and anti-inflammatory cytokine IL-10 (Supplementary Figure 2A). An increase in IL-17 expressing Tregs in the PBMC of all NSCLC patients compared to HC was seen in Fr. I and Fr. II Tregs, but not Fr. III (Fig. 4a). This was true for both ADC and SCC when compared to HC. No difference was observed between ADC and SCC in any of the Tregs subpopulations in expression of IL-17. Interestingly, a decrease in expression of IL-10 was seen in all three fractions in NSCLC patients compared to HC (Fig. 4b), in line with previously reported loss of expression of IL-10 by Tregs in CC patients [8]. When analyzed by histologic type, there was a decrease in IL-10 expression among all three fractions for ADC compared to HC, but only in Fr. I for SCC compared to HC. There was no significant difference in expression of IL-10 observed between Fr. I, Fr. II or Fr. III for ADC compared to SCC (data not shown).
T regulatory cells have increased IL-17 expression and decreased IL-10 expression in peripheral blood in patients with NSCLC. a Frequency of IL-17+ Tregs among FoxP3+ T cells in healthy donors compared to NSCLC patients, divided into Tregs fractions I–III. Tregs fractions I and II demonstrate a significant increase in IL-17+ cells in NSCLC patients compared with HC (***p < 0.001). b Frequency of IL-10+ Tregs in HC compared to NSCLC patients, divided into Tregs fractions I–III. All three Tregs fractions demonstrated a significant decrease in IL-10+ cells in patients with NSCLC compared with HC (***p < 0.001)
Tregs fractions I and II have increased RORγt expression in peripheral blood in patients with NSCLC
Tregs were assessed for the expression of the transcription factor that directs expression of the pro-inflammatory cytokine IL-17, namely RORγt, and co-expression of Foxp3 (Supplementary Figure 2B). Expansion of Tregs that co-express RORγt and Foxp3 has been described to occur in a stage-dependent manner in patients with CC [8]. In NSCLC patients compared to HC, we observed a notable expansion of RORγt+ Fr. II Tregs and less robust increase among Fr. I; we did not observe any increase in RORγt+ cells among Fr. III, suggesting that these were either not TH17 cells or had not completed their lineage commitment (Fig. 5). No difference was seen when ADC was compared to SCC.
T regulatory cell fractions I and II have increased RORγt expression in peripheral blood in patients with NSCLC. Frequency of RORγt+ Tregs among FoxP3+ T cells in HC compared to NSCLC, divided into Tregs fractions I–III. Tregs fractions I and II demonstrated a significantly increased population of RORγt+ cells in NSCLC patients compared to HC (***p < 0.001)
Tregs in peripheral blood in patients with NSCLC suppress proliferation of naïve T cells
To demonstrate that Tregs in peripheral blood of those patients with NSCLC are in fact suppressive, naïve T cells were mixed with Tregs from HD blood and NSCLC blood. Tregs from NSCLC blood showed a stronger suppression of proliferation of naïve T cells as compared to Tregs from HD blood (Fig. 6), in line with their activated state in cancer patients.
T regulatory cells from peripheral blood of NSCLC patients are potently T cell suppressive. Peripheral blood was collected prior to tumor removal at the time of surgery. Mononuclear cells were isolated and subsequently analyzed for suppression of proliferation of naïve conventional CD4 T cells from the same donors pre-labeled with CFSE. Dilution of CFSE was used to measure cell proliferation. a Representative flow cytometry histogram overlays show CFSE staining of naïve T cells that were either left unstimulated (blue) or were stimulated in vitro with CD3/CD28 (red). Stimulation was in the absence or in the presence of an equal number (1/1) of Tregs. Tregs and conventional CD4 T cells were isolated from control healthy donors (n = 5) or lung cancer patients (n = 5) as indicated. b Cumulative analysis (triplicates) of the fraction of CD3/CD28 stimulated naïve T cells that divided as in a. Data are representative of two independent experiments involving four lung cancer patients. (**p ≤ 0.01)
Discussion
Tregs are an important mediator in the control of the immune system, both in autoimmune diseases and suppression of anti-tumor immunity [11]. Tregs typically comprise 5–10 % of the CD4+ T cells in the peripheral blood of healthy humans and are known to be expanded in the peripheral blood of a variety of cancers, including NSCLC [6, 12–14]. The discovery of sublineages of Tregs, as defined by their abilities to suppress or promote inflammation, has shed new light on the precise role Tregs play in carcinogenesis. Sagakuchi et al. [7] defined three subpopulations of Foxp3+ T cells in the PB of healthy individuals. These subpopulations expressed different levels of IL-17 and were present in different proportions in inflammatory disease. Our laboratory recently reported that PB of CC patients have higher levels of IL-17-producing Tregs than that of healthy donors [15]. Tregs from healthy donors suppressed degranulation of MC. Tregs from CC patients, however, had gained pro-inflammatory properties as assayed by increased MC degranulation, but maintained effector T cell suppression. By targeted ablation of RORγt in T cells of mice with hereditary polyposis, we were able to hinder polyposis and thus verify the contribution of pro-inflammatory Tregs to the carcinogenesis process [8]. Treg subpopulations have not previously been described in NSCLC. In the current study, we demonstrate that Tregs Fr. II is responsible for the increased proportion of Tregs observed in NSCLC patients and that these cells have increased expression of RORγt and IL-17 and decreased IL-10, potentially marking them as pro-inflammatory cells.
Increased total Tregs, as defined by CD4+CD25+Foxp3+, in the PB of NSCLC patients have been previously reported to occur in a stage-dependent manner [6]. Studies identifying increased CD4+CD25+ cells in the PB of NSCLC have also been published [16, 17]. The findings of our study are in line with these previous findings, but further demonstrate that the increase in Tregs can be attributed to the population of cells that are CD4+CD45RAlowCD45ROhighCD25highFoxp3high and that a high proportion of these cells express RORγt compared to corresponding populations in HC.
Voo et al. [18] reported a population of IL-17-producing Tregs in healthy donor PB which co-express RORγt and have full T cell-suppressive properties. RORγt is a transcription factor that promotes TH17 differentiation and up-regulates the production of IL-17 [19]. TH17 and IL-17 have also been found to play a critical pathogenic role in lung cancer due to pro-inflammatory characteristics [20]. Findings of IL-17-producing Tregs in both healthy donors and cancer patients suggest that these Tregs either expand from a distinct sublineage or have some level of plasticity with the ability to be either anti-inflammatory or pro-inflammatory. It is significant that the pro-inflammatory Tregs have potent T cell-suppressive properties [4, 8]. The promotion of inflammation and suppression of T cells would provide a formidable advantage for cancer cell growth.
It has recently been reported that among patients with NSCLC, those with ADC demonstrate an increased Tregs infiltrate in the tumor and N1 station lymph nodes compared to SCC [21]. This raises the possibility that tumor histology is related to the body’s lymphocyte response. Tregs are known to be more prevalent in the regional lymph nodes of NSCLC patients than the peripheral blood, and a higher proportion of Tregs in the regional lymph nodes of stage I patients has been shown to be an independent negative prognostic indicator [14]. The above studies, however, evaluated total Tregs and did not investigate subpopulations. While the current study did not find a difference in Tregs subpopulations between ADC and SCC in the PB, it is possible that a difference does exist in the tumor microenvironment or at the regional lymph node level. Further investigation of Tregs subpopulations at the tissue level is needed to determine the impact these cells play in creating a permissive milieu for tumor growth, invasion and metastasis.
RORγt+ Tregs have increased expression of IL-17 and β-catenin but undetectable expression of IL-10 in CC patients [8, 22]. Unlike conventional Tregs, these cells are unable to suppress mast cell degranulation, but retain T cell-suppressive capabilities [8]. Loss of expression of IL-10 by T cells is highly detrimental for CC [23]. Recently, it was noted that following resection for CC, the abundance of RORγt+IL-10− Tregs decreased and effectively normalized [9]. If this were to hold true for NSCLC, it would create the possibility of a simple surveillance tool for detecting subclinical recurrence. Additionally, RORγt+ Tregs monitoring could be used to help guide decisions regarding those patients who would benefit from adjuvant therapy or close observation. Finally, novel RORγt inhibitors are commercially available, and preliminary data show that these molecules have the ability to restore the anti-inflammatory properties of Tregs isolated from CC patients [9]. While the clinical significance of this is yet to be determined, the current study demonstrates that a distinct subset of Tregs is increased in patients with NSCLC and that further investigations of these cells, the role they play in lung cancer, and their potential target for immune-modulating therapies is warranted.
A potential weakness of the current study is the slight difference in mean age between the HC and NSCLC groups. The impact of age on the frequency of Tregs subpopulations has been previously reported [7]. Aged donors (79–90 years old) had a slightly decreased proportion of Tregs Fr. I (1.1 vs. 2.4 %) and increased proportion of Fr. II (2.5 vs. 1.6 %) compared to younger donors (18–40 years old). Given these small differences, it is unlikely that the small difference in age between the two groups in our study would account for the dramatic increase in Fr. II we observed in patients with NSCLC, particularly given that we found no difference between the two groups in Fr. I and only a slight difference in Fr. III for ADC compared to HC.
In conclusion, the findings of the present study not only fit in with the current literature demonstrating an increase in Tregs in the peripheral blood of patients with NSCLC, but add insight into the specific subset of Tregs that account for this increase. Further investigations into the roles that specific Tregs subsets play in lung cancer may aid in the understanding of the increasingly complex role, and these cells play in carcinogenesis, tumor immune-surveillance and the advancement of immunotherapy for NSCLC.
Abbreviations
- ADC:
-
Adenocarcinoma
- CC:
-
Colon cancer
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- FACS:
-
Fluorescence-activated cell sorting
- Foxp3:
-
Forkhead box P3
- Fr:
-
Fraction
- HC:
-
Healthy control
- IL-10:
-
Interleukin 10
- IL-17:
-
Interleukin 17
- MC:
-
Mast cells
- MNC:
-
Mononuclear cells
- NSCLC:
-
Non-small cell lung cancer
- PB:
-
Peripheral blood
- PBMC:
-
Peripheral blood mononuclear cells
- RORγt:
-
Retinoic acid receptor related orphan receptor gamma T
- SCC:
-
Squamous cell cancer
- TH17:
-
T helper 17 cells
- Tregs:
-
T regulatory cells
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Sakaguchi S, Miyara M, Costantino CM, Hafler DA (2010) FOXP3 + regulatory T cells in the human immune system. Nat Rev Immunol 10(7):490–500
Khazaie K, von Boehmer H (2006) The impact of CD4+CD25+ Treg on tumor specific CD8 + T cell cytotoxicity and cancer. Semin Cancer Biol 16(2):124–136. doi:10.1016/j.semcancer.2005.11.006
Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K (2009) T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res 69(13):5490–5497. doi:10.1158/0008-5472.CAN-09-0304
Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ, Khazaie K (2010) In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA 107(14):6430–6435
Erfani N, Mehrabadi SM, Ghayumi MA, Haghshenas MR, Mojtahedi Z, Ghaderi A, Amani D (2012) Increase of regulatory T cells in metastatic stage and CTLA-4 over expression in lymphocytes of patients with non-small cell lung cancer (NSCLC). Lung Cancer 77(2):306–311
Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S (2009) Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30(6):899–911
Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, Halverson AL, Stryker SJ, Boller AM, Singal A, Sneed RK, Sarraj B, Ansari MJ, Oft M, Iwakura Y, Zhou L, Bonertz A, Beckhove P, Gounari F, Khazaie K (2012) Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med 4(164):164ra159
Blatner NR, Gounari F, Khazaie K (2013) The two faces of regulatory T cells in cancer. Oncoimmunology 2(5):e23852
Bos PD, Rudensky AY (2012) Treg cells in cancer: a case of multiple personality disorder. Sci Transl Med 4(164):164fs144
Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T (2006) Foxp3 + CD25 + CD4 + natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 212:8–27
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9(2):606–612
Li L, Chao QG, Ping LZ, Xue C, Xia ZY, Qian D, Shi-ang H (2009) The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC. Cancer Biother Radiopharm 24(3):357–367
Hanagiri T, Shigematsu Y, Shinohara S, Takenaka M, Oka S, Chikaishi Y, Nagata Y, Iwata T, Uramoto H, So T, Tanaka F (2013) Clinical significance of the frequency of regulatory T cells in regional lymph node lymphocytes as a prognostic factor for non-small-cell lung cancer. Lung Cancer 81(3):475–479
Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ, Khazaie K (2010) In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA 107(14):6430–6435
Liu L, Yao J, Ding Q (2006) Huang S (2006) CD4 + CD25high regulatory cells in peripheral blood of NSCLC patients. J Huazhong Univ Sci Technolog Med Sci 26(5):548–551
Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T (2005) CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep 14(5):1269–1273
Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ (2009) Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA 106(12):4793–4798
Ji Y, Zhang W (2010) Th17 cells: positive or negative role in tumor? Cancer Immunol Immunother 59(7):979–987
Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C (2014) T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci USA 111(15):5664–5669
Black CC, Turk MJ, Dragnev K, Rigas JR (2013) Adenocarcinoma contains more immune tolerance regulatory t-cell lymphocytes (versus squamous carcinoma) in non-small-cell lung cancer. Lung 191(3):265–270
Keerthivasan S, Aghajani K, Dose M, Molinero L, Khan MW, Venkateswaran V, Weber C, Emmanuel AO, Sun T, Bentrem DJ, Mulcahy M, Keshavarzian A, Ramos EM, Blatner N, Khazaie K, Gounari F (2014) Beta-catenin promotes colitis and colon cancer through imprinting of proinflammatory properties in T cells. Sci Transl Med 6(225):225ra228
Dennis KL, Wang Y, Blatner NR, Wang S, Saadalla A, Trudeau E, Roers A, Weaver CT, Lee JJ, Gilbert JA, Chang EB, Khazaie K (2013) Adenomatous polyps are driven by microbe-instigated focal inflammation and are controlled by IL-10-producing T cells. Cancer Res 73(19):5905–5913
Acknowledgments
David Bentrem is supported by a Career Development Award from the Health Services Research and Development Service of the Department of Veterans Affairs. Khashayarsha Khazaie is supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) Grant 1R01CA160436 and NIH/NIAID R01 AI108682-01. Fotini Gounari is supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) R01 AI108682-01. We would like to acknowledge Dr. Alberto de Hoyos for his contribution in obtaining lung cancer tissue.
Conflict of interest
The authors have no disclosures of conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Joseph D. Phillips and Lawrence M. Knab have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Phillips, J.D., Knab, L.M., Blatner, N.R. et al. Preferential expansion of pro-inflammatory Tregs in human non-small cell lung cancer. Cancer Immunol Immunother 64, 1185–1191 (2015). https://doi.org/10.1007/s00262-015-1725-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1725-1