Abstract
Objective
To review the congenital anomalies of the pancreas with their main clinical manifestations and key imaging findings on CT and MRI.
Background and clinical significance
Anomalies of pancreatic development are frequent and generally asymptomatic, but can mimic and predispose individuals to pancreatic or peripancreatic pathologies, such as pancreatitis or malignancy. Their correct diagnosis may help avoid unnecessary further investigations and procedures, or establish adequate treatment when they manifest clinically. Differentiating pancreatic congenital anomalies from their main radiological mimics constitutes a challenge for the radiologist and requires familiarity with key imaging findings.
Conclusion
The imaging findings of CT and MRI are essential for the correct diagnosis of congenital pancreatic anomalies.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alterations of pancreatic development result in pancreatic congenital anomalies. Some of them are asymptomatic, but others may be associated with pathological conditions. On cross-sectional imaging, pancreatic anomalies can mimic pathological conditions; therefore, accurate diagnosis is essential to avoid unnecessary additional investigations or invasive procedures. Conversely, these anomalies may predispose individuals to pancreatic or peripancreatic pathologies, making their recognition crucial in order to establish an adequate treatment when they manifest clinically [1, 2]. This review covers pancreatic embryology, the different anomalies in which altered pancreatic development can result as well as their radiological features on CT and MRI and some pathological conditions that may be associated with them.
CT and MR imaging of the pancreas
Multidetector computed tomography (MDCT) is usually the initial imaging modality for the assessment of most pancreatic diseases, including acute pancreatitis, trauma, and pancreatic masses, especially in their acute onset. This is likely attributable to its broad availability, relative affordability, and high diagnostic performance [3]. A typical MDCT protocol includes an unenhanced phase and a dynamic acquisition consisting of a pancreatic phase performed approximately 35–40 s after IV contrast agent injection and a portal venous phase performed subsequently after a 70-s delay. MDCT has limitations due to low contrast resolution in the assessment of small focal lesions, ductal delineation, especially in normal caliber ducts, initial signs of chronic pancreatitis, and characterization of cystic lesions. Consequently, the sensitivity of MDCT in identifying pancreatic ductal variations is low (50–60%) [4]. Another limitation is radiation exposure, which underscores the necessity of dose optimization, especially for young patients and those who require follow-up scans. Recent advances such as dual-energy CT may have the potential to overcome some of these limitations and further expand the utility and value of CT in pancreatic imaging [5].
Magnetic resonance imaging (MRI) provides an optimal contrast resolution for soft tissues and for the bile and pancreatic ducts [6]. MRI is increasingly being used to scan pancreatic diseases in patients with suspected biliopancreatic pain, to stage chronic pancreatitis, and to diagnose and follow-up some pancreatic tumors [6, 7]. In some instances, these conditions are more effectively depicted with this technique. Magnetic resonance cholangiopancreatography (MRCP) is the gold standard in the evaluation of the pancreatic duct and biliary tract. It has displaced endoscopic retrograde cholangiopancreatography (ERCP) which, due to its invasiveness and morbidity, is now mostly reserved for patients requiring procedures and interventions. MRCP provides high contrast resolution of stationary liquids, such as intrabiliary and intrapancreatic juices through long T2 relaxation time of fluid to image the biliary tree and pancreatic ducts with suppression of adjacent tissue signal. MRCP is often combined with MRI sequences as T1-weigthed (T1w) and T2-weighted (T2w) images, diffusion-weighted images (DWI), and dynamic contrast-enhanced (DCE) sequences [8, 9]. In routine protocols, the acquisition of DCE sequences is performed typically in the arterial phase (20–25 s post contrast injection), portal venous phase (55–60 s), and delayed venous phase (90–180 s) [10]. When hepatobiliary contrast medium is used, acquisitions at 10 and 20 min can also be performed [11]. MRCP can be enhanced with secretin, a physiologic polypeptide exocrine hormone excreted by the duodenum in response to the emptying of the gastric content after a meal. Secretin induces the release of bicarbonate rich fluid from the pancreas, increasing fluid signal in the pancreatic ducts that progresses into the duodenum. Secretin-enhanced MRCP (S-MRCP) takes advantage of fluid-sensitive MR sequences to improve the visualization of ductal anatomy [12].
Developmental anomalies of the pancreas
Normal pancreas is formed from the fusion of two buds, the ventral and dorsal anlagen (Fig. 1). The ventral anlage arises from the hepatic diverticulum adjacent to the biliary system, while the dorsal anlage originates from the dorsal mesogastrium. Around the 37th day of gestation, the rotation of the stomach and the duodenum during embryogenesis results in the displacement of the ventral bud toward the dorsal area and from the embryo’s right to its left side, positioning the ventral bud inferiorly and posteriorly to the dorsal bud [13]. The two buds, with their respective ducts, fuse during the 7th week of gestation; the ventral anlage forms the posterior portion of the head and the uncinate process, while the dorsal anlage forms the anterior portion of the head, the body, and the tail of the gland. The duodenal segment of the ventral duct may remain patent or may involute partially or completely. If patent, it constitutes the duct of Santorini, draining into the minor papilla. Ultimately, the gland drains predominantly through the main pancreatic duct or duct of Wirsung, which results from the fusion of the ventral duct and the remaining part of the dorsal duct, along with the common bile duct in the major papilla [2, 13] (Fig. 1).
Around the 37th day of gestation, the rotation of the stomach and the duodenum displaces the ventral pancreatic bud, along with the common bile duct (CBD) positioning it under and behind the dorsal bud (a). The arrow in b represents the course of the main pancreatic duct, which drains into the major papilla (MP) after the fusion of both ducts around the seventh week of gestation to form the anatomical pancreas (c). GB gallbladder, MiP minor papilla, MPD main pancreatic duct, S duct of Santorini, W duct of Wirsung. All drawings by Javier Rubín ©
Developmental anomalies of the pancreas can be classified according to the embryological process involved [14]. Alterations of fusion and formation of the duct can manifest as conditions such as pancreas divisum, anomalous biliopancreatic junction, and others, such as ansa pancreatica, meandering main pancreatic duct, wirsungocele, santorinicele, bifid tail, or congenital cysts. Rotation and migration anomalies manifest as annular pancreas and ectopic pancreas, while anomalies of parenchymal and cellular differentiation can result in pancreatic agenesis and hypoplasia.
Pancreas divisum
Pancreas divisum is the most common congenital pancreatic anatomical anomaly, with a prevalence of 4–15% [15]. A four-fold higher incidence has been observed in European and American populations compared to Asian populations [16]. This condition results from a failure in fusion of the dorsal and ventral ducts during the seventh week of intrauterine development. Depending on the extent of the fusion failure, three distinct variants are recognized: complete, incomplete, and reverse pancreas divisum. The complete, or classic, variant is the most prevalent, accounting for 70% of the cases [17]. In this form, most of the gland drains into the minor papilla through the duct of Santorini while the head and the uncinate process drain into the major papilla through the duct of Wirsung, in conjunction with the common bile duct (Fig. 2).
Pancreas divisum (a). MRCP imaging of classic pancreas divisum, coronal reconstruction (b) displays the ventral duct (VD) joining the common bile duct (CBD) to drain into the major papilla, separately from the main pancreatic duct (MPD) which drains into the minor papilla (arrow). Axial section (c) shows the MPD crossing anteriorly to the CBD before reaching the minor papilla (arrow) and the duodenum (D)
The incomplete variant, which accounts for approximately 15% of cases is similar to the classic form, except for the presence of an additional communicating branch between the ventral and dorsal systems (Fig. 3a–c). The reverse variant is very uncommon and is characterized by a dorsal isolated main pancreatic duct draining through the major papilla alongside the common bile duct and a separate ventral duct of Wirsung draining through the minor papilla (Fig. 3d, e). The key imaging finding of the classical form of pancreas divisum is the identification of a prominent dorsal duct, coursing anterior and superior to the common bile duct (Fig. 2c). Secretin-enhanced MRCP has demonstrated a higher diagnostic sensitivity for pancreas divisum than plain MRCP (86% vs 52%) in a meta-analysis published by Rustagi et al. [18]. Pancreas divisum should not be diagnosed when a tumor or focal pancreatitis obstruct the main pancreatic duct downstream from the origin of the duct of Santorini in an otherwise normal pancreas. This may lead to an enlargement of the accessory duct that drains the body and tail of the pancreas, mimicking pancreas divisum [17].
Incomplete (a–c) and reverse (d and e) variants of pancreas divisum are less frequent. MRCP in a coronal oblique projection (b) depicts a small ventral duct (VD) joining the common bile duct (CBD) to drain into the major papilla, while the main pancreatic duct (MPD) crosses anteriorly over the CBD to drain into the minor papilla. S-MRCP (c) reveals a small ductal connection (arrow) between the ventral duct (VD) and the dorsal duct (MPD), which is diagnostic of incomplete pancreas divisum. MRCP of reverse pancreas divisum in a coronal oblique projection (e) demonstrates a small ventral duct (VD) draining into the minor papilla, while the MPD joins the common bile duct (CBD) to drain into the major papilla
Pancreas divisum is asymptomatic in most patients (95%) and is usually discovered incidentally on abdominal imaging. However, it can hinder the drainage of the gland, as the dominant dorsal duct, which is larger and longer, opens through a relatively smaller minor papilla. This can predispose to the formation of a santorinicele and lead to chronic abdominal pain [19, 20] (Fig. 4).
Pancreas divisum is associated to santorinicele. 3D-reformatted MRCP (a) depicts a pancreas divisum configuration with the main pancreatic duct (MPD) traversing anteriorly over the CBD to drain into the minor papilla. S-MRCP (b) shows a saccular dilatation of the most distal portion of the MPD (arrow) corresponding to a santorinicele
Despite this, the association between pancreas divisum and pancreatitis has not been conclusively established. When chronic pancreatitis occurs in a patient with pancreas divisum, the resultant morphological changes in the pancreas can present an atypical distribution [21] (Fig. 5).
When chronic pancreatitis occurs in pancreas divisum, the morphological changes in the pancreas can present an atypical distribution. MRI coronal sections diagnostic of pancreas divisum (a, b), show a dilated main pancreatic duct (MPD) running anteriorly to the common bile duct (CBD) and ventral duct (VD). Coronal (c) and axial (d) MDCT sections of the same patient demonstrate a dorsal distribution of morphological changes with atrophy and calcifications affecting the region of the dorsal anlage (DA) (d), while the region of the ventral anlage (VA) remains preserved (c)
Asymptomatic patients do not require any further therapeutic management, but when pancreas divisum is clearly symptomatic, treatments such as endoscopic papillotomy, stent placement, or surgery may be indicated [22].
Ansa pancreatica
Ansa pancreatica (Fig. 6) is characterized by an inverted S-shaped duct of Santorini that drains into the minor papilla and connects with a lateral branch to the duct of Wirsung, which typically maintains a normal configuration. Unlike annular pancreas, in ansa pancreatica the duct does not cross the duodenum [22, 23]. The prevalence of ansa pancreatica has not been reliably determined; however, various studies have estimated it to be close to 1% [24, 25]. Some studies indicate that ansa pancreatica may be a predisposing factor to recurrent acute pancreatitis, especially among heavy alcohol consumers. However, the precise mechanism of this association remains unclear [24].
Meandering main pancreatic duct
Meandering main pancreatic duct refers to an abnormal curvature of the ventral duct within the pancreatic head. Two types have been described: the ‘loop’ type, where the main pancreatic duct creates a localized hairpin-like curve (Fig. 7a, b), and the ‘reverse-Z’ type where the main pancreatic duct forms two pronounced angles when viewed in a coronal projection (Fig. 7c, d). A prevalence of approximately 2% has been documented. Loop and reverse-Z subtypes were found more frequently in patients with idiopathic recurrent acute pancreatitis. According to a series by Gonoi et al., patients with both types of meandering main pancreatic duct have a significantly increased risk of idiopathic acute pancreatitis and idiopathic recurrent acute pancreatitis, with odds ratios of 4.01 and 26.2, respectively [26].
Santorinicele and wirsungocele
Santorinicele and wirsungocele are focal cystic dilatations of the terminal part of the dorsal and ventral pancreatic ducts, respectively. Santorinicele (Fig. 4) is frequently associated with pancreas divisum and may be an acquired condition. It is also associated with recurrent episodes of acute pancreatitis [27].
Bifid pancreatic duct
Duplication of the main pancreatic duct is a benign branching anomaly that usually occurs at the tail level (Fig. 8). As pancreatic buds fuse, a primitive ductal branch system develops, resulting in the formation of a dominant branch that later becomes the main pancreatic duct. Variations in the number of ducts occur when more than one primary channel forms [28]. Bifurcation of the main pancreatic duct does not necessarily result in morphological changes in the pancreas, as variations in the number of main pancreatic ducts can also occur in a normally shaped pancreas. However, bifid main pancreatic duct should be inferred in cases of a ‘fish tail’ pancreas morphology [29] (Fig. 8a). The clinical significance of main pancreatic duct duplication remains unclear, with it often presenting as an incidental finding in patients undergoing imaging for unrelated conditions. Regardless of its clinical importance, a detailed anatomical depiction of the pancreatic duct system is crucial prior to pancreatectomy, as ductal anomalies can lead to leakage and postoperative complications [29].
Anomalous biliopancreatic junction
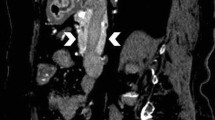
Normally, the duct of Wirsung and the common bile duct converge within the ampulla of Vater forming a common duct approximately 1–12 mm in length, with an average length of about 4 mm, surrounded by the sphincter of Oddi. This configuration prevents the reflux of pancreatic secretions into the biliary tree [30]. In anomalous biliopancreatic junction (ABPJ) these ducts merge before reaching the duodenal wall, forming a longer common duct. This anomaly occurs in 1.5–3.2% of individuals [31] and is associated with the loss of the regulating sphincteric function, which allows the reflux of pancreatic secretions into the bile ducts that normally operate under lower pressure (Fig. 9). ABPJ is considered an etiological factor for the development of choledochal cysts. It has been proposed that enzymes activated in refluxed pancreatic secretions induce chronic inflammation, damage to the biliary wall, and subsequent cystic dilatation of the lumen. However, other factors must also contribute to the presence of choledochal cysts, as they can also be present in newborn infants [31].
Coronal MRCP of a patient with an anomalous biliopancreatic junction depicts a loop-type meandering main pancreatic duct (MPD) that joins prematurely with the common bile duct (CBD), within the head of the pancreas forming a common duct (arrow) that exceeds 15 mm in length before entering the major papilla. This case also presents dilatation of the intrahepatic biliary tract with intrahepatic biliary cysts (IBC) and a choledocal cyst (CHC), consistent with a type IVA cystic dilatation of the biliary tract in the Todani’s classification [60, 61]. BTC: biliary tract cyst; GB: gallbladder
Patients with ABPJ and reflux of pancreatic juice into the bile ducts may not have a choledochal cyst. These patients are at increased risk of developing gallbladder cancer. The stagnation and concentration of bile mixed with pancreatic juice in the gallbladder are believed to have carcinogenic effects, leading to recommendations for preventive cholecystectomy, sometimes combined with extrahepatic bile duct resection [32, 33]. Adult patients with ABPJ, both with and without choledochal cysts, can present clinical symptoms, such as abdominal pain, vomiting, acute pancreatitis, and cholangitis [33]. Pancreatobiliary reflux can also occur in individuals with a normal pancreatobiliary junction (Fig. 10). This condition is thought to result from the dysfunction of the sphincter of Oddi, although the presence of periampullary diverticula or invasive procedures such as sphincterotomy or balloon papillary dilatation may also contribute to reflux [30, 34].
Coronal MRCP (a) in a patient with a history of pancreatitis demonstrates a normal configuration of the BPJ and a moderate biliary dilatation of the CBD, measuring 12 mm in diameter. After secretin stimulation (b), the diameter of the CBD expands to 16 mm, indicating pancreatic-biliary reflux. The diffuse dilatation of the extrahepatic bile duct is consistent with a type IC choledochal cyst according to Todani’s classification
Congenital cysts of the pancreas
Congenital cysts of the pancreas result from the failure of embryonic ducts to regress during their replacement by permanent ducts, leading to obstruction and subsequent cyst formation that can fill with fluid. These cysts can be solitary or multiple and may occur independently or in association with systemic diseases, such as cystic fibrosis, Von Hippel–Lindau syndrome (Fig. 11), and autosomal dominant renal polycystosis. These associated conditions are beyond the scope of this work. Congenital cysts are extremely rare and can be encountered in the fetus, infant, child, or adult [35]. Their antenatal discovery is typically incidental during routine screening. Although usually asymptomatic, larger cysts may exert a mass effect and become clinically manifest. Treatment options include complete excision or, if located in the pancreatic head where excision is not feasible, drainage can be considered as a therapeutic option [36].
Annular pancreas
Annular pancreas is a consequence of altered migration of the ventral pancreatic bud. It is thought that the adhesion of the ventral pancreatic anlage to the duodenum during migration hinders it from rotating completely, resulting in a ring of pancreatic tissue encircling the descending duodenum, in connection with the anatomical pancreas [37]. Annular pancreas can be either complete or incomplete and is considered incomplete when the annulus does not encircle the duodenum entirely, which has been reported in approximately one third of cases [38]. A rare variant known as portal annular pancreas occurs when pancreatic parenchyma fuses around the portal and superior mesenteric veins, thus encircling the portal vein. While portal annular pancreas is typically asymptomatic, its identification prior to surgery is important to prevent potential operative complications [39].
Postmortem examination series have suggested a prevalence of annular pancreas of around 0.01%, whereas ERCP studies have reported a frequency of 0.4%. The true prevalence is uncertain due to potential biases, but it is likely to fall somewhere between these reported values [38]. In abdominal imaging, the presence of pancreatic tissue extending posterolaterally to the duodenum should raise concerns about the presence of an annular pancreas. Accurate radiological diagnosis requires differentiation from a primary thickening of the duodenal wall, such as adenocarcinoma or leiomyoma [40]. This involves assessing the continuity with the anatomical pancreatic tissue and identifying an annular duct running within it [37] (Fig. 12).
Annular pancreas (a). Axial MDCT (c) of a patient with annular pancreas reveals a ring of pancreatic tissue (AP) encircling the descending duodenum (D), in continuity with the anatomical pancreas (P). Axial MRCP (b) of the same patient shows the main pancreatic duct (MPD) crossing anterior to the duodenum (D) and entering the intestinal lumen on its lateral aspect
Annular pancreas is usually asymptomatic. In newborns, it can manifest as gastrointestinal or biliary obstruction, with 70% of affected newborns presenting with other associated malformations [41]. In adults, it is most often detected between the third and the sixth decades of life and may manifest clinically with symptoms, such as upper gastrointestinal bleeding due to peptic ulceration, postprandial fullness, and vomiting due to duodenal obstruction, acute or chronic pancreatitis and, infrequently, biliary obstruction [38, 42]. Surgical treatment may be required for symptomatic cases of annular pancreas.
Ectopic pancreas
Ectopic or heterotopic pancreas is caused by an anomaly of migration that results in the presence of pancreatic tissue outside the gland, lacking anatomical or vascular connection to the normal pancreas [2] (Figs. 13 and 14). Its prevalence ranges from 0.3 to 13.7% of individuals [43]. The upper gastrointestinal tract is the most common location, with gastric and duodenal involvement reported at frequencies of 24–38% and 9–36%, respectively [44]. But it has been identified in other locations, such as the jejunum, liver, gallbladder, colon, appendix, spleen, mesentery, retroperitoneum, female reproductive system, mediastinum, and lungs [44]. Gastric and duodenal ectopic pancreas is most frequently found in the submucosa along the greater curvature of the antrum and in the proximal duodenum, typically with an endophytic growth pattern. The first 50 cm of the jejunum is the third most frequent location, estimated in 0.5–35% of cases. They are also typically located in the submucosa although exophytic growth patterns are more common [45].
MRI of a patient with a history of intestinal obstruction and an endoscopic finding of a stenosing anthropyloric lesion shows a cystic lesion with thick walls in the antral region (E). Following partial gastrectomy, the pathological examination identified pancreatic heterotopia with the formation of a pancreatic pseudocyst within the gastric mucosa
Imaging of ectopic pancreas typically reveals a nonspecific endophytic submucosal solid mass with an enhancement pattern similar to the normal pancreas. Sometimes, a small central umbilication, a remnant of the primitive pancreatic duct, can be identified [23]. Heterotopic pancreas in the mesentery is a very rare condition [46] that presents as a mesenteric mass, with morphological features closely resembling the main pancreas (a homogeneous, well-enhancing mass with an elongated appearance and pancreas-like clefts or lobulations) often found in close association with the jejunum. Mesenteric ectopia is usually larger than ectopic pancreas found in other locations [47, 48] and its unique association with the jejunum leads some to consider it as a variant of jejunal pancreatic ectopia with exophytic growth [46].
Submucosal heterotopic pancreas can be easily misidentified as gastrointestinal stromal tumors (GIST). Unlike GISTs, which often exhibit exophytic or mixed growth patterns, globular contours, and a well-defined border, ectopic pancreas usually presents with an endoluminal pattern, flat contours, and poorly defined borders, in addition to the previously described characteristics.
Most cases of ectopic pancreas are asymptomatic and are identified incidentally on pathological specimens or during autopsies [44]. However, it can manifest clinically with symptoms resulting from mass effect, such as obstruction or intussusception, as well as underlying pathologies, including ulceration, bleeding, and acute or chronic pancreatitis. Ectopic pancreas is susceptible to the same pathological conditions that affect the normal pancreas. For instance, there have been reported cases of ectopic insulinomas in patients presenting with hypoglycemia [49]. Cystic degeneration (Fig. 14) and, on rare occasions, malignant transformation can also occur.
Groove pancreatitis is a rare form of chronic pancreatitis that affects the ‘groove’ between the head of the pancreas, the duodenum, and the common bile duct (Fig. 15). The exact cause is unknown, but long-term alcohol abuse and smoking are frequently observed in patients with this condition. Ectopic pancreas has been associated with groove pancreatitis when heterotopic pancreatic tissue is located between the medial duodenal wall and the head of the pancreas [50, 51]. When ectopic pancreas manifests symptoms, the clinical presentation can be severe, requiring surgical intervention [50, 52].
Groove pancreatitis and cystic dystrophy of the pancreas. Axial and coronal CT images of the pancreas (a and b) shows hypodense fibrotic tissue in the pancreatoduodenal groove. On MRI (c) this tissue appears slightly hypointense on T2-weighted image. Additionally, cysts are visible in both the pancreatic head and the medial duodenal wall (arrows) indicative of pancreatic ectopia with cystic changes
Pancreatic hypoplasia and agenesis
Total pancreatic agenesis is extremely rare, as it is incompatible with life. Pancreatic hypoplasia results from the total or partial absence of one of the pancreatic anlagen with the absence of the dorsal pancreatic anlage being the most frequent variation [2]. A morphological classification based on the presence of agenesis or hypoplasia of the dorsal pancreas (type 1), the uncinate process (type 2), or both (type 3) [53] has been proposed. Dorsal pancreatic agenesis or hypoplasia has been reported as part of polysplenia syndrome, likely due to the close relationship between the developing pancreas and the spleen in the dorsal mesogastrium [54]. It can occur in isolation or in association with heterotaxia syndrome [55]. Conversely, an association between intestinal malrotation and aplasia or hypoplasia of the ventral pancreas has been suggested, probably due to the relationship between the ventral anlage and the rotation of the duodenojejunal loop during pancreatic development [56].
Pancreatic hypoplasia is often asymptomatic, but can manifest with nonspecific abdominal pain, pancreatitis, and diabetes mellitus, as most islets and beta cells are located in the pancreatic body and tail [1]. The key image finding in the most frequent variation is a truncated and short pancreas, where the tail and part of the body are absent, but the minor papilla and a remnant of the duct of Santorini may be present. It is crucial to differentiate pancreatic hypoplasia from glandular lipomatosis and atrophy and to rule out the presence of pancreatic malignancy with associated glandular atrophy [57]. In lipomatosis and atrophy, the pancreatic duct is present and the glandular space is replaced by fat. In contrast, in hypoplasia and agenesis the duct is absent and the stomach or intestine occupy the anatomical space of the normal pancreas, ventral to the splenic vein [58] (Fig. 16).
Pancreatic hypoplasia (a). MDCT coronal (b) and axial (c) sections of a patient with partial agenesis of the dorsal pancreas show a truncated pancreas (P), consisting only of the head and a small segment of the body. The anatomical space of the body and tail, located ventrally to the splenic vein (SV), is occupied by the jejunum (J) and stomach (S). CBD common bile duct
Other anatomical variants
Anatomical variants can lead to diagnostic confusion. Pancreatic contour alterations, such as lobulations in the head and neck of the pancreas, are present in approximately 34% of individuals. They appear as pseudomasses and can simulate pancreatic neoplasms [59]. Intrapancreatic masses, such as intrapancreatic accessory spleen, can be mistaken for pancreatic neoplasms; however, unlike neoplasms, intrapancreatic accessory spleen presents the same radiological characteristics as the anatomical spleen [1] (Fig. 17).
Conclusion
Pancreatic congenital anomalies are frequent and result from alterations in the processes involved in glandular embryology, with the most frequent variants resulting from abnormalities in fusion and duct formation. While most of these anomalies are asymptomatic, they can mimic pathological conditions and predispose individuals to specific pancreatic or peripancreatic pathologies. Their correct diagnosis is critical to avoid unnecessary investigations or invasive procedures and to provide adequate treatment when they manifest clinically. Furthermore, it is essential to consider these anomalies when planning pancreatic and peripancreatic surgeries to prevent potential surgical complications. Distinguishing pancreatic congenital anomalies from their main radiological pitfalls is a challenge for the radiologist, for which findings from computed tomography and magnetic resonance are essential.
References
Srisajjakul S, Prapaisilp P, Bangchokdee S. Imaging of congenital pancreatic lesions: emphasis on key imaging features. Jpn J Radiol. 2015;33(9):525-532. https://doi.org/10.1007/s11604-015-0458-6
Mortelé KJ, Rocha TC, Streeter JL, Taylor AJ. Multimodality Imaging of Pancreatic and Biliary Congenital Anomalies. RadioGraphics. 2006;26(3):715-731. https://doi.org/10.1148/rg.263055164
Agostini A, Borgheresi A, Bruno F, et al. New advances in CT imaging of pancreas diseases: a narrative review. Gland Surg. 2020;9(6):2283-2294. https://doi.org/10.21037/gs-20-551
Asayama Y, Fang W, Stolpen A, Kuehn D. Detectability of pancreas divisum in patients with acute pancreatitis on multi-detector row computed tomography. Emerg Radiol. 2012;19(2):121-125. https://doi.org/10.1007/s10140-011-1008-x
Almeida RR, Lo GC, Patino M, Bizzo B, Canellas R, Sahani D V. Advances in Pancreatic CT Imaging. Am J Roentgenol. 2018;211(1):52-66. https://doi.org/10.2214/AJR.17.18665
Sandrasegaran K, Lin C, Akisik FM, Tann M. State-of-the-art pancreatic MRI. Am J Roentgenol. 2010;195(1):42-53. https://doi.org/10.2214/AJR.10.4421
O’Neill E, Hammond N, Miller FH. MR Imaging of the Pancreas. Radiol Clin North Am. 2014;52(4):757-777. https://doi.org/10.1016/j.rcl.2014.02.006
Harrington KA, Shukla‐Dave A, Paudyal R, Do RKG. MRI of the Pancreas. J Magn Reson Imaging. 2021;53(2):347-359. https://doi.org/10.1002/jmri.27148
Itani M, Lalwani N, Anderson MA, Arif-Tiwari H, Paspulati RM, Shetty AS. Magnetic resonance cholangiopancreatography: pitfalls in interpretation. Abdom Radiol. 2021;48(1):91-105. https://doi.org/10.1007/s00261-021-03323-1
Patel BN. Routine MR Imaging for Pancreas. Magn Reson Imaging Clin N Am. 2018;26(3):315-322. https://doi.org/10.1016/j.mric.2018.03.009
Canellas R, Rosenkrantz AB, Taouli B, et al. Abbreviated MRI Protocols for the Abdomen. RadioGraphics. 2019;39(3):744-758. https://doi.org/10.1148/rg.2019180123
Swensson J, Zaheer A, Conwell D, Sandrasegaran K, Manfredi R, Tirkes T. Secretin-Enhanced MRCP: How and Why— AJR Expert Panel Narrative Review. Am J Roentgenol. 2021;216(5):1139-1149. https://doi.org/10.2214/AJR.20.24857
Henry BM, Skinningsrud B, Saganiak K, Pękala PA, Walocha JA, Tomaszewski KA. Development of the human pancreas and its vasculature — An integrated review covering anatomical, embryological, histological, and molecular aspects. Ann Anat - Anat Anzeiger. 2019;221:115-124. https://doi.org/10.1016/j.aanat.2018.09.008
Adda G, Hannoun L, Loygue J. Development of the human pancreas: variations and pathology. A tentative classification. Anat Clin. 1984;5(4):275-283. https://doi.org/10.1007/BF01798752
Agha FP, Williams KD. Pancreas Divisum: Incidence, Detection, and Clinical Significance. Am J Gastroenterol. 1987;82(4):315-320. https://doi.org/10.1111/j.1572-0241.1987.tb01666.x
Liao Z, Gao R, Wang W, et al. A systematic review on endoscopic detection rate, endotherapy, and surgery for pancreas divisum. Endoscopy. 2009;41(05):439-444. https://doi.org/10.1055/s-0029-1214505
Gutta A, Fogel E, Sherman S. Identification and management of pancreas divisum. Expert Rev Gastroenterol Hepatol. 2019;13(11):1089-1105. https://doi.org/10.1080/17474124.2019.1685871
Rustagi T, Njei B. Magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: A systematic review and meta-analysis. Pancreas. 2014;43(6):823-828. https://doi.org/10.1097/MPA.0000000000000143
Cotton P. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatitis. Gut. 1980;21:105-114.
Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc. 2004;60(3):419-425. https://doi.org/10.1016/S0016-5107(04)01815-2
Dutta S, Sowmiya S V., Chapa UK, Jain A, Reddy A, Nelamangala Ramakrishnaiah VP. Pancreatic divisum with chronic pancreatitis of the ventral pancreas. ANZ J Surg. 2021;91(3):E126-E127. https://doi.org/10.1111/ans.16152
Borghei P, Sokhandon F, Shirkhoda A, Morgan DE. Anomalies, Anatomic Variants, and Sources of Diagnostic Pitfalls in Pancreatic Imaging. Radiology. 2013;266(1):28-36. https://doi.org/10.1148/radiol.12112469
Alexander LF. Congenital Pancreatic Anomalies, Variants, and Conditions. Radiol Clin North Am. 2012;50(3):487-498. https://doi.org/10.1016/j.rcl.2012.03.006
Hayashi TY, Gonoi W, Yoshikawa T, Hayashi N, Ohtomo K. Ansa pancreatica as a predisposing factor for recurrent acute pancreatitis. World J Gastroenterol. 2016;22(40):8940. https://doi.org/10.3748/wjg.v22.i40.8940
Adibelli ZH, Adatepe M, Imamoglu C, Esen OS, Erkan N, Yildirim M. Anatomic variations of the pancreatic duct and their relevance with the Cambridge classification system: MRCP findings of 1158 consecutive patients. Radiol Oncol. 2016;50(4):370-377. https://doi.org/10.1515/raon-2016-0041
Gonoi W, Akai H, Hagiwara K, et al. Meandering Main Pancreatic Duct as a Relevant Factor to the Onset of Idiopathic Recurrent Acute Pancreatitis. Algül H, ed. PLoS One. 2012;7(5):e37652. https://doi.org/10.1371/journal.pone.0037652
Srisajjakul S, Prapaisilp P, Bangchokdee S. Diagnostic clues, pitfalls, and imaging characteristics of ‘-celes’ that arise in abdominal and pelvic structures. Abdom Radiol. 2020;45(11):3638-3652. https://doi.org/10.1007/s00261-020-02546-y
Dinter D, Löhr J-M, Neff KW. Bifid Tail of the Pancreas: Benign Bifurcation Anomaly. Am J Roentgenol. 2007;189(5):W251-W253. https://doi.org/10.2214/AJR.05.1453
Giarraputo L, Savastano S, Napetti S. Trifidum anomaly of the main pancreatic duct. Pancreatology. 2020;20(3):569-570. https://doi.org/10.1016/j.pan.2020.01.018
Sugita R. Pancreaticobiliary reflux as a high-risk factor for biliary malignancy: Clinical features and diagnostic advancements. World J Hepatol. 2015;7(13):1735. https://doi.org/10.4254/wjh.v7.i13.1735
Yamauchi S, Koga A, Matsumoto S, Tanaka M, Nakayama F. Anomalous junction of pancreaticobiliary duct without congenital choledochal cyst: a possible risk factor for gallbladder cancer. Am J Gastroenterol. 1987;82(1):20–24. http://www.ncbi.nlm.nih.gov/pubmed/3799576.
Le Roy B, Gagnière J, Filaire L, Fontarensky M, Hordonneau C, Buc E. Pancreaticobiliary maljunction and choledochal cysts: from embryogenesis to therapeutics aspects. Surg Radiol Anat. 2016;38(9):1053-1060. https://doi.org/10.1007/s00276-016-1669-y
Morine Y, Shimada M, Takamatsu H, et al. Clinical features of pancreaticobiliary maljunction: Update analysis of 2nd Japan-nationwide survey. J Hepatobiliary Pancreat Sci. 2013;20(5):472-480. https://doi.org/10.1007/s00534-013-0606-2
Sai JK, Suyama M, Kubokawa Y, et al. Occult pancreatobiliary reflux in patients with a normal pancreaticobiliary junction. Gastrointest Endosc. 2003;57(3):364-368. https://doi.org/10.1067/mge.2003.53
Auringer ST, Ulmer JL, Sumner TE, Turner CS. Congenital cyst of the pancreas. J Pediatr Surg. 1993;28(12):1570-1571. https://doi.org/10.1016/0022-3468(93)90100-Y
Warnock WT, Khoshnam N, Bird KM, et al. Congenital Cyst of the Pancreas: A Case Report and Review of Literature. Fetal Pediatr Pathol. 2016;35(4):265-271. https://doi.org/10.3109/15513815.2016.1166535
Leyendecker JR, Elsayes KM, Gratz BI, Brown JJ. MR Cholangiopancreatography: Spectrum of Pancreatic Duct Abnormalities. Am J Roentgenol. 2002;179(6):1465-1471. https://doi.org/10.2214/ajr.179.6.1791465
Sandrasegaran K, Patel A, Fogel EL, Zyromski NJ, Pitt HA. Annular Pancreas in Adults. Am J Roentgenol. 2009;193(2):455-460. https://doi.org/10.2214/AJR.08.1596
Joseph P, Raju RS, Vyas FL, Eapen A, Sitaram V. Portal annular pancreas. A rare variant and a new classification. J Pancreas. 2010;11(5):453-455.
Vaughn DD, Jabra AA, Fishman EK. Pancreatic disease in children and young adults: evaluation with CT. RadioGraphics. 1998;18(5):1171-1187. https://doi.org/10.1148/radiographics.18.5.9747614
Nijs E, Callahan MJ, Taylor GA. Disorders of the pediatric pancreas: Imaging features. Pediatr Radiol. 2005;35(4):358-373. https://doi.org/10.1007/s00247-004-1326-1
Yogi Y, Shibue T, Hashimoto S. Annular pancreas detected in adults, diagnosed by endoscopic retrograde cholangiopancreatography: Report of four cases. Gastroenterol Jpn. 1987;22(1):92-99. https://doi.org/10.1007/BF02806340
Fékété F, Noun R, Sauvanet A, Fléjou JF, Bernades P, Belghiti J. Pseudotumor Developing in Heterotopic Pancreas. World J Surg. 1996;20(3):295-298. https://doi.org/10.1007/s002689900047
Kim DU, Lubner MG, Mellnick VM, Joshi G, Pickhardt PJ. Heterotopic pancreatic rests: imaging features, complications, and unifying concepts. Abdom Radiol. 2017;42(1):216-225. https://doi.org/10.1007/s00261-016-0874-9
Kim DW, Kim JH, Park SH, et al. Heterotopic pancreas of the jejunum: associations between CT and pathology features. Abdom Imaging. 2015;40(1):38-45. https://doi.org/10.1007/s00261-014-0177-y
Seo N, Kim JH. Characteristic CT features of heterotopic pancreas of the mesentery: “another pancreas” in the mesentery. Clin Imaging. 2014;38(1):27-30. https://doi.org/10.1016/j.clinimag.2013.09.008
Shin SS, Jeong YY, Kang HK. Giant Heterotopic Pancreas in the Jejunal Mesentery. Am J Roentgenol. 2007;189(5):W262-W263. https://doi.org/10.2214/AJR.05.1142
Borghol S, Diris B, Alberti N, Crombe A, Laurent F. Ectopic pancreatic tissue in the jejunal mesentery. Diagn Interv Imaging. 2015;96(11):1233-1236. https://doi.org/10.1016/j.diii.2015.01.009
Guerrero-Pérez F, Vilarrasa N, Huánuco L V., et al. Ectopic insulinoma: a systematic review. Rev Endocr Metab Disord. 2023;24(6):1135-1146. https://doi.org/10.1007/s11154-023-09824-2
LeCompte MT, Mason B, Robbins KJ, et al. Clinical classification of symptomatic heterotopic pancreas of the stomach and duodenum: A case series and systematic literature review. World J Gastroenterol. 2022;28(14):1455-1478. https://doi.org/10.3748/wjg.v28.i14.1455
Raman SP, Salaria SN, Hruban RH, Fishman EK. Groove Pancreatitis: Spectrum of Imaging Findings and Radiology-Pathology Correlation. Am J Roentgenol. 2013;201(1):W29-W39. https://doi.org/10.2214/AJR.12.9956
Sai Prasad TR, Datta Gupta S, Bhatnagar V. Ectopic pancreas associated with a choledochal cyst and extrahepatic biliary atresia. Pediatr Surg Int. 2001;17(7):552-554. https://doi.org/10.1007/s003830100607
Cho HS, Woo JY, Hong H, et al. Morphologic Classification of Congenital Short Pancreas on Multidetector Computed Tomography. J Comput Assist Tomogr. 2013;37(5):797-804. https://doi.org/10.1097/RCT.0b013e31829ce256
Herman TE, Siegel MJ. Polysplenia syndrome with congenital short pancreas. Am J Roentgenol. 1991;156(4):799-800. https://doi.org/10.2214/ajr.156.4.2003448
Low JP, Williams D, Chaganti JR. Polysplenia syndrome with agenesis of the dorsal pancreas and preduodenal portal vein presenting with obstructive jaundice—a case report and literature review. Br J Radiol. 2011;84(1007):e219-e222. https://doi.org/10.1259/bjr/27680217
Inoue Y, Nakamura H. Aplasia or hypoplasia of the pancreatic uncinate process: comparison in patients with and patients without intestinal nonrotation. Radiology. 1997;205(2):531-533. https://doi.org/10.1148/radiology.205.2.9356640
Güçlü M, Serin E, Ulucan Ş, et al. Agenesis of the dorsal pancreas in a patient with recurrent acute pancreatitis: case report and review. Gastrointest Endosc. 2004;60(3):472-475. https://doi.org/10.1016/S0016-5107(04)01733-X
Lal H, Yadav P, Mourya C. Dependent stomach sign and dependent intestine sign of dorsal pancreatic agenesis. Abdom Radiol. 2017;42(2):667-669. https://doi.org/10.1007/s00261-016-0911-8
Ross BA, Jeffrey RB, Mindelzun RE. Normal variations in the lateral contour of the head and neck of the pancreas mimicking neoplasm: evaluation with dual-phase helical CT. Am J Roentgenol. 1996;166(4):799-801. https://doi.org/10.2214/ajr.166.4.8610553
Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts. Am J Surg. 1977;134(2):263-269. https://doi.org/10.1016/0002-9610(77)90359-2
Brown ZJ, Baghdadi A, Kamel I, Labiner HE, Hewitt DB, Pawlik TM. Diagnosis and management of choledochal cysts. HPB. 2023;25(1):14-25. https://doi.org/10.1016/j.hpb.2022.09.010
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amseian, G., Ayuso, JR. Pancreatic congenital anomalies and their features on CT and MR imaging: a pictorial review. Abdom Radiol 49, 1734–1746 (2024). https://doi.org/10.1007/s00261-024-04229-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-024-04229-4