Abstract
Epoprostenol is a prostacyclin (prostaglandin I2) analog that causes vasodilation and inhibits platelet aggregation and is used in the management of severe pulmonary arterial hypertension (PAH). We herein report a patient with PAH who developed pancreatic enlargement after the initiation of therapy including epoprostenol. Although it is well known that thyroid enlargement occurs in patients with PAH receiving epoprostenol therapy, the pancreatic findings associated with epoprostenol therapy have not been well described. Although the size of the pancreas was clearly increased, there was no blood data or symptoms suggestive of abnormal pancreatic function and pancreatitis, and the patient’s abdominal complaints improved quickly, despite the continuation of epoprostenol therapy. Eleven months after the start of continuous intravenous epoprostenol infusion therapy, the pancreatic enlargement was still evident on imaging, but there were no abdominal symptoms or elevated pancreatic enzymes. This case highlights the fact that epoprostenol therapy may cause pancreatic changes that mimic acute or autoimmune pancreatitis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary arterial hypertension (PAH) is a rare but potentially lethal disease that causes severe pulmonary hypertension due to progressive narrowing of the small pulmonary arteries [1]. This peripheral pulmonary artery stenosis results from the proliferation of endothelial cells and hyperplasia of pulmonary artery smooth muscle cells of the vascular wall [2], giving this condition the potential to respond to vasodilators. Single-agent or combination therapy with pulmonary vasodilators that modulate the three mechanistic pathways (nitric oxide, endothelin, and prostacyclin) is expected to improve the symptoms, exercise tolerance, and survival of patients with PAH [3, 4]. For high-risk patients with PAH, the recommended treatment is an intravenous infusion of a prostacyclin (prostaglandin I2; PGI2) analog (epoprostenol) [5] that acts as a powerful vasodilator and platelet aggregation inhibitor.
As extrapulmonary imaging findings associated with intravenous epoprostenol infusion, diffuse enlargement of the thyroid gland is well known; however, the specific findings in other organs, including other gland tissues, have not been well described. It is generally reported that intravenous epoprostenol therapy can be a trigger for thyroid dysfunctions [6,7,8]. As hyperthyroidism is an aggravating factor in PAH, the thyroid function should be screened during epoprostenol treatment. In addition, patients with PAH may experience several nonspecific abdominal symptoms such as abdominal distension, nausea, and diarrhea; these symptoms are not clinically problematic in most cases, and abdominal imaging examinations are rarely performed. It is difficult to identify the cause of such abdominal symptoms because these symptoms can be caused by either right heart failure due to PAH or adverse treatment effects.
We herein report a patient with PAH who developed pancreatic enlargement after the initiation of combination-agent therapy with vasodilators including intravenous epoprostenol infusion treatment.
Case report
A previously healthy 27-year-old woman developed dyspnea on exertion that worsened after childbirth and was referred to our institution for suspected pulmonary hypertension. Cardiologists confirmed the diagnosis of idiopathic PAH. Right heart catheterization findings indicated severe PAH, as the mean pulmonary arterial pressure (mPAP) was 102 mmHg (PAH is defined as an mPAP of ≥ 25 mmHg at rest [4]) and the cardiac index was 2.16 L/min m2. A tunneled central line (Hickman®) catheter was implanted and intravenous epoprostenol was initiated at a dose of 0.33 ng/kg min. The patient responded quickly to epoprostenol and the dosage was gradually increased. When the target dose of 81 ng/kg min was achieved at 6 months after the initiation of epoprostenol treatment, her mPAP had decreased to 45 mmHg. After the initiation of epoprostenol infusion, an endothelin receptor antagonist (macitentan, 10 mg once daily) was started 1 day later, and a soluble guanylate cyclase stimulator (riociguat, 2.5 mg three times daily) was started 7 days later.
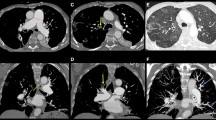
Eight months after the initiation of continuous intravenous epoprostenol, the patient developed abdominal distension and diarrhea. Follow-up chest CT including the upper abdomen incidentally revealed that the pancreas was enlarged compared with pretreatment CT images (Fig. 1). At that time, laboratory data showed no pancreatic enzyme abnormalities (serum amylase level 129 U/L, normal range 45–140 U/L; serum lipase was not measured), no elevation in IgG (IgG concentration 716 mg/dL, normal range 826–1840 mg/dL; IgG4 was not measured), and no evidence of diabetes mellitus. Plain CT performed 8 months later revealed that the size of the pancreas had clearly increased. Peripancreatic fluid and increased peripancreatic fat density were not detected. Abdominal MRI performed 1 month later (9 months after the start of epoprostenol treatment) revealed that the pancreas was diffusely enlarged and lacked lace-like contours. However, in contrast to the imaging findings of acute pancreatitis, there was no blurring of the pancreatic boundaries. The signal intensity of the pancreatic parenchyma was slightly hypointense on T1-weighted images (both in-phase and opposed-phase three-dimensional gradient echo Dixon sequence images) and slightly hyperintense on T2-weighted half-Fourier acquisition snapshot turbo spin-echo images (HASTE) (Fig. 2a, d–f). Because MRI examination was performed mainly on the liver to confirm the presence of a congested liver, (fat-suppressed) T1-weighted image was not obtained. Unlike in typical autoimmune pancreatitis, the T2-weighted HASTE and magnetic resonance cholangiopancreatography (MRCP) revealed that the main pancreatic duct had a normal uniform diameter without irregularities (Fig. 2a, g). The enlarged homogeneous pancreatic parenchyma and thin smooth pancreatic duct seemed incongruous. Diffusion-weighted imaging revealed diffuse hyperintensity throughout the whole pancreas, and the apparent diffusion coefficient (ADC) value was 1.23 × 10–3 mm2/s (average of three circular regions of interest as shown in Fig. 2b and c).
Magnetic resonance imaging during epoprostenol treatment. a T2-weighted (half-Fourier acquisition snapshot turbo spin-echo; HASTE) images show a slightly hyperintense signal of the pancreatic parenchyma. b Diffusion-weighted imaging (b = 1000) reveals mild hyperintensity in the whole pancreas, with c an apparent diffusion coefficient value of 1.23 × 10–3 mm2/s (average of three circular regions of interest). The signal intensity of the pancreatic parenchyma is slightly hypointense on T1-weighted d in-phase, e opposed-phase, and f water images using the three-dimensional gradient echo Dixon sequence. The main pancreatic duct has a normal uniform diameter without irregularities on a T2-weighted HASTE images and g magnetic resonance cholangiopancreatography
A review of the seven CT examinations the patient had undergone since starting epoprostenol treatment revealed that the size of the pancreas had already increased at 7 days after the initiation of epoprostenol compared with pretreatment imaging (Fig. 1). For each CT scan, we measured the pancreatic tail area in a free-hand region of interest drawn around the margins of the pancreatic tail on the single axial slice that included the largest area of the pancreatic tail. Because these CT images were obtained to assess the lung parenchyma and pulmonary arteries, the whole pancreas was not scanned in most cases. Hence, we decided to measure the area of only the tail of the pancreas, defined as the area between the left border of the aorta and hilum of the spleen. Figure 3 shows the changes in the pancreatic size since the day of the initiation of epoprostenol treatment (defined as day 0).
Left: Timeline of the change in pancreatic size in accordance with the administered dose of epoprostenol. Right: CT image showing the method used to measure the pancreatic tail area. The region of interest around the pancreatic tail is drawn in a free-hand manner on a single axial slice. The tail of the pancreas is defined as the area between the left border of the aorta and the hilum of the spleen
Incidentally, CT of the neck demonstrated a diffusely enlarged thyroid gland (Fig. 4); however, the thyroid function test results were within normal limits, with a TSH concentration of 0.736 µIU/mL (normal range 0.5–5.0 µIU/mL), free T4 concentration of 1.360 ng/dL (normal range 0.88–1.62 ng/dL), and free T3 concentration of 2.75 pg/mL (normal range 2.33–4.00 pg/mL).
The patients’ abdominal symptoms rapidly improved. Eleven months after the start of treatment, right heart catheterization showed that the PAH had improved (mPAP 39 mmHg, cardiac index 5.69 L/min m2). Continuous intravenous epoprostenol infusion and the other two medications (macitentan and riociguat) have been continued to date. The pancreatic enlargement is still evident on imaging, but the patient has no abdominal symptoms or elevated pancreatic enzymes.
Discussion
We reported a patient receiving combination-agent treatment for PAH including continuous intravenous epoprostenol infusion developed the pancreatic enlargement, without clinical symptoms suggestive of pancreatic dysfunction and acute pancreatitis. The size of the pancreas varies widely among healthy individuals in accordance with age and body size. After increasing relatively rapidly in childhood, the total pancreas volume shows minimal change from 20 to 60 years of age and then declines thereafter [9]. The presence of a large pancreas is often of unclear pathological significance because of the difficulty in distinguishing individual differences. However, a large pancreas must be investigated when there is active inflammation (such as in acute pancreatitis or autoimmune pancreatitis [AIP]) or diffuse tumor cell infiltration. In the present case, we excluded pancreatic infiltration with tumor cells based on the time course of the changes in pancreatic size, as the pancreas rapidly enlarged within 7 days, continued to swell, and then remained a similar size for nearly 200 days (Fig. 3). In addition, the condition of active inflammation was not consistent with the clinical information in the present case; however, other than acute pancreatitis or AIP, a relatively rapid increase in the size of the pancreas is not often experienced and these imaging findings can bother the radiologist. Therefore, based on the time course, clinical data, and blood data of this case, it is highly likely that the pancreatic enlargement is a secondary change due to medication treatment such as epoprostenol. It is hard to conclude that epoprostenol is the sole cause of these pancreatic findings in the case presented here because the other two medications were used. However, we assumed that epoprostenol is the most suspicious for the following reasons: first, as shown in Fig. 3, changes in pancreas size showed some similarities with changes in the dose of epoprostenol, while the doses of the other two drugs have not changed; second, diffuse pancreatic enlargement suspected to be AIP in PAH patient receiving epoprostenol therapy have been previously reported by Shirai et al. [10], but to the best of our knowledge, no abnormal pancreatic findings other than acute pancreatitis have been reported with the other two medications. Since the possibility of acute pancreatitis was denied in our case, drug-induces acute pancreatitis was not listed in the differential diagnosis; third, at least in the thyroid gland, increase in the size of the organ was well known [6,7,8]. This is an effect not seen in other oral medications. Further investigation is needed in a larger number of cases to discuss whether epoprostenol is the sole cause of the pancreatic enlargement.
Epoprostenol is a synthetic PGI2 analog that acts as a vasodilator of stenotic pulmonary arteries. PGI2 activates adenylate cyclase and causes increases in cyclic adenosine monophosphate (cAMP) levels, leading to vascular smooth muscle relaxation [11]. The thyroid gland is a well-known target for other organs that show increased organ size in patients with PAH receiving epoprostenol infusion. It has been hypothesized that diffuse thyroid hyperplasia is caused by thyroid hormone production stimulated by the cAMP produced by PGI2-stimulated receptors (IP) in the follicular epithelium [6,7,8]. In the pancreas, PGI2 receptors are reported to be expressed in the smooth muscle of small arteries, but their accurate distribution within the pancreas remains unknown. Previous studies have reported non-inflammatory and non-tumor-related pancreatic enlargement in rats fed raw soybean meal containing trypsin inhibitor [12] and rats receiving long-term bethanechol, a muscarinic acetylcholine receptor agonist [13]. In both cases, the acinar cells increased in size and the number of zymogen granules increased and these results suggest that an increase in the volume of the exocrine part increases the overall size of the pancreas. PGI2 dose-dependent dilatation of the pancreatic vasculature and an increase in blood flow to the pancreas has been reported in a dog model [14]. Because the pancreas has an abundant capillary network consisting of interlobular vessels [15], increased blood flow (and edema) may cause pancreatic enlargement. However, PGI2 analogs can also act on other prostanoid receptors because of receptor homology, which complicates the assessment of the effect of PGI2 on the pancreas.
A mild form of acute edematous pancreatitis can be the differential diagnosis based on the CT findings and is histologically characterized by interlobular interstitial edema and inflammatory infiltration in the early stages [16]. An increase in vascular permeability and the accumulation of fluid in the perilobular space results in an increased signal intensity on T2-weighted images [17], reflecting the degree of edema. In our case, the signal intensity of the pancreatic parenchyma was slightly hypointense on T1-weighted images and slightly hyperintense on T2-weighted images, consistent with edematous pancreatic parenchyma. The absence of elevated serum amylase during epoprostenol treatment suggests that the pancreatic digestive enzymes are not activated, unlike in acute pancreatitis. However, considering that interlobular edema and dehiscence during early acute pancreatitis can contribute to an increase in pancreatic size, we hypothesized that the increase in pancreatic blood flow caused by prostacyclin administration (possibly in conjunction with mild congestion due to arterial dominance dilation) might cause interlobular edema, resulting in pancreatic enlargement. In addition, the pancreas does not have a well-developed fibrous capsule and is surrounded by thin loose connective tissue, which may cause a more obvious change in the size of the organ due to edema. This may explain why epoprostenol treatment does not cause an increase in the size of the liver or spleen, which have tight fibrous capsules. AIP is characterized by dense lymphoplasmacytic inflammation and storiform fibrosis, resulting in a macroscopically enlarged pancreas with a sausage-like appearance and pancreatic duct narrowing with duct irregularity on imaging [18, 19]. In our case, although the presence of pancreatic swelling with a diffuse hypointense signal on T1-weighted images was consistent with AIP, there were no main pancreatic duct irregularities. In addition, although AIP is associated with a significantly lower ADC value (1.01 ± 0.11 × 10–3 mm2/s) than the ADC values in pancreatic cancer (1.25 ± 0.11 × 10–3 mm2/s) and the normal pancreas (1.49 ± 0.16 × 10−3 mm2/s) [20], the ADC value in this case (1.23 × 10−3 mm2/s) is not as low as that seen in AIP. We considered that this ADC value suggested that there was no marked infiltration of lymphocytes or inflammatory cells. Shirai et al. have reported the potential link between PAH and IgG4 related disease (diagnosed from the serum IgG4 level or histopathological examination of the lacrimal gland) [10]. They noted that five of 75 PAH patients were complicated by IgG4 related disease with symmetrical lacrimal and salivary gland enlargement, revealing that PAH patients were at higher risk for developing IgG4 related disease. Interestingly, two of these five patients had pancreatic enlargement on plain CT and were diagnosed as AIP (no pathology proven). We believe that our case is different from AIP, based on the time course, clinical data and blood data. CT and MRI findings were also different from those of typical AIP, such as findings of pancreatic duct without ductal irregularity and ADC values. Further studies and accumulation of cases are needed to exclude the possibility of AIP. On the contrary, if the combination between PAH and IgG4 related disease is considered to have a higher prevalence, the patients diagnosed as AIP could increase based on imaging findings.
In conclusion, this report describes pancreatic enlargement in a patient receiving continuous intravenous epoprostenol infusion therapy for PAH. This pancreatic enlargement could be mistaken for acute pancreatitis or autoimmune pancreatitis, which were not consistent with the clinical presentation. The accumulation of such cases and further studies are warranted to clarify the mechanism of pancreatic enlargement and the association with pancreatic function over time.
References
McLaughlin VV, McGoon MD (2006) Pulmonary arterial hypertension. Circulation 114:1417–1431
Prins KW, Thenappan T (2016) World health organization group I pulmonary hypertension: Epidemiology and pathophysiology. Cardiol Clin 34:363–374
Galiè N, Humbert M, Vachiery J-L, et al (2015) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 46:903–975
Fukuda K, Date H, Doi S, et al (2019) Guidelines for the Treatment of Pulmonary Hypertension (JCS 2017/JPCPHS 2017). Circ J 83:842–945
Barst RJ, Rubin LJ, Long WA, et al (1996) A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 334:296–301
Chadha C, Pritzker M, Mariash CN (2009) Effect of epoprostenol on the thyroid gland: enlargement and secretion of thyroid hormone. Endocr Pract 15:116–121
Satoh M, Aso K, Nakayama T, Saji T (2017) Effect of treatment with epoprostenol and endothelin receptor antagonists on the development of thyrotoxicosis in patients with pulmonary arterial hypertension. Endocr J 64:1173–1180
Menon AA, Sahay S, Braverman LE, Farber HW (2019) Thyroid Dysfunction in Patients with Pulmonary Artery Hypertension (PAH): The Effect of Therapies Affecting the Prostanoid Pathway. Lung 197:761–768
Saisho Y, Butler AE, Meier JJ, et al (2007) Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat 20:933–942
Shirai Y, Tamura Y, Yasuoka H, et al (2014) IgG4-related disease in pulmonary arterial hypertension on long-term epoprostenol treatment. Eur Respir J 43:1516–1519
Majed BH, Khalil RA (2012) Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev 64:540–582
Beswick IP, Pirola RC, Bouchier IA (1971) The cause of pancreatic enlargement in rats fed raw soybean. Br J Exp Pathol 52:252–255
Kato M, Ohkuma S, Kataoka K, et al (1994) Mechanisms for pancreatic hypertrophy induced by long-term administration of bethanechol. Eur J Pharmacol (Environ Toxicol Pharmacol Sect) 292:47–55
Homma T, Malik KU (1982) Effect of prostaglandins on pancreatic circulation in anesthetized dogs. J Pharmacol Exp Ther 222:623–628
Dolenšek J, Pohorec V, Rupnik MS, Stožer A (2017) Pancreas Physiology. In: Seicean A (ed) Challenges in Pancreatic Pathology. IntechOpen, Rijeka
Glasbrenner B, Adler G (1993) Pathophysiology of acute pancreatitis. Hepatogastroenterology 40:517–521
Zhang X-M, Feng Z-S, Zhao Q-H, et al (2006) Acute interstitial edematous pancreatitis: Findings on non-enhanced MR imaging. World J Gastroenterol 12:5859–5865
Notohara Kenji ZL (2013) Histology of autoimmune pancreatitis. The Pancreapedia: Exocrine Pancreas Knowledge Base
Takahashi M, Fujinaga Y, Notohara K, et al (2020) Diagnostic imaging guide for autoimmune pancreatitis. Jpn J Radiol 38:591–612
Kamisawa T, Takuma K, Anjiki H, et al (2010) Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol 105:1870–1875
Acknowledgements
We thank Kelly Zammit, BVSc, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was waived in this case report in accordance with the opt-out method used at our institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Someya, Y., Koyasu, S., Ohnishi, Y. et al. Pancreatic enlargement in a patient receiving therapy with vasodilators for pulmonary arterial hypertension: a case report. Abdom Radiol 47, 1948–1953 (2022). https://doi.org/10.1007/s00261-022-03458-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03458-9