Abstract
Purpose
To compare outcomes in patients with T1b and T2a renal cell carcinoma (RCC) treated with percutaneous cryoablation (PCA) who underwent transarterial embolization (TAE) of the RCC prior to PCA (TAE + PCA) to patients who were treated with PCA alone.
Methods
Retrospective review of all adult patients with T1b (4.1–7 cm) and T2a (7.1–10 cm) RCC treated with PCA from 2008 to 2021. Data collected included age, sex, tumor diameter, RENAL nephrometry score, technical success, adverse events (AEs), changes in serum creatinine, local control, and recurrence rates. A p value of 0.05 was considered the threshold for statistical significance.
Results
13 patients with 13 RCCs (mean age: 72.7 ± 10.4; 54% male) and 35 patients with 37 RCCs (mean age: 66.7 ± 10.6; 60% male) were included in the TAE + PCA and PCA groups, respectively. The TAE + PCA group had larger mean tumor diameter (5.7 ± 1.1 cm vs. 4.7 ± 0.6 cm; p < 0.0001) and higher mean RENAL nephrometry score (8.9 ± 1.1 vs. 7.8 ± 1.5; p = 0.02). There were no differences between the groups with respect to technical success of PCA (p = 0.46), local tumor control (p = 0.3), or mean number of procedures to achieve local tumor control (p = 0.85). Mean increase in serum creatinine was not significantly different between the two groups (p = .63). Major AEs were similar between the groups (p = 1); however, the TAE + PCA group had no major hemorrhagic AEs while the PCA alone group had three (8.3%).
Conclusion
TAE + PCA in patients with T1b or T2 RCC is technically feasible without significant added detriment to renal function. This combined approach may help to reduce hemorrhagic AEs but larger patient cohorts are needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous ablation is a well-accepted therapeutic approach for patients with T1a renal cell carcinoma (RCC) (< 4 cm in maximal diameter) who are either unwilling or unable to undergo partial or total nephrectomy [1, 2]. However, for larger tumors, including T1b (4.1–7 cm) and T2 (> 7 cm) masses, the technical and clinical outcomes of percutaneous ablation are worse [3], suggesting additional strategies are needed for this patient population. Clear cell RCC, the most common histologic variant [4], is a hypervascular tumor on contrast-enhanced imaging [5]. This feature has led several investigators to report retrospective case series evaluating transarterial embolization (TAE) prior to percutaneous ablation as a mechanism to improve outcomes in non-surgical patients with T1b or T2 RCC [6,7,8,9,10,11,12,13]. While these reports address the technical feasibility and safety of the combined approach, there are a paucity of data comparing clinical outcomes in patients with T1b or T2 RCC who underwent TAE prior to percutaneous ablation versus those who underwent percutaneous ablation alone. Along these lines, one retrospective study in patients with RCC measuring ≥ 5 cm compared outcomes from four patients who underwent TAE + percutaneous cryoablation (PCA) to those of six patients who received PCA alone found that post-procedural hematoma volume was reduced in the TAE + PCA patients [14]. More recently, a retrospective comparative study matched nine patients with RCC treated with TAE + PCA by age, sex, and tumor size to 18 patients treated with PCA alone [15]. However, no significant differences were seen between the groups with regard to number of probes required, complication rates, changes in renal function, or post-procedural hematocrit levels; although, patients with both T1a and T1b RCC were included in this analysis which may have lessened the impact of TAE. As such, additional comparative data specifically for patients with either T1b or T2 RCC is needed. The purpose of this study is to compare technical and clinical outcomes of patients with T1b and T2 RCC treated with TAE + PCA to those treated with PCA alone.
Materials and methods
Patients
The approval for this retrospective study was granted by the Institutional Review Board (IRB) and was Health Insurance Affordability and Accountability Act compliant. The Radiology Information System (RIS) was searched for all adult patients with RCC treated with PCA from January 2009 to July 2021. Patients with either T1b (4.1–7 cm) or T2 (7.1–10 cm) RCC were identified for further analysis. This included 47 patients with 48 T1b or T2 RCCs. Patient characteristics, such as age, sex, Charlson comorbidity index, international normalized ratio (INR), platelet count, and baseline serum creatinine, are detailed in Table 1. 12 (25%) of the tumors were treated with TAE + PCA, while 36 (75%) of the tumors were treated with PCA alone. The decision to treat with TAE + PCA or PCA alone was at the discretion of the attending interventional radiologist.
Tumor characteristics
Maximal tumor diameter was measured on either the most recent ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) prior to PCA. Tumor complexity was assessed by the RENAL nephrometry score [16]. Both the maximal tumor diameter and RENAL nephrometry score were recorded by either a medical student or interventional radiology resident. This was overseen by a board-certified interventional radiologist with 4 years of experience in practice. The results for each group are summarized in Table 1. Biopsy specimens were available in 26/48 (54%) of tumors with results showing RCC in 16/26 (62%) and low-grade oncocytic renal neoplasm in 3/26 (12%). Seven biopsy samples (26%) had no malignancy identified, from inadvertently obtaining a sample from normal renal parenchyma instead of the mass at the time of procedure.
Embolization procedure
All TAEs were performed by board-eligible or board-certified interventional radiologists with post-training experience ranging from 1 to 26 years (Fig. 1). All TAEs were performed with moderate sedation. Vascular access was obtained under sonographic guidance in either the right common femoral artery, left common femoral artery, or left radial artery depending on patient characteristics and operator preference. A diagnostic angiogram from the main renal artery was performed to identify the artery (or arteries) supplying the RCC. After selecting the artery (or arteries) supplying the RCC with a microcatheter, TAE was performed to stasis in the target vessel(s). Embolic agents utilized included Embozene™ microspheres alone (n = 4; 33%) (75–900 µm; Varian, Palo Alto, CA, USA), Embozene™ microspheres followed by a gelatin sponge slurry (n = 2; 17%) (75–400 µm; Varian), an alcohol and ethiodized oil emulsion (n = 2; 17%), Embosphere® microspheres alone (n = 2; 17%) (300–500 µm; Merit, South Jordan, UT, USA), Embosphere® microspheres followed by a gelatin sponge slurry (n = 1; 8%) (300–500 µm; Merit), and Ekobi® microspheres (n = 1; 8%) (180–212 µm; IMBIOTECHNOLOGIES, Edmonton, AB, CA). Embolic selection was at the discretion of the operator. TAE were performed at a mean 10.25 days prior to PCA (range: 0–46; inter-quartile range: 13).
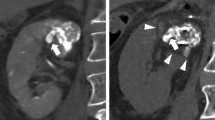
a Axial slice from a contrast-enhanced CT of the abdomen demonstrates a T1b renal cell carcinoma on the anterior portion of the left kidney (white arrow). b Digital subtraction angiogram (DSA) with the microcatheter in the distal left renal artery demonstrates the hypervascular RCC in the left kidney (white arrow). c DSA with the microcatheter in a branch of the left renal artery demonstrates lack of perfusion to the tumor after embolization (white arrows). d Axial slice from a non-contrast CT of the abdomen obtained with the patient in right lateral decubitus position during the ablation procedure shows one of four ablation needles used during the procedure with the resultant iceball (white arrow). e Axial slice from a contrast-enhanced CT of the abdomen obtained approximately 5 years after the ablation procedure continues to demonstrate the hyperdense embolic material within the mass without evidence of residual enhancing tissue (white arrow), consistent with a continued complete response to therapy
Cryoablation procedure
All PCAs were performed by board-eligible or board-certified interventional radiologists with post-training experience ranging from 1 to 26 years (Fig. 1). General anesthesia or conscious sedation were used based on operator and patient preference. All procedures were performed with computed tomography (CT) and CT-fluoroscopy guidance alone. Peri-procedural antibiotics were administered, and anti-coagulation therapies were withheld according to guidelines [17, 18]. A preliminary scan was performed to determine appropriate percutaneous access. Specific ablation platforms and probes were left to operator discretion but both ICEFx™ (Boston Scientific, Marlborough, Massachusetts, USA) and Endocare® (Varian) were employed. Regardless, probe positioning and number were at the discretion of the operator with the goal of achieving at least a 5-mm margin. The number of probes used in each procedure was recorded. With the probes in place, the ablation zone is estimated. If the operator determined that a critical structure such as colon would encroach on the anticipated ablation zone, hydrodissection was performed. Subsequently, PCA was performed according to the manufacturer’s instructions for use. Most commonly, this included a 10-min freeze, an active thaw, a second 10-min freeze, and then an active thaw. Intermittent imaging was performed throughout the ablation cycle to assess progress. Adequate coverage of the lesion and immediate adverse events (AEs) were assessed on a final intraprocedural CT scan.
Outcomes
Per institutional practice, imaging follow-up, consisting of either contrast-enhanced US, CT, or MRI, was obtained at approximately three months after PCA to assess response. If there is no residual disease, patients were seen again with imaging in approximately six months. After that, imaging follow-up occurred annually for up to 5 years. All follow-up imaging was interpreted by board-eligible or board-certified abdominal radiologists. Technical and oncologic outcome parameters such as primary technical success, secondary technical success, and local recurrence were defined by published standards [19]. AEs were classified according to published criteria from the Society of Interventional Radiology (SIR) [20]. Volumes of any post-PCA hematomas were calculated by a board-certified interventional radiologist with approximately 4 years of post-procedure training using commercially available software (TeraRecon, Foster City, CA, USA).
Statistical analysis
Statistical analysis was performed utilizing commercially available software (GraphPad, v9, San Diego, CA, USA). An unpaired t test and Chi-squared test were used to analyze continuous and categorical data, respectively. A p value of ≤ 0.05 was considered to be statistically significant.
Results
Patient and tumor characteristics
Patient and tumor characteristics are summarized in Table 1. Regarding tumor characteristics, patients in the TAE + PCA cohort had both larger (mean (SD): 57.33 mm (11.88) vs. 46.69 mm (6.55); p = 0.0003) and more complex (mean RENAL score (SD): 7.58 (2.23) vs. 6.64 (2.13); p = 0.0192) tumors.
Technical outcomes
Technical outcomes are summarized in Table 2. Imaging follow-up was available on 11/12 (92%) tumors in the TAE + PCA group and 33/36 (92%) tumors in the PCA along group. Both patients that did not achieve primary technical success in the TAE + PCA group underwent repeat PCA without additional TAE, both achieving local control (secondary technical success = 100%). Of the 11 patients that did not achieve primary technical success in the PCA alone group, six underwent repeat PCA without TAE to achieve local control (secondary technical success = 84.8%, n = 28/33). The remaining five patients in this group are under active surveillance for residual disease.
Clinical outcomes
Mean follow-up in the TAE + PCA and PCA alone groups was 37 months (range: 2–71) and 29 months (range: 1–103), respectively. Clinical outcomes, including major AEs, hemorrhagic major AEs, volume of post-PCA hematomas, post-procedural serum creatinine, and percentage change in serum creatinine after PCA, are detailed in Table 3. No differences in the rates of total major AEs were identified (p = 0.76). The single major AE in the TAE + PCA group consisted of a perinephric abscess that was managed with a percutaneous drain and ureteral stent placement. The four major AEs in the PCA group included a perinephric abscess complicated by a colo-ureteral fistula requiring percutaneous drain and ureteral stent placement, a hemorrhagic pseudoaneurysm in the kidney requiring coil embolization, a retroperitoneal hematoma with active extravasation on CT requiring embolization, and a retroperitoneal hematoma causing hemorrhagic shock and pulseless electrical activity (PEA) arrest. In this last patient, hemorrhage from a lumbar artery was treated after achieving return of spontaneous circulation (ROSC). Patients in the TAE + PCA group experienced no major hemorrhagic AEs with a post-procedural hematoma volume that was less than half that in the PCA alone group although this did not reach statistical significance (p = 0.29). The combined TAE + PCA group did not experience a greater detriment to renal function than the PCA alone group (p = 0.51). One patient (n = 1/11; 9%) in the TAE + PCA group had local recurrence during the follow-up period, which was identified at 40 months. Six additional tumors in the PCA alone group were lost to follow-up after their initial imaging. Among these patients, local recurrence was identified in one patient (n = 1/27; 3.7%) at 19 months. No difference was seen in rates of local recurrence between the two groups (p = 0.5).
Discussion
In this retrospective comparative study, the technical and clinical outcomes of patients with T1b and T2 RCC treated with TAE + PCA were compared to patients treated with PCA alone. Given that the combined TAE + PCA cohort consisted of both larger (57.33 ± 11.88 vs 46.69 ± 6.55, p = 0.003) and more complex tumors (mean nephrometry scores: 8.92 ± 1.16 vs 7.78 ± 1.48, p = 0.0192) that required a higher number of cryoablation probes for adequate coverage during the initial PCA procedure (4.3 (1.5) vs. 3.3 (1.4); p = 0.05), these patients should expect to have lower rates of primary and secondary technical success, higher rates of major AEs, larger post-procedural hematomas, and higher rates of local recurrence than patients in the PCA alone group who had smaller and less complex tumors if TAE had no clinical benefit [16, 21, 22]. Yet, despite these baseline differences between the two groups, no significant differences were identified with regards to primary technical success, secondary technical success, major AEs, number of sessions needed to achieve local tumor control, and local recurrence. Furthermore, there were no major hemorrhagic AEs in the TAE + PCA group, while 8.3% of patients in the PCA alone group had a major hemorrhagic event after ablation. This difference did not reach statistical significance in this cohort likely due to sample size but is an important consideration as hemorrhage is the most common complication after PCA [3]. Along these lines, patients in the TAE + PCA group had post-PCA hematoma volumes that were less than half the size of the hematomas observed in the PCA alone group; although, again, this did not reach statistical significance likely due to sample size. Importantly, the data show that the combined approach does not worsen renal function to a greater degree than monotherapy. Thus, the current data demonstrate that the combined approach is safe and may confer technical and clinical benefits.
The majority of clinical investigations into the utility of TAE as an adjunct to PCA have demonstrated the relative safety and technical feasibility of the combined approach even though the reports are limited due to small sample size, retrospective nature, absence of a true efficacy endpoint, and lack of a comparator group (i.e., PCA alone) [6,7,8,9,10,11,12,13]. For instance, one of the largest single-arm, retrospective series reported 31 patients with 36 T1 RCCs who were treated with TAE + PCA with approximately 2 years of follow-up [10]. Patients in this study experienced no major AEs related to either procedure or no evidence of recurrence during the follow-up period in 30/36 tumors (83.3%). Of note, all local recurrence occurred in patients with T1b RCC. To improve on single-arm data, one retrospective study compared patients with RCC ≥ 5 cm treated with TAE + PCA (n = 4) to those treated with PCA alone (n = 6) [14]. Similar to the current data, this study reported that the mean post-PCA hematoma volume in the combined cohort was smaller than that in the PCA alone group (18.3 ml ± 25.9 vs. 359.3 ml ± 460.9; p < 0.01), with only one patient from the PCA alone group requiring a post-PCA transfusion and hospitalization. While encouraging, these early comparative results needed to be confirmed with larger patient samples. To this end, a larger, more recent retrospective, comparative study where propensity-score matching based on patient age, sex, and tumor diameter was performed between patients treated with TAE + PCA and PCA alone to identify objective benefits to adjunctive TAE [15]. In this study, nine patients treated with TAE + PCA were matched in a 2:1 ratio with 18 patients treated with PCA alone; however, in contrast to prior reports, the authors reported no significant differences between the groups with regards to number of probes used, technical success, complication rates, changes in renal function, or changes in hematocrit levels. One limitation of this study is that patients with both T1a and T1b RCC were included. The inclusion of T1a RCC may have reduced the impact of TAE. As such, the current report was limited to patients with T1b or T2 RCC. Finally, a recent single-arm prospective study sought to evaluate the safety and efficacy of TAE + RCC in patients with tumors ≥ 3 cm in size [23]. 19 patients were enrolled over approximately 3 years with a mean tumor diameter of 39 mm. No major AEs were identified from either procedure while two patients (10.5%) developed local recurrence during the follow-up period. Unfortunately, the study provided no control group of patients who underwent PCA without TAE, thus limiting direct comparisons of the efficacy of TAE in these patients.
There are several limitations of the current study. First, because of its retrospective design, operator bias could not be controlled for and likely contributed to the significantly larger tumor diameter and increased nephrometry scores of the combined TAE + PCA group. Likewise, the operator’s decision-making process about when to treat with TAE + PCA or PCA alone could not be controlled for and was not always readily available in clinic notes. Second, sampling bias may limit generalizability of these results as they were obtained from a single tertiary academic medical center. Third, while the lack of differences between oncologic outcomes and AEs between the groups may suggest that TAE has clinical utility, it is important to remember that the lack of evidence for worse outcomes is not in of itself evidence that TAE improves clinical outcomes. Fourth, the type of embolic agent and timing of TAE prior to PCA in each case was at the discretion of the operator. It is possible that different embolic agents may have different outcomes in RCC; although, this has never been specifically studied. Additionally, whether the timing of the two procedures has an effect on technical or clinical outcomes is also unknown. Fifth, there is pathologic confirmation of RCC in only 54% of patients in the study. Finally, the current investigation is limited to PCA. It is unknown whether the findings can be generally applied to other ablation modalities, such as radiofrequency ablation, microwave ablation, or irreversible electroporation.
In conclusion, TAE + PCA in non-surgical patients with T1b or T2 RCC is technically feasible without significant added detriment to renal function. The combined approach may help to reduce hemorrhagic AEs, which are relatively common in this patient population [3]; although, larger patient cohorts are needed to provide statistical validity to this observation. Even though patients in the TAE + PCA cohort had larger and more complex tumors, their technical and clinical outcomes were no different than the PCA alone group, suggesting some benefit to adjunctive TAE. Future prospective studies utilizing matched control groups of patients are needed to identify the exact patient population that would most benefit from the combined approach.
References
[1] Campbell SC, Clark PE, Chang SS, Karam JA, Souter L, Uzzo RG. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J Urol. 2021;206(2):199-208.
[2] Motzer RJ, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(1):71-90.
[3] Gunn AJ, Parikh NS, Bhatia S. Society of Interventional Radiology Quality Improvement Standards on Percutaneous Ablation in Renal Cell Carcinoma. J Vasc Interv Radiol. 2020;31(2):195-201.e3.
[4] Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
[5] Abou Elkassem AM, Lo SS, Gunn AJ, Shuch BM, Dewitt-Foy ME, Abouassaly R, et al. Role of Imaging in Renal Cell Carcinoma: A Multidisciplinary Perspective. Radiographics. 2021;41(5):1387-407.
[6] Winokur RS, Pua BB, Madoff DC. Role of combined embolization and ablation in management of renal masses. Semin Intervent Radiol. 2014;31(1):82-5.
[7] Hall WH, McGahan JP, Link DP, deVere White RW. Combined embolization and percutaneous radiofrequency ablation of a solid renal tumor. AJR Am J Roentgenol. 2000;174(6):1592-4.
[8] Gebauer B, Werk M, Lopez-Hänninen E, Felix R, Althaus P. Radiofrequency ablation in combination with embolization in metachronous recurrent renal cancer in solitary kidney after contralateral tumor nephrectomy. Cardiovasc Intervent Radiol. 2007;30(4):644-9.
[9] Tacke J, Mahnken A, Becker A, Rohde D, Gunther RW. Nephron-sparing percutaneous ablation of a 5 cm renal cell carcinoma by superselective embolization and percutaneous RF-ablation. Rofo. 2001;173(11):980-3.
[10] Arima K, Yamakado K, Kinbara H, Nakatsuka A, Takeda K, Sugimura Y. Percutaneous radiofrequency ablation with transarterial embolization is useful for treatment of stage 1 renal cell carcinoma with surgical risk: results at 2-year mean follow up. Int J Urol. 2007;14(7):585-90.
[11] Mondshine RT, Owens S, Mondschein JI, Cizman B, Stavropoulos SW, Clark TW. Combination embolization and radiofrequency ablation therapy for renal cell carcinoma in the setting of coexisting arterial disease. J Vasc Interv Radiol. 2008;19(4):616-20.
[12] Nakasone Y, Kawanaka K, Ikeda O, Tamura Y, Yamashita Y. Sequential combination treatment (arterial embolization and percutaneous radiofrequency ablation) of inoperable renal cell carcinoma: single-center pilot study. Acta Radiol. 2012;53(4):410-4.
[13] Yamakado K, Nakatsuka A, Kobayashi S, Akeboshi M, Takaki H, Kariya Z, et al. Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc Intervent Radiol. 2006;29(3):389-94.
[14] Woodrum DA, Atwell TD, Farrell MA, Andrews JC, Charboneau JW, Callstrom MR. Role of intraarterial embolization before cryoablation of large renal tumors: a pilot study. J Vasc Interv Radiol. 2010;21(6):930-6.
[15] Gunn AJ, Mullenbach BJ, Poundstone MM, Gordetsky JB, Underwood ES, Raid-Bahrami S. Trans-arterial embolization of renal cell carcinoma as an adjunctive therapy prior to cryoablation: a propsensity score matching analysis. Diagn Interv Radiol. 2018;24(6):357-63.
[16] Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844-53.
[17] Chehab MA, Thakor AS, Tulin-Silver S, et al. Adult and pediatric antibiotic prophylaxis during vascular and IR procedures: a Society of Interventional Radiology Practice Parameter update endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J Vasc Interv Radiol. 2018;29(11):1483-1501.
[18] Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions - Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-84.
[19] Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273(1):241-60.
[20] Khalilzadeh O, Baerlocher MO, Shyn PB, Connolly BL, Devane AM, Morris CS, et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2017;28(10):1432-7.
[21] Maxwell AWP, Baird GL, Iannuccilli JD, Mayo-Smith WW, Dupuy DE. Renal Cell Carcinoma: Comparison of RENAL Nephrometry and PADUA Scores with Maximum Tumor Diameter for Prediction of Local Recurrence after Thermal Ablation. Radiology. 2017;283(2):590-7.
[22] Schmit GD, Schenck LA, Thompson RH, Boorjian SA, Kurup AN, Weisbrod AJ, et al. Predicting Renal Cryoablation Complications: New Risk Score Based on Tumor Size and Location and Patient History. Radiology. 2014;272(3):903-10.
[23] Gobara H, Matsui Y, Uka M, Tomita K, Umakoshi N, Araki M, et al. Percutaneous cryoablation combined with prior transcatheter arterial embolization for renal cell carcinomas of 3 cm or larger: a prospective study. Int J Clin Oncol. 2022.
Acknowledgements
Findings from this investigation were presented as an oral scientific abstract at the Society of Interventional Radiology Annual Scientific Meeting in June 2022. The preliminary abstract can be found in a June 2022 supplementary issue of the Journal of Vascular of Interventional Radiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Huang is a consultant for Varian Medical Systems; Dr. Caridi is a consultant and speaker for Varian Medical Systems and Boston Scientific Corporation; Dr. Gunn is a consultant and speaker for Varian Medical Systems and Boston Scientific Corporation and receives grant funding from Penumbra, Inc and Varian Medical Systems.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salei, A., Raymond, M., Savage, C. et al. Transarterial embolization of T1b and T2a renal cell carcinoma prior to percutaneous cryoablation: a retrospective comparative study. Abdom Radiol 48, 773–779 (2023). https://doi.org/10.1007/s00261-022-03755-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03755-3