Abstract
The purpose of this article is to review the current molecular classification of endometrial cancer, the imaging findings in early and advanced disease, and the current management strategies, focusing on the new systemic therapies for advanced EC. In recent years, the management of endometrial cancer has significantly changed. The molecular characterization of endometrial cancer has shed new light into the biologic behavior of this disease, the International Federation of Gynecology and Obstetrics staging system was recently revised, and imaging was formally incorporated in the management of endometrial cancer. Recent genomic analysis of endometrial cancer led to the approval of new molecular-targeted therapies and immune checkpoint inhibitors. Imaging allows assessment of myometrial invasion, cervical stromal extension, lymph node involvement and distant metastases, and has a crucial role for treatment planning. Treatment strategies, which include surgery, radiation and systemic therapies are based on accurate staging and risk stratification.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial Cancer (EC) is the most common cancer of the female reproductive organs in the United States [1, 2]. The incidence has been rising in recent years, due to various factors, including increased obesity and increasing age of the population [3]. The age-adjusted death rate for uterine cancer has also increased in the past years, shifting from 4.08 for 100.000 women in 1998 to 5.10 for 100.000 women in 2018 [4]. There are substantial racial disparities in EC: The average death rate per 100.000 from 2012 to 2016 was 8.8 for black women and 4.5 for white women [1]. These disproportions have been accredited, among multiple causes, to an increased incidence of tumors with advanced-stage and aggressive histology along with decreased use of surgery for black women [5].
Pathogenesis and clinical behavior of EC is heterogeneous. Based on clinical and histological variables, two main types of EC have been described: type I tumors, which are frequently associated with hyperestrogenism, mostly show well-differentiated endometrioid histology, and express moderate to high levels of estrogen receptor; type II tumors, which are not associated with metabolic or endocrine disorders and often arise in nonobese women, are poorly differentiated, most commonly of serous or clear cell histology. Although the vast majority of EC diagnosed are type I and early stage, which has a favorable prognosis, with 86% overall survival rate, type II EC often presents with advanced disease, has an 80–90% recurrence rate at three years and carries a poor prognosis, with 56% overall survival rate [5,6,7,8,9].
Traditional pathological reporting of EC has limitations due to poor reproducibility of tumor typing and to accurately identify patients at risk for recurrence or metastatic disease. The identification of the underlying molecular background of EC has resulted in the development of new molecular-based classifications of EC and in the design of new clinical trials for new systemic therapies, which include molecular-targeted therapies and immune checkpoint inhibitors for the various molecular subtypes [10, 11].
The International Federation of Gynecology and Obstetrics (FIGO) staging system is the generally accepted method for EC staging. An accurate determination of cancer extent is important for appropriate application of treatment [12]. The initial imaging workup depends on whether the patient will follow nonfertility-sparing or fertility-sparing treatment. When available, MRI is the study of choice for the anatomical study of the pelvis since it can accurately depict the depth of myometrial invasion and cervical stromal invasion [13]. Imaging is also crucial for adjuvant treatment planning, detection of post-operative residual disease in high-risk patients, and post-treatment surveillance of asymptomatic patients with high risk of recurrence [14].
The ability to reduce mortality of EC ultimately depends on adequate diagnosis and management of advanced and recurrent disease [7]. The management of EC should be in a multidisciplinary setting, where clinical, histologic and imaging findings are discussed, allowing for individualized treatment [12]. Radiologists, as part of the multidisciplinary team, should be knowledgeable about the recent advances in classification, diagnosis and management of EC. In this article, we will review the basis for the molecular classification, the imaging findings in early and advanced disease, and the current management strategies, focusing on the new systemic therapies for advanced EC, and ultimately, we will present the most recent advances in radiomics of EC.
Molecular background of EC
In recent years, various molecular-based classifications of EC have been proposed [15, 16]. Most notably, The Cancer Genome Atlas (TCGA) stratifies EC into four distinct prognostic groups: polymerase ε (POLE) ultramutated, microsatellite instability (MSI) hypermutated, copy-number low, and copy-number high [17, 18].
POLE-mutated/ultramutated
EC with missense mutations in the POLE proofreading domains are characterized as POLE ultramutated. Approximately 7% of EC present this type of mutation. DNA polymerases have a proofreading domain responsible for DNA replication fidelity. Loss-of-function mutations in POLE proofreading domains increase the number of DNA replication errors and the incidence of neoplasms [19, 20]. These EC are typically high-grade, with prominent lymphocytic infiltration, and generally difficult to classify reliably based on histology alone. In TCGA study, these tumors were found to have a more favorable progression-free survival as compared with the other three groups of ECs [20, 21]. The excellent survival outcome in POLE subtypes is poorly understood: it has been postulated that the large number of mutations renders these cancers highly immunogenic for the host, due to the high number of neoantigens binding to the histocompatibility complexes, which ultimately induces an enhanced anti-tumor T-cell response [20, 21].
Microsatellite instability
The MSI group accounts for approximately 30% of EC and comprises defects in post-replicative DNA mismatch repair (MMR) system, resulting in genomic instability by facilitating the accumulation of somatic mutations [22]. Most of these tumors are sporadic, arising as a result of epigenetic silencing of MLH1 by promoter methylation. However, a minority is caused by germline mutations in the MMR genes, resulting in Lynch syndrome, an autosomal-dominant syndrome associated with a markedly increased risk of colorectal carcinoma and EC [23]. Like POLE-mutated EC, these tumors typically display a prominent lymphocytic infiltration, which in conjunction of high mutation load, make them potential candidates for immune checkpoint inhibitors therapy [24, 25].
Copy-number low
Copy-number low tumors comprise the majority of EC, have low mutation frequency, are microsatellite stable and include low-grade endometrioid carcinomas. The progression-free survival of this group was lower than the POLE-mutated/ultramutated group in TCGA. Multiple mutated genes were identified, including PTEN, PIK3CA, CTNNB1 [16]. Somatic alteration of the PI3K pathway was present in 92% of copy-number low cases in TCGA study. The frequent activation of the PI3K–PTEN–AKT–mTOR pathway makes it an attractive therapeutic target, with many clinical trials evaluating the efficacy of mTOR inhibitors, PI3K inhibitors, AKT inhibitors, and dual PI3K–mTOR inhibitors [15].
The RAS/beta catenin pathway is also frequently mutated in this group, and within this pathway CTNNB1 mutations are frequent [26, 27]. Some studies have identified associations between CTNNB1 mutations and poor outcome in low-risk endometrioid carcinomas, suggesting the possibility of a prognostic significance for the CTNNB1 mutation [15].
Copy-number high (serous-like)
This group consists primarily of serous-like tumors with extensive somatic copy-number alterations and a low mutation rate. Approximately 25% of the grade 3 endometrioid cancers were in this category. This group exhibits TP53 mutations and amplifications in oncogenes MYC and HER2, which are all involved in cell-cycle regulation [27]. The presence of amplifications of HER2 suggests a potential role for human epidermal growth factor receptor 2 (HER2)-targeted therapy [5].
Staging of EC-role of imaging
EC is commonly staged through the FIGO staging system (Fig. 1). Surgical staging is the standard of care, and consists of total hysterectomy, bilateral salpingo-oophorectomy, peritoneal washing, and lymph node assessment. Preoperative imaging is crucial because it can optimize the treatment plan by allowing differentiation between early and advanced stage. Imaging is also useful to identify recurrence and assess local treatment response [28, 29].
Illustrated FIGO staging system for endometrial cancer. a Stage 1 disease is subdivided according to depth of myometrial invasion. b Stage II disease is confined to the uterus. c Stage III disease depicted by extrauterine disease. d Stage IVA disease with bladder or rectum involvement and distant organ spread of Stage IVB disease
In patients undergoing nonfertility-sparing treatment, the National Comprehensive Cancer Network (NCCN) guidelines for EC imaging consider pelvic MRI to assess local disease extent and pelvic transvaginal ultrasound (TVUS) to assess uterine size if not clear on clinical exam [30,31,32]. For patients undergoing fertility-sparing treatment, pelvic MRI is preferred to preoperatively stage and determine local disease extent to exclude myometrial invasion. In addition, chest imaging, either chest x-ray or chest CT should be considered at diagnosis [32]. Based upon symptoms or physical exam findings, a metastatic workup including abdominal/pelvic and/or chest CT and/or whole-body PET/CT, may be indicated [30,31,32].
Imaging of early-stage disease
Gynecological examination is usually completed by TVUS, which is performed with a high frequency probe and a small field of view, allowing good local evaluation. Several authors indicate an endometrial thickness of < 5 mm as the normal cut-off value in post-menopausal women in the presence of bleeding [33]. In post-menopausal women without bleeding, the role of TVUS is less clear, but an endometrial thickness of < 5 mm often does not need further evaluation, given that an endometrial thickness of < 5 mm has greater than 99% negative predictive value for EC [34].
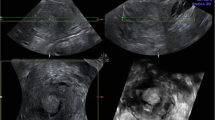
At TVUS, myometrial invasion appears as an iso- or hyperechoic tissue compared to the surrounding myometrium. An intact subendometrial halo (inner layer of myometrium) usually indicates no more than superficial invasion, whereas obliteration of the halo indicates deep invasion (Fig. 2). The cervix should be examined in a sagittal plane for tumor invasion into the cervical stroma [35]. The distance from the outer cervical os to the lower tumor margin is the only parameter that might have the potential to predict cervical invasion [14, 35].
66-year-old woman with post-menopausal bleeding. TVUS transverse image showing an endometrial mass with obliteration of subendometrial halo (arrows), reflecting myometrial invasion. Histopathology analysis confirmed the presence of a 5 cm endometrial adenocarcinoma invading 20 mm (> 50%) into the myometrium
On MRI, EC usually appears of intermediate signal intensity on T2-weighted images (T2WI). The subjacent myometrium is composed of an inner layer or “junctional zone”, which demonstrates hypointense signal on T2WI, while the outer layer is more variable in signal but usually shows intermediate signal (Fig. 3). MRI is the modality of choice to evaluate for the presence of myometrial invasion of EC with reported accuracy ranging from 83 to 92% [29]. T2-weighted MRI is the fundamental sequence for evaluating myometrial invasion, allowing the distinction between the intermediate signal intensity tumor against the hypointense junctional zone [29, 36]. On diffusion-weighted imaging (DWI), EC exhibits restricted diffusion compared with the normal myometrium and endometrial tissue [29, 37].
On contrast-enhanced sequences, the inner myometrial layer enhances uniformly during the early dynamic phase. Disruption of this layer or breach of the junctional zone is indicative of myometrial invasion. Therefore, an intact junctional zone with continuous subendometrial enhancement can exclude deep myometrial invasion with a diagnostic accuracy ranging from 83 to 96% [38, 39].
The incidence of nodal metastasis in patients with less than 50% and greater than 50% myometrial invasion is reported to be 5% and 18%, respectively. Depth of myometrial invasion also correlates with higher tissue grades, higher risk of recurrence, and decreased survival rates [40].
Tumors confined to the uterus are classified as stage I or II. Stage IA disease has absent or less than 50% of myometrial invasion (Fig. 4). Stage IB has more than 50% myometrial invasion (Fig. 5). When the tumor invades the cervical stroma but does not extend beyond the uterus, corresponds to a stage II (Fig. 6) [29]. On T2WI, the cervical mucosal invasion is visualized as a widened endocervical canal with preservation of the T2-hypointense cervical stroma. When there is stromal invasion, the lesion with higher signal intensity disrupts the T2-hypointense cervical stroma. The presence of cervical invasion increases the risk of recurrence and worsens outcome [40].
Imaging of advanced-stage disease
Tumors beyond stage II are no longer confined to the uterus and there is local and/or regional spread of the tumor. In stage III disease, the tumor extends beyond the uterus. Stage IIIA tumors invade the uterine serosa or the adnexa. There is disruption of the serosal hypointense signal in T2WI. Stage IIIB tumors invade the vagina or the parametrium (Fig. 7). Parametrial involvement appears as disruption of the cervical serosa with direct extension into the surrounding parametrial fat. It is demonstrated as disruption of the normally smooth outer contour of the cervix on T2WI and a change in the parametrial fat signal intensity on T1-weighted images (T1WI). Invasion of the vaginal wall may be identified as a hyperintense thickened lower vaginal wall on T2WI [33]. Stage IIIC disease (Fig. 8) is characterized by the presence of lymphadenopathy and is subdivided in either pelvic (stage IIIC1) and/or para-aortic (stage IIIC2) lymph node involvement [29, 41].
68-year-old woman with uterine serous carcinoma. FIGO stage IIIB: Invasion of parametrium and/or vagina. Pelvic MRI shows a an endometrial mass extending to the level of the cervix (arrow), where it disrupts the cervical stroma and b left lateral wall of the cervix (arrow), in keeping with parametrial extension
44-year-old woman with progressive endometrioid adenocarcinoma of the endometrium. FIGO stage IIIC: Invasion of pelvic (IIIC1) and/or para-aortic (IIIC2) lymph nodes. Postsurgical CT scan shows new para-aortic and pelvic adenopathy (arrows). Next generation sequencing demonstrated MSI-H status. Radiotherapy and pembrolizumab were added
TVUS can be used to diagnose metastatic disease to the ovaries with a sensitivity and specificity of subjective evaluation of greyscale and Doppler ultrasound findings of 84–91% and 94–100%, respectively [42]. However, TVUS has limitations inherent to the restricted field of view and for assessing nodal disease due to low accuracy and sensitivity; as such, it can only detect large metastatic lymph nodes [43].
The risk factors for lymph node involvement include presence of high-risk histologic subtypes, lymphovascular space invasion, deep myometrial invasion, and cervical stromal invasion [44]. Periuterine, common iliac, internal iliac, external iliac and para-aortic adenopathy inferior to the renal veins are all considered regional metastatic disease in EC. Inguinal adenopathy is considered nonregional adenopathy in EC and constitutes distant disease [45].
The detection of metastatic lymph nodes relies greatly on size. Usually, to determine lymph node enlargement, a 1 cm cut-off for short axis is used. However, there is limited evaluation in normal-sized lymph nodes with micrometastasis. For such cases, the use of DWI and apparent diffusion coefficient (ADC) could be beneficial, since small metastatic lymph nodes may show diffusion restriction and ADC values similar to that of the primary tumor [46,47,48].
Stage IV disease is tumor that extends beyond the true pelvis or invades the bladder or rectum. The loss of the T2-hypointense signal of the bladder or rectal wall with mucosa invasion indicates stage IVA disease (Fig. 9). Tumors with distant metastases are classified as stage IVB [49]. Such distant metastasis can be from hematogenous, lymphatic (to inguinal lymph nodes or para-aortic adenopathy above the renal veins) or peritoneal spread (to the omentum, upper abdomen).
74-year-old woman with poorly differentiated endometrial carcinoma. FIGO stage IVA: Tumor invades mucosa of rectum or bladder. a Heterogeneous intermediate signal mass occupying the endometrial cavity extending into the cervix (white arrow). Obstruction of the endometrial cavity at the fundus secondary to the mass (blue arrow). b Extension to the uterine serosa in the anterior aspect of the corpus with urinary bladder wall invasion (white arrow). Bilateral ovarian solid and cystic lesions which could represent large adnexal implants (blue arrows)
Pulmonary metastases are the most common site of hematogenous spread from EC with an incidence of 2.3–4.6% [50, 51]. Frequently multiple, bilateral nodules are seen. Pleural involvement in the form of effusions, nodularity and thickening are seen infrequently (Fig. 10). Dissemination to the liver, the most common intra-abdominal solid organ involved has been reported in 2.3% [52]. Hepatic metastases are single or multiple lesions with hypovascular enhancement. Other atypical intra-abdominal organ involvement includes the adrenal glands and spleen (1%). EC metastases to the bone are generally restricted to the axial skeleton, including the pelvis and thoracolumbar vertebrae (Fig. 11). Brain involvement in EC is rare, occurring in < 1% of EC, and may present as enhancing lesions in cerebral and cerebellar regions (Fig. 12) [53].
Peritoneal involvement may be seen as peritoneal masses, nodules and/or serosal implants that may cause extrinsic compression of the bowel [54,55,56].
Imaging of recurrent and metastatic disease
Most tumor recurrences occur within 3 years post-treatment. Recurrent disease most commonly affects regional lymph nodes (46%) and the vaginal vault (42%). Extra pelvic recurrence commonly involves the peritoneum and lungs. The peritoneum is one of the most common sites of recurrent EC, occurring in 28% of recurrent EC, and constitutes a poor prognostic factor [54]. Atypical metastatic sites include extra-abdominal lymph nodes, liver, adrenals, brain, bones, and soft tissue. Therefore, post-therapy surveillance imaging may include evaluation of the abdomen and pelvis based on symptoms and physical exam findings. Additionally, CT may be used to follow up certain high-risk individuals to detect recurrence in distant organs such as the lungs. However, relative to MRI, CT has inferior soft tissue resolution and is not beneficial in assessment of the vaginal vault [28, 30].
Overview of treatment
Role of surgery
Total hysterectomy and bilateral salpingo-oophorectomy are the basis of treatment of EC unless the patient is an appropriate candidate for fertility preservation. The method by which the surgery is performed has changed substantially and minimally invasive approaches (laparoscopic and robotic) have been shown to be feasible. Intraperitoneal fragmentation or morcellation of the uterus should be avoided.
Role of lymphadenectomy
The main route of spread of EC is through lymphatic dissemination; however, the role of lymphadenectomy for women with early-stage EC is controversial. Some groups advocate selective lymph node sampling in women at high risk for nodal metastases (high-grade or deeply invasive tumors), whereas others recommend routine systematic lymphadenectomy in all patients. Strategies using selective nodal assessment are limited by the difficulty in predicting depth of tumor invasion and final tumor grade intraoperatively. The necessary extent of nodal dissection is debated as some studies have recorded no benefit for lymphadenectomy in either overall recurrence-free survival as well as increased perioperative complications and operative times with lymphadenectomy [57,58,59]. To overcome the morbidity of lymphadenectomy, many advocate for sentinel lymph node dissection [60].
Early-stage disease
Patients with early-stage disease can be divided in two groups: the vast majority of patients who are comprehensively staged with nodal evaluation, and a minority of patients who are not comprehensively staged. Patients with FIGO grade 1 or 2 endometrioid carcinomas limited to the inner half of the endometrium may not benefit from post-operative therapy. For others, some form of adjuvant therapy has been considered. Adjuvant radiation in early-stage disease depends on risk factors such as age, tumor grade, lymphovascular invasion and depth of myometrial invasion, as it reduces locoregional recurrence, however, with no impact in overall survival. Adjuvant chemotherapy in early-stage disease is based on risk factors. [61].
In younger patients, a fertility-sparing approach can be considered if these women are selected carefully. Although younger women could present with early-stage and low-grade malignancies, this may not be the case, thus complete workup will be necessary. For instance, in the Geneva Cancer Registry, only 18% of women younger than 45 years old had stage IA disease at final pathology [62].
Fertility sparing options are not the standard of care and are reserved for women who wish to retain fertility presenting with noninvasive grade 1 EC. However, this topic is still subject to much debate. Continuous progestin-based therapy such as megestrol acetate, medroxyprogesterone, or an intrauterine device containing levonorgestrel are the mainstay conservative hormonal treatment for EC in these patients [63, 64].
Advanced-stage endometrial cancer
The optimal treatment for advanced stages of EC is difficult to define as this is comprised of heterogeneous disease and presentation ranging from micro or macroscopic lymph node metastases, implants, pulmonary metastases and inoperable disease. In general, optimal cytoreductive therapy has therapeutic benefit. Even if metastases are resected or minimized to microscopic residual disease, the risk of recurrence is high and adjuvant treatment is typically recommended [65]. Adjuvant therapy with combination chemotherapy with or without radiation therapy is typically stratified based on risk of recurrence [66]. In terms of treatment planning, imaging further helps in determining the radiation field [67, 68].
Role of radiation
Radiotherapy may be considered for: adjuvant treatment (guided by surgical stage, tumor histology, and adverse risk factors), definitive treatment of nonsurgical patients, local recurrence, and palliative treatment. There are two modalities: external beam radiation therapy (EBRT) and vaginal brachytherapy (VBT) [69]. VBT has low morbidity because a small source of radiation is delivered directly to the targeted area. EBRT can have more side-effects since the beam of radiation is directed from outside the body but covers a broader area. Such side-effects may include enteritis, diarrhea, lymphedema, strictures, and fistulas [70,71,72].
Systemic therapy
Molecular characterization of EC is becoming crucial in directing treatment for advanced and recurrent disease. In addition to histologic analysis, assessment of hormone receptor status, MSI analysis, and assessment of human epidermal growth factor receptor 2 (HER2) status for uterine serous cancers are critical. For recurrent and advanced EC, combination chemotherapy with carboplatin and paclitaxel is considered the standard of care. For patients with uterine serous carcinomas that overexpress HER2, the addition of trastuzumab to carboplatin and paclitaxel has been shown to improve survival [72, 73].
Hormonal therapy is indicated in women with advanced or recurrent endometrioid EC with positive hormone receptors. However, this is typically reserved for patients with limited performance status or for second- or third-line treatment [74, 75].
Also, in the second- and third-line of treatment, the evaluation of MSI status guides indication of targeted therapies and next generation sequencing panels are frequently useful. Pembrolizumab, an immune checkpoint inhibitor monoclonal antibody targeting programmed death receptor-1 (PD-1), combined with Lenvatinib, a multi-tyrosine kinase inhibitor, has been approved for the treatment of MSI–high (MSI-H)/MMR–deficient (dMMR) EC that have progressed after prior therapy and have no satisfactory alternative treatment options [76]. The rationale for the use of immune checkpoint inhibitors in these EC subtypes is that ECs with higher mutation rates are more infiltrated with lymphocytes which cause upregulation of immune checkpoint molecules -PD-1 and PDL1 (negative regulators of immune response). Therefore, immune checkpoint inhibitors can restore anti-tumor immunity, leading to tumor regression [77, 78].
The optimal management of patients with advanced EC has yet to be defined mainly based on studied algorithms that could improve survival with acceptable toxicity. Most recently, the comprehensive genomic characterization has improved the understanding of EC and has revealed these new strategies for targeted therapy.
Radiomics
Improved methods for preoperative risk stratification in EC are highly requested nowadays. Radiomic tumor profiling involves the extraction of large amounts of quantitative imaging data that can be utilized in risk stratification models to predict clinical outcomes and biological behavior [79, 80].
Many studies explored the role of MRI-based radiomics in EC [79,80,81,82,83,84,85]. These mostly focused on predicting high-risk disease, identifying molecular subtypes of EC, and predicting nodal status [79,80,81,82,83,84,85].
Identifying high-risk EC is crucial to identify which patient would benefit from lymphadenectomy [56,57,58,59,60]. A retrospective multicenter study on 717 patients with EC showed that a radiomics nomogram based on radiomics features, clinical and histopathologic parameters, including age, cancer antigen 125 levels, and histological grade following curettage, could predict high-risk EC with good diagnostic performance [81]. Notably, the model performed better in identifying high-risk EC than the actual surgical procedure performed [81]. A study on 102 patients with stage I EC, showed that MRI-based radiomics predicted low-grade EC with higher accuracy compared to a model including clinical and conventional MRI characteristics [82]. Fasmer et al. showed that MRI-based whole-volume tumor radiomic signatures predicted high-risk pathological features and poor outcomes with medium-to-high diagnostic performance. Specifically, whole-tumor radiomic features outperformed single-slice features for prediction of advanced FIGO stage and nodal status, possibly due to the incorporation of information from the entire tumor, thus better assessing heterogeneity of tumors [80].
Regarding molecular characterization of EC, a study on 150 EC patients showed that contrast-enhanced CT-based radiomics distinguished MSI from copy-number low and copy-number high EC with moderate accuracy [AUC of 0.78 (95% CI 0.58–0.91)] [83].
Among the various studies on the role of radiomics to predict nodal status, a multicenter study on 622 EC patients showed that an MRI-based radiomics model including radiomic features and conventional MRI findings showed higher diagnostic performance in assessing nodal status compared to a model based only on conventional MRI findings [84, 85]. In a study on 200 patients with EC, Xu et al. showed that a predictive model combining MRI-based radiomics, lymph node size on MRI and cancer antigen 125 predicted nodal disease with high accuracy in normal-sized nodes [85].
Conclusion
The recent molecular characterization of EC has led to approval of new systemic therapies, resulting in therapeutic advances and in ongoing changes in management strategies. Imaging can help optimizing treatment of EC by allowing differentiation between early and advanced stages and allow prompt identification of recurrent disease. Radiologists should be familiar with the recent advances in molecular characterization of EC, the role of imaging for EC and the current management strategies for EC.
Availability of data and material
Not datasets were generated or analyzed for this article.
References
American Cancer Society. Key statistics for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html. Last revised: January 12, 2021. Visited December 5, 2020
Siegel RL, Miller KD, Jemal A (2020). Cancer statistics. CA: a cancer journal for clinicians; 70(1), 7–30. https://doi.org/10.3322/caac.21590
Clarke MA, Devesa SS, Harvey SV, Wentzensen N (2019). Hysterectomy-Corrected Uterine Corpus Cancer Incidence Trends and Differences in Relative Survival Reveal Racial Disparities and Rising Rates of Nonendometrioid Cancers. J Clin Oncol; 37(22):1895-1908. https://doi.org/10.1200/JCO.19.00151
US Surveillance, Epidemiology, and End Results (SEER). Cancer Stat Facts - Uterine Cancer: https://www.seer.cancer.gov/statfacts/html/corp.html. Accessed December 5, 2020
Brooks RA, Fleming GF, Lastra RR, et al (2019). Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin; 69(4):258-279. https://doi.org/10.3322/caac.21561
Connor EV, Rose PG (2018). Management Strategies for Recurrent Endometrial Cancer. Expert Rev Anticancer Ther; 18(9):873-885. https://doi.org/10.1080/14737140.2018.1491311
Sorbe B, Juresta C, Ahlin C (2014). Natural history of recurrences in endometrial carcinoma. Oncol Lett; 8(4):1800-1806. https://doi.org/10.3892/ol.2014.2362
Jemal A, Siegel R, Ward E, et al (2008). Cancer statistics, 2008. CA Cancer J Clin; 58(2):71-96. https://doi.org/10.3322/CA.2007.0010
Van Nyen T, Moiola CP, Colas E, Annibali D, Amant F (2018). Modeling Endometrial Cancer: Past, Present, and Future. Int J Mol Sci; 19(8):2348. https://doi.org/10.3390/ijms19082348
Carlson J, McCluggage WG (2019). Reclassifying endometrial carcinomas with a combined morphological and molecular approach. Curr Opin Oncol; 31(5):411-419. https://doi.org/10.1097/CCO.0000000000000560
Arora S, Balasubramaniam S, Zhang W, et al (2020). FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin Cancer Res; 26(19):5062-5067. https://doi.org/10.1158/1078-0432.CCR-19-3979
Freeman SJ, Aly AM, Kataoka MY, Addley HC, Reinhold C, Sala E (2012). The revised FIGO staging system for uterine malignancies: implications for MR imaging. Radiographics; 32(6):1805-1827. https://doi.org/10.1148/rg.326125519
Amant F, Mirza MR, Koskas M, Creutzberg CL (2018). Cancer of the corpus uteri. Int J Gynaecol Obstet; 143 Suppl 2:37–50. https://doi.org/10.1002/ijgo.12612
Lin MY, Dobrotwir A, McNally O, Abu-Rustum NR, Narayan K (2018). Role of imaging in the routine management of endometrial cancer. Int J Gynaecol Obstet; 143 Suppl 2(Suppl 2):109–117. https://doi.org/10.1002/ijgo.12618
Bell DW, Ellenson LH (2019). Molecular Genetics of Endometrial Carcinoma. Annu Rev Pathol; 14:339-367. https://doi.org/10.1146/annurev-pathol-020117-043609
Piulats JM, Guerra E, Gil-Martín M, et al (2017). Molecular approaches for classifying endometrial carcinoma. Gynecol Oncol; 145(1):200-207. https://doi.org/10.1016/j.ygyno.2016.12.015
Talhouk A, McConechy MK, Leung S, et al (2015). A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer; 113(2):299-310. https://doi.org/10.1038/bjc.2015.190
Levine, D., The Cancer Genome Atlas Research Network., Genome sequencing centres: Broad Institute, et al (2013). Integrated genomic characterization of endometrial carcinoma. Nature; 497:67–73. https://doi.org/10.1038/nature12113
Shinbrot E, Henninger EE, Weinhold N, et al (2014). Exonuclease mutations in DNA polymerase epsilon reveal replication strand specific mutation patterns and human origins of replication. Genome Res; 24(11):1740-1750. https://doi.org/10.1101/gr.174789.114
Bellone S, Centritto F, Black J, et al (2015). Polymerase ε (POLE) ultra-mutated tumors induce robust tumor-specific CD4+ T cell responses in endometrial cancer patients. Gynecol Oncol; 138(1):11-17. https://doi.org/10.1016/j.ygyno.2015.04.027
Chang Z, Talukdar S, Mullany SA, Winterhoff B (2019). Molecular characterization of endometrial cancer and therapeutic implications. Curr Opin Obstet Gynecol; 31(1):24-30. https://doi.org/10.1097/GCO.0000000000000508
Zighelboim I, Goodfellow PJ, Gao F, et al (2007). Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol; 25(15):2042-2048. https://doi.org/10.1200/JCO.2006.08.2107
Liccardo R, De Rosa M, Izzo P, Duraturo F (2017). Novel Implications in Molecular Diagnosis of Lynch Syndrome. Gastroenterol Res Pract; 2017:2595098. https://doi.org/10.1155/2017/2595098
Drakes ML, Czerlanis CM, Stiff PJ (2020). Immune Checkpoint Blockade in Gynecologic Cancers: State of Affairs. Cancers (Basel); 12(11):3301. https://doi.org/10.3390/cancers12113301
Howitt BE, Shukla SA, Sholl LM, et al (2015). Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol; 1(9):1319-1323. https://doi.org/10.1001/jamaoncol.2015.2151
Akhtar M, Al Hyassat S, Elaiwy O, Rashid S, Al-Nabet ADMH (2019). Classification of Endometrial Carcinoma: New Perspectives Beyond Morphology. Adv Anat Pathol; 26(6):421-427. https://doi.org/10.1097/PAP.0000000000000251
Le Gallo M, Bell DW (2014). The emerging genomic landscape of endometrial cancer. Clin Chem; 60(1):98-110. https://doi.org/10.1373/clinchem.2013.205740
Expert Panel on GYN and OB Imaging, Reinhold C, Ueno Y, et al (2020). ACR Appropriateness Criteria® Pretreatment Evaluation and Follow-Up of Endometrial Cancer. J Am Coll Radiol; 17(11S):S472-S486. https://doi.org/10.1016/j.jacr.2020.09.001
Nougaret S, Horta M, Sala E, et al (2019). Endometrial Cancer MRI staging: Updated Guidelines of the European Society of Urogenital Radiology. Eur Radiol; 29(2):792-805. https://doi.org/10.1007/s00330-018-5515-y
Saleh M, Virarkar M, Bhosale P, El Sherif S, Javadi S, Faria SC (2020). Endometrial Cancer, the Current International Federation of Gynecology and Obstetrics Staging System, and the Role of Imaging. J Comput Assist Tomogr; 44(5):714-729. https://doi.org/10.1097/RCT.0000000000001025
Sala E, Wakely S, Senior E, Lomas D (2007). MRI of malignant neoplasms of the uterine corpus and cervix. AJR Am J Roentgenol; 188(6):1577-1587. https://doi.org/10.2214/AJR.06.1196
National Comprehensive Cancer Network. NCCN Guidelines Version 1.2021 Endometrial Carcinoma. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1473. Visited January 24, 2021
Rizzo S, Femia M, Buscarino V, et al (2018). Endometrial cancer: an overview of novelties in treatment and related imaging keypoints for local staging. Cancer Imaging; 18(1):45. https://doi.org/10.1186/s40644-018-0180-6
ACOG Committee Opinion No. 734: The Role of Transvaginal Ultrasonography in Evaluating the Endometrium of Women With Postmenopausal Bleeding. Obstet Gynecol. 2018;131(5):e124-e129. https://doi.org/10.1097/AOG.0000000000002631
Leone FP, Timmerman D, Bourne T, et al (2010). Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: a consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet Gynecol; 35(1):103-12. https://doi.org/10.1002/uog.7487
Andreano A, Rechichi G, Rebora P, Sironi S, Valsecchi MG, Galimberti S (2014). MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: a systematic review and meta-analysis. Eur Radiol; 24(6):1327-1338. https://doi.org/10.1007/s00330-014-3139-4
Frei KA, Kinkel K, Bonél HM, Lu Y, Zaloudek C, Hricak H (2000). Prediction of deep myometrial invasion in patients with endometrial cancer: clinical utility of contrast-enhanced MR imaging-a meta-analysis and Bayesian analysis. Radiology; 216(2):444-449. https://doi.org/10.1148/radiology.216.2.r00au17444
Lee JH, Dubinsky T, Andreotti RF, et al (2011). ACR appropriateness Criteria® pretreatment evaluation and follow-up of endometrial cancer of the uterus. Ultrasound Q; 27(2):139-145. https://doi.org/10.1097/RUQ.0b013e31821b6f73
Chi DS, Barakat RR, Palayekar MJ, et al (2008). The incidence of pelvic lymph node metastasis by FIGO staging for patients with adequately surgically staged endometrial adenocarcinoma of endometrioid histology. Int J Gynecol Cancer; 18(2):269-273. https://doi.org/10.1111/j.1525-1438.2007.00996.x
Murakami T, Kurachi H, Nakamura H, et al (1995). Cervical invasion of endometrial carcinoma--evaluation by parasagittal MR imaging. Acta Radiol; 36(3):248-253
Nougaret S, Lakhman Y, Vargas HA, et al (2017). From Staging to Prognostication: Achievements and Challenges of MR Imaging in the Assessment of Endometrial Cancer. Magn Reson Imaging Clin N Am; 25(3):611-633. https://doi.org/10.1016/j.mric.2017.03.010
Sokalska A, Timmerman D, Testa AC, et al (2009). Diagnostic accuracy of transvaginal ultrasound examination for assigning a specific diagnosis to adnexal masses. Ultrasound Obstet Gynecol; 34(4):462-470. https://doi.org/10.1002/uog.6444
Wang L, Sui Y, Zhang G, et al (2019). Comparative study of B-ultrasound and CT in preoperative diagnosis of myometrial invasion and lymph node metastasis in endometrial carcinoma. Int J Clin Exp Med; 12:1018–1024
Solmaz U, Mat E, Dereli ML, et al (2015). Lymphovascular space invasion and positive pelvic lymph nodes are independent risk factors for para-aortic nodal metastasis in endometrioid endometrial cancer. Eur J Obstet Gynecol Reprod Biol; 186:63-67. https://doi.org/10.1016/j.ejogrb.2015.01.006
McMahon CJ, Rofsky NM, Pedrosa I (2010). Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology; 254(1):31-46. https://doi.org/10.1148/radiol.2541090361
Lin G, Ho KC, Wang JJ, et al (2008). Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging; 28(1):128-135. https://doi.org/10.1002/jmri.21412
Rechichi G, Galimberti S, Signorelli M, et al (2011). Endometrial cancer: correlation of apparent diffusion coefficient with tumor grade, depth of myometrial invasion, and presence of lymph node metastases. AJR Am J Roentgenol; 197(1):256-262. https://doi.org/10.2214/AJR.10.5584
Kim JK, Kim KA, Park BW, Kim N, Cho KS (2009). Feasibility of diffusion-weighted imaging in the differentiation of metastatic from nonmetastatic lymph nodes: early experience. J Magn Reson Imaging; 29(5):1242. https://doi.org/10.1002/jmri.21480
Barwick TD, Rockall AG, Barton DP, Sohaib SA (2006). Imaging of endometrial adenocarcinoma. Clin Radiol; 61(7):545-555. https://doi.org/10.1016/j.crad.2006.03.011
Paik ES, Yoon A, Lee YY, et al (2015). Pulmonary metastasectomy in uterine malignancy: outcomes and prognostic factors. J Gynecol Oncol; 26(4):270-276. https://doi.org/10.3802/jgo.2015.26.4.270
D'Orsi CJ, Bruckman J, Mauch P, Smith EH (1979). Lung metastases in cervical and endometrial carcinoma. AJR Am J Roentgenol; 133(4):719-722. https://doi.org/10.2214/ajr.133.4.719
Descamps P, Calais G, Moire C, et al (1997). Predictors of distant recurrence in clinical stage I or II endometrial carcinoma treated by combination surgical and radiation therapy. Gynecol Oncol; 64(1):54-58. https://doi.org/10.1006/gyno.1996.4511
Chura JC, Marushin R, Boyd A, Ghebre R, Geller MA, Argenta PA (2007). Multimodal therapy improves survival in patients with CNS metastasis from uterine cancer: a retrospective analysis and literature review. Gynecol Oncol; 107(1):79-85. https://doi.org/10.1016/j.ygyno.2007.05.027
Fujiwara H, Saga Y, Takahashi K, et al (2008). Omental metastases in clinical stage I endometrioid adenocarcinoma. Int J Gynecol Cancer; 18(1):165-167. https://doi.org/10.1111/j.1525-1438.2007.00961.x
Kurra V, Krajewski KM, Jagannathan J, Giardino A, Berlin S, Ramaiya N (2013). Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer Imaging; 13(1):113-122. https://doi.org/10.1102/1470-7330.2013.0011
Levy AD, Shaw JC, Sobin LH (2009). Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics; 29(2):347-373. https://doi.org/10.1148/rg.292085189
DiSaia PJ, Creasman WT, Boronow RC, Blessing JA (1985). Risk factors and recurrent patterns in Stage I endometrial cancer. Am J Obstet Gynecol; 151(8):1009-1015. https://doi.org/10.1016/0002-9378(85)90371-0
Benedetti Panici P, Basile S, Maneschi F, et al (2008). Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst; 100(23):1707–1716. https://doi.org/10.1093/jnci/djn397
ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK (2009). Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomized study. Lancet; 373(9658):125-136. https://doi.org/10.1016/S0140-6736(08)61766-3
Khoury-Collado F, Glaser GE, Zivanovic O, et al (2009). Improving sentinel lymph node detection rates in endometrial cancer: how many cases are needed? Gynecol Oncol; 115(3):453-455. https://doi.org/10.1016/j.ygyno.2009.08.026
SGO Clinical Practice Endometrial Cancer Working Group, Burke WM, Orr J, et al (2014). Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol; 134(2):385-392. https://doi.org/10.1016/j.ygyno.2014.05.018
Navarria I, Usel M, Rapiti E, et al (2009). Young patients with endometrial cancer: how many could be eligible for fertility-sparing treatment? Gynecol Oncol; 114(3):448-451. https://doi.org/10.1016/j.ygyno.2009.05.038
Shah MM, Wright JD (2011). Management of endometrial cancer in young women. Clin Obstet Gynecol; 54(2):219-225. https://doi.org/10.1097/GRF.0b013e318218607c
Won S, Kim MK, Seong SJ (2020). Fertility-sparing treatment in women with endometrial cancer. Clin Exp Reprod Med; 47(4):237-244. https://doi.org/10.5653/cerm.2020.03629
Barlin JN, Puri I, Bristow RE (2010). Cytoreductive surgery for advanced or recurrent endometrial cancer: a meta-analysis. Gynecol Oncol; 118(1):14-18. https://doi.org/10.1016/j.ygyno.2010.04.005
Alvarez Secord A, Havrilesky LJ, Bae-Jump V, et al (2007). The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol; 107(2):285-291. https://doi.org/10.1016/j.ygyno.2007.06.014
Onda T, Yoshikawa H, Mizutani K, et al (1997). Treatment of node-positive endometrial cancer with complete node dissection, chemotherapy and radiation therapy. Br J Cancer; 75(12):1836-1841. https://doi.org/10.1038/bjc.1997.313
Vaidya AP, Littell R, Krasner C, Duska LR (2006). Treatment of uterine papillary serous carcinoma with platinum-based chemotherapy and paclitaxel. Int J Gynecol Cancer; 16 Suppl 1:267–272. https://doi.org/10.1111/j.1525-1438.2006.00413.x
Martell K, Doll C, Barnes EA, Phan T, Leung E, Taggar A (2019). Radiotherapy practices in postoperative endometrial cancer: A survey of the ABS membership. Brachytherapy; 18(6):741-746. https://doi.org/10.1016/j.brachy.2019.07.004
Koh WJ, Abu-Rustum NR, Bean S, et al (2018). Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw; 16(2):170–199. https://doi.org/10.6004/jnccn.2018.0006
Emons G, Vordermark D (2019). Adjuvant treatment for endometrial cancer. Curr Opin Oncol; 31(5):404-410. https://doi.org/10.1097/CCO.0000000000000558
Passarello K, Kurian S, Villanueva V (2019). Endometrial Cancer: An Overview of Pathophysiology, Management, and Care. Semin Oncol Nurs; 35(2):157-165. https://doi.org/10.1016/j.soncn.2019.02.002
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E (2016). Endometrial cancer. Lancet; 387(10023):1094-1108. https://doi.org/10.1016/S0140-6736(15)00130-0
Kelley RM, Baker WH (1961). Progestational agents in the treatment of carcinoma of the endometrium. N Engl J Med; 264:216-222. https://doi.org/10.1056/NEJM196102022640503
van Weelden WJ, Massuger LFAG; ENITEC, Pijnenborg JMA, Romano A (2019). Anti-estrogen Treatment in Endometrial Cancer: A Systematic Review. Front Oncol; 9:359. https://doi.org/10.3389/fonc.2019.00359
Makker V, Rasco D, Vogelzang NJ, et al (2019). Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol; 20(5):711-718. https://doi.org/10.1016/S1470-2045(19)30020-8
Makker V, Taylor MH, Aghajanian C, et al (2020). Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol; 38(26):2981-2992. https://doi.org/10.1200/JCO.19.02627
Barrington DA, Dilley SE, Smith HJ, Straughn JM Jr (2019). Pembrolizumab in advanced recurrent endometrial cancer: A cost-effectiveness analysis. Gynecol Oncol; 153(2):381-384. https://doi.org/10.1016/j.ygyno.2019.02.013
Ytre-Hauge S, Dybvik JA, Lundervold A, et al (2018). Preoperative tumor texture analysis on MRI predicts high-risk disease and reduced survival in endometrial cancer. J Magn Reson Imaging; 48(6):1637-1647. https://doi.org/10.1002/jmri.26184
Fasmer KE, Hodneland E, Dybvik JA, et al (2021). Whole-Volume Tumor MRI Radiomics for Prognostic Modeling in Endometrial Cancer. J Magn Reson Imaging; 53(3):928–937. https://doi.org/10.1002/jmri.27444
Yan BC, Li Y, Ma FH, Feng F, Sun MH, Lin GW, Zhang GF, Qiang JW (2020). Preoperative Assessment for High-Risk Endometrial Cancer by Developing an MRI- and Clinical-Based Radiomics Nomogram: A Multicenter Study. J Magn Reson Imaging; 52(6):1872-1882. doi: https://doi.org/10.1002/jmri.27289.
Chen J, Gu H, Fan W, et al (2021). MRI-Based Radiomic Model for Preoperative Risk stratification in Stage I Endometrial Cancer. J Cancer; 12(3):726-734. https://doi.org/10.7150/jca.50872
Veeraraghavan H, Friedman CF, DeLair DF, et al (2020). Machine learning-based prediction of microsatellite instability and high tumor mutation burden from contrast-enhanced computed tomography in endometrial cancers. Sci Rep; 10(1):17769. https://doi.org/10.1038/s41598-020-72475-9
Yan BC, Li Y, Ma FH, et al (2021). Radiologists with MRI-based radiomics aids to predict the pelvic lymph node metastasis in endometrial cancer: a multicenter study. Eur Radiol; 31(1):411–422. https://doi.org/10.1007/s00330-020-07099-8
Xu X, Li H, Wang S, et al (2019). Multiplanar MRI-Based Predictive Model for Preoperative Assessment of Lymph Node Metastasis in Endometrial Cancer. Front Oncol; 9:1007. https://doi.org/10.3389/fonc.2019.01007
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
FA had the idea for the article. The literature research and the first draft of the manuscript was written by CL, PB and FA. PC and MH reviewed and edited the paper. All authors read, drafted and critically revised the work with approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luna, C., Balcacer, P., Castillo, P. et al. Endometrial cancer from early to advanced-stage disease: an update for radiologists. Abdom Radiol 46, 5325–5336 (2021). https://doi.org/10.1007/s00261-021-03220-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03220-7