Abstract
Objectives
To update the 2009 ESUR endometrial cancer guidelines and propose strategies to standardize image acquisition, interpretation and reporting for endometrial cancer staging with MRI.
Methods
The published evidence-based data and the opinion of experts were combined using the RAND-UCLA Appropriateness Method and formed the basis for these consensus guidelines. The responses of the experts to 81 questions regarding the details of patient preparation, MR imaging protocol, image interpretation and reporting were collected, analysed and classified as “RECOMMENDED” versus “NOT RECOMMENDED” (if at least 80% consensus among experts) or uncertain (if less than 80% consensus among experts).
Results
Consensus regarding patient preparation, MR image acquisition, interpretation and reporting was determined using the RAND-UCLA Appropriateness Method. A tailored MR imaging protocol and a standardized report were recommended.
Conclusions
These consensus recommendations should be used as a guide for endometrial cancer staging with MRI.
Key points
• MRI is recommended for initial staging of endometrial cancer.
• MR imaging protocol should be tailored based on the risk of lymph node metastases.
• Myometrial invasion is best assessed using combined axial-oblique T2WI, DWI and contrast-enhanced imaging.
• The mnemonic “Clinical and MRI Critical TEAM” summarizes key elements of the standardized report.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2009, the European Society of Urogenital Radiology (ESUR) published guidelines for the staging of endometrial cancer (EC). The new guidelines recognized magnetic resonance imaging (MRI) as the imaging modality of choice for evaluating disease extent in patients with newly diagnosed EC [1].
More recently, the European Society for Medical Oncology (ESMO) recommended that the initial surgical treatment of patients with EC should be tailored based on the risk of lymph node metastases, addressing the ongoing controversy regarding the role of lymphadenectomy [2,3,4]. The major clinical challenge in the initial management of EC is to distinguish patients who are at intermediate to high risk of lymph node metastases from those at low risk to avoid overtreatment. The ESMO guidelines advise against lymphadenectomy in low risk patients, i.e. grade 1 or 2 endometrioid adenocarcinoma without deep myometrial invasion (MI) [2, 3]. In contrast, lymphadenectomy is suggested or recommended for intermediate and high-risk groups, respectively. Additionally, while further studies are needed, sentinel lymph node (SLN) sampling is now recognized as a potential alternative to lymphadenectomy [5].
MRI can accurately assess the depth of MI and, thus, it is useful to stratify patients into low versus intermediate to high-risk groups before the surgery. Until recently, a combination of T2-weighted (T2WI) and dynamic contrast-enhanced imaging (DCE-MRI) was accepted as the best approach for local staging of EC [6, 7]. Recent evidence suggests that diffusion-weighted imaging (DWI) improves the evaluation of MI. As a result, DWI is now routinely used as an adjunct to T2WI and DCE-MRI [8,9,10,11,12,13]. It remains to be determined whether combined T2WI and DWI is superior to DCE-MRI.
The aim of this manuscript is to present updated ESUR recommendations for the initial staging of EC, reflecting recent clinical and imaging developments. The value of a tailored MR imaging protocol and a standardized imaging report is emphasized.
Materials and methods
The initial ESUR guidelines for staging of EC with MRI were published in 2009 [1]. Over the past year, members of the ESUR Female Pelvic Imaging working group have re-examined the current literature and clinical standards in management of EC, resulting in this update.
We chose the RAND-UCLA Appropriateness Method (RAM) because of its strength in combining evidence-based data and expert judgments to attain consensus on a variety of clinically pertinent questions. RAM was previously used to develop the European Society of Gastrointestinal and Abdominal Radiology consensus guidelines for MR imaging assessment of rectal cancer [14, 15].
The methodological steps were as follows:
-
Step 1: Literature review
Medline (Ovid), EMBRASE (embrace.com) and the Cochrane Library were searched for original manuscripts published between 2007 and 2017 pertaining to MR imaging and staging of EC (Supplementary material – File 1).
-
Step 2: Questionnaire development
A questionnaire consisting of 81 questions was developed by the two lead authors (SN and RF) and later refined with input from three advising members (ES, AR, ITN). The questions in the survey focused on the key MR imaging requirements, integration of functional MR imaging sequences and development of the standardized imaging report.
-
Step 3: Panel selection
The panel was comprised of all members of the ESUR Female Pelvic Imaging working group.
-
Step 4: Survey prior to the first meeting of the panel
The questionnaire was distributed to all members of the panel via electronic mail in November 2016; responses were recorded using a dedicated survey platform (surveymonkey.com).
-
Step 5: Data extraction and analysis
The answers to the questionnaire were collected in January 2017 and analysed by one lead author (SN). On the basis of the answers to the survey, each item was classified as follows: (1) “RECOMMENDED” (at least 80% agreement in favour), (2) “NOT RECOMMENDED” (at least 80% agreement in opposition) or (3) “UNCERTAIN”, i.e. consensus was not reached (less than 80% agreement). The results were presented to and discussed with the ESUR Female Pelvic Imaging working group at the Annual European Congress of Radiology Meeting in March 2017.
-
Step 6: Second survey
Five extra questions were added to the survey and distributed to all members via electronic mail to clarify any potentially conflicting answers that arose after the first survey and the first meeting.
-
Step 7: Second and final meeting of the panel
The members of the ESUR Female Pelvic Imaging working group met again at the Annual European Symposium on Urogenital Radiology in September 2017 with two lead authors (SN and RF) serving as moderators. Final results of the survey were circulated among all members of the panel 2 weeks prior to this meeting and formed the basis of the discussion at the meeting. The focus was on the questions with no consensus among experts.
-
Step 8: Data reporting
The final data analysis was performed by the two lead authors (SN and RF). Each item was ultimately classified as (1) “RECOMMENDED” versus (2) “NOT RECOMMENDED” if at least 80% agreement among experts or (3) “UNCERTAIN” (no consensus defined as less than 80% agreement among experts).
Results update
The panel included 28 experts from 26 different institutions. Twenty-three members were from centres in Europe: Portugal (n = 2), France (n = 3), Spain (n = 2), United Kingdom (n = 5), Switzerland (n = 3), Germany (n = 3), Austria (n = 1), Sweden (n = 1), Italy (n = 2) and Greece (n = 1). Five panellists were from the three institutions outside Europe: Japan, USA and Brazil.
The panel’s recommendations (based on at least 80% consensus among experts) are summarized in Table 1.
Discussion
Update on role of DCE-MRI and DWI
The literature search described in the “Materials and methods” section resulted in 271 relevant publications. A flowchart summarizing the selection process is shown in the supplementary materials. One extra study was added to this list after review of the references from the retrieved studies. Thus, a total of 26 studies (25 + 1 retrieved) were identified that evaluated the accuracy of DCE-MRI and/or DWI for primary staging of EC. No prospective randomized clinical trials or meta-analyses of prospective clinical trials were identified. The majority were well-conducted, non-randomized, single-centre comparative studies and cohort studies and/or retrospective studies.
Role of DCE-MRI
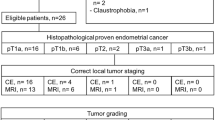
Multiple studies have compared the performance of combined DCE-MRI + T2WI versus T2WI alone to stage EC, focusing in particular on the depth of myometrial invasion (MI) [8, 9, 11,12,13, 16,17,18,19,20,21,22,23,24,25,26] (Table 2). A meta-analysis demonstrated that DCE-MRI had similar sensitivity to T2WI but was more specific for the detection of deep MI [27]. On DCE-MRI, MI was best depicted during the equilibrium phase (2 min 30 s after the injection) [28, 29]. The panel discussed the value of single-phase high spatial resolution contrast-enhanced imaging at 2 min 30 sec versus DCE-MRI. DCE-MRI allows to determine the presence of uninterrupted enhancement of the subendometrial zone which is best seen approximately 35–40 s following contrast injection. This information is useful when fertility-sparing management is being considered because it helps to exclude any MI, a key finding to confirm patient eligibility for conservative management [30]. Moreover, delayed DCE-MRI images (4–5 min after the injection) are optimal for the detection of cervical stromal invasion (CSI). On the basis of the above considerations, the panel recommends performing either DCE-MRI or single-phase contrast-enhanced imaging at 2 min 30 s depending on the provided clinical history (i.e. patient’s age and desire for fertility preservation) and the radiologist’s availability to supervise image acquisition (Figs. 1 and 2).
Role of DWI
Multiple studies have demonstrated the added value of DWI for EC staging, particularly for assessing the depth of MI [8, 9, 11,12,13, 18, 20,21,22,23, 26, 30,31,32,33,34] (Table 3). DWI is particularly useful in patients who cannot receive intravenous injection of gadolinium-based contrast agents or have tumors that are isointense or hyperintense to the myometrium on contrast-enhanced images [18, 35]. DWI is also useful in evaluating the depth of MI in the setting of concurrent adenomyosis [13]. The ESUR panel recommends including DWI to stage EC; at a minimum the acquisition should include an axial oblique plane with the same orientation as axial oblique T2WI (i.e. perpendicular to the long axis of the uterus).
The ESUR panel endorses the National Cancer Institute and prior ESUR consensus recommendations that advise a minimum of two b values with an optimal high b value of 800 to 1000 s/mm2 [36, 37].
Several recent studies have compared DWI and DCE-MRI for the assessment of MI. Some found DWI to be superior to DCE-MRI; for example, Takeuchi et al. reported that DWI had the accuracy of 94% while DCE-MRI had the accuracy of 88% for detecting deep MI [13, 18, 20, 35]. Other studies found no difference in the accuracy between the two techniques [11]. A meta-analysis by Andreano et al. reported no significant difference in the sensitivity or specificity between DWI and DCE-MR for diagnosing deep MI [10]. A more recent and larger meta-analysis by Deng et al. confirmed similar diagnostic performance of DWI to DCE-MRI; however, they also found that combined T2WI + DWI were superior to either DWI or DCE-MRI alone [38].
Tips for MRI interpretation
Diagnosis
EC is typically intermediate in signal intensity (SI) on T2WI and is hyperintense compared with the myometrium.
FIGO MRI stage IA/IB
Stage IA is diagnosed if the tumor invades less than 50% of the myometrial thickness whereas stage IB is present if the tumor involves 50% or more of the myometrial thickness [39].
The depth of MI is best measured on the axial oblique images acquired perpendicular to the endometrial cavity. First, a line is drawn parallel to the presumed inner edge of the myometrium. Then, two lines are drawn: one measuring the entire thickness of the myometrium and the other measuring the maximum tumor extension into the myometrium. The ratio of the two lengths corresponds to the depth of MI [40]. The assessment of the depth of MI may be a challenge (Figs. 3 and 4) if a large endometrial tumor distends and thins the myometrium (Fig. 3), if an endometrial tumor is relatively isointense to myometrium on T2WI (Fig. 4), if a tumor involves a cornu of the uterus where myometrium is physiologically thinner than elsewhere in the uterus (Fig. 4), or if the uterine anatomy is distorted by leiomyomas and/or adenomyosis. In these clinical scenarios, radiologists should be aware of possible tumor overstaging; under these circumstances DCE-MRI and DWI may be of particular value to improve the delineation of tumor margins and to avoid the overestimation of tumor extent.
a and b. Axial-oblique T2WI (a) and fused axial oblique T2-DWI (b) show a large tumor distending the endometrial cavity (white arrows) and compressing the myometrium; a continuous low signal intensity junctional zone is seen on both sets of images. c A smooth uninterrupted band of early subendometrial enhancement on DCE-MRI helps to exclude myometrial invasion (arrows)
Axial oblique T2WI (a) shows a large endometrial mass involving the right cornu of the uterus (white arrow). The tumor (black arrow) is isointense to the myometrium making it difficult to detect its margins. On T2WI, the tumor possibly extends to and abuts uterine serosa (white arrow). Contrast-enhanced T1WI (b) and fused axial T2WI-DWI (c) improve the delineation of tumor margins and show tumor extension into the outer half of the myometrium but no involvement of the uterine serosa consistent with FIGO IB disease (white arrow)
FIGO MRI stage II
CSI is best assessed by evaluating both the sagittal and axial oblique planes that are acquired parallel and perpendicular to the long axis of the cervix, respectively. CSI is diagnosed when intermediate-SI tumor disrupts low-SI fibrous cervical stroma (CS) on T2WI. On DCE-MRI, CSI is indicated when the normal enhancement of CS is disrupted by a hypo-enhancing tumor, best seen on delayed phase images (4–5 min). On DWI, CSI is suspected when a tumor (high SI on high b value DWI and low SI on the apparent diffusion coefficient (ADC) map) disrupts low-SI CS. Potential pitfalls for assessing CSI on MRI are summarized in Fig. 5.
FIGO MRI stage III
-
Stage IIIA tumors invade the uterine serosa. They appear as intermediate-to-high-SI lesions that disrupt normally smooth outer contour of the uterus on T2WI. One should be careful not to overcall stage IIIA when the tumor is isointense to the myometrium on T2WI. Contrast-enhanced imaging and DWI can improve the delineation of tumor margins and facilitate MR staging (Fig. 4). Stage IIIA also includes direct tumor spread to the adnexa or ovarian metastases.
-
Stage IIIB tumors involve the parametria or the vagina by either direct invasion or metastatic spread. DWI is particularly useful for detecting small tumor deposits in the cervix and/or vagina.
-
Stage IIIC disease is characterized by the presence of lymph node metastases and is subdivided on the basis of pelvic (stage IIIC1) and/or para-aortic (stage IIIC2) lymph node involvement. The risk factors for lymph node metastases include presence of high-risk histologic subtypes (grade 3 endometrioid adenocarcinoma and non-endometrioid histologic types, i.e. carcinosarcoma, serous carcinoma or clear cell carcinoma), lymphovascular space invasion, deep MI, and CSI [41, 42]. MRI has low sensitivity for the detection of lymph node metastases [43]. The assessment is largely based on size criteria where a short axis diameter of greater than 8 mm in pelvic nodes and 10 mm in para-aortic nodes is taken to indicate tumoral involvement [29, 43]. Other morphological features including round shape, spiculated margins, heterogeneous SI, SI similar to that of the primary tumor, or the presence of necrosis can be used to suggest the involvement in smaller nodes [44]. DWI aids in the detection of lymph nodes owing to their high SI on high b value images. However, there is significant overlap between the ADC values of benign and malignant nodes and therefore the technique is currently used only as an adjunct to T2WI [45,46,47,48,49].
FIGO MRI stage IV
Stage IV disease manifests with direct invasion of the bladder or rectal mucosa (stage IVA) or distant metastases (stage IVB). Preserved fat planes between the tumor and bladder or rectum exclude stage IVA with high accuracy, alleviating the need for cystoscopy or rectosigmoidoscopy.
Key components of MRI report (Fig. 6)
The panel members unanimously agreed on the need for a structured MRI report because structured reporting improves the report quality and facilitates the communication of clinical relevant information to a referring physician [37, 50,51,52,53,54,55]. The recommended structured report is presented in Fig. 6; the elements of the report are summarized as a short mnemonic, “Clinical and MRI Critical TEAM” (Clinical for clinical information, MRI for MI, Critical for CSI, TE for tumor extension, A for adnexa and M for metastasis).
Role of MRI in initial clinical decision-making
Role of MRI in selection of patients prior to fertility-sparing therapy
Approximately 5% of women are diagnosed with EC under the age of 40 [56]. If a patient is of childbearing age, desires fertility preservation and has endometrium-confined grade 1 endometrioid adenocarcinoma or premalignant conditions (for example, atypical hyperplasia), conservative medical treatment with progestins (administered orally or via an intra-uterine device) may be an option. Fertility-sparing management is controversial in patients with Lynch syndrome since their disease is due to genetic predisposition and may be less responsive to progestins [57]. In addition, there is evidence that patients with BMI greater than 25 kg/m2 before or after progestogen treatment have a worse response to treatment and a high recurrence rate [58]. MRI is useful prior to initiating conservative management to confirm that the disease is confined to the endometrium.
The eligibility criteria for fertility-preserving management are summarized in Table 4.
Role of MRI for initial treatment planning
The standard surgical procedures that simultaneously stages and treats EC include total hysterectomy, bilateral salpingo-oophorectomy with peritoneal washings, and pelvic plus para-aortic lymph node dissection. However, most patients present with FIGO stage I disease and are at low risk for lymph node metastases. The clinical benefit of lymphadenectomy in early-stage EC is controversial. Lymphadenectomy allows complete surgical staging and facilitates adjuvant treatment selection, potentially reducing the morbidity of unnecessary radiation therapy. However, lymphadenectomy carries a 7–10% risk of lymphocele development and a 23% risk of lower-extremity lymphedema [59]. Several recent large prospective trials showed no survival benefit after lymphadenectomy in patients with early-stage grade 1 and 2 endometrioid adenocarcinoma [60,61,62,63]. Therefore, in patients with clinical stage I disease, the need for lymphadenectomy may be determined based on the presence of risk factors that increase the likelihood of lymph node metastases and subsequent recurrence [63,64,65,66].
The ESUR endorses the ESMO recommendations to stratify stage I EC into four risk categories [2, 4]. Accordingly, lymphadenectomy is not recommended in the low-risk group, i.e. stage I grade 1 or 2 endometrioid adenocarcinoma with less than 50% MI [2, 4]. Lymphadenectomy is suggested or recommended for all other patients with newly diagnosed EC. In this schema, preoperative information regarding the depth of MI and histologic subtype is essential to tailor the surgical approach. MRI can assess the depth of MI, while histologic type and grade are determined by endometrial sampling [67].
The panel recommends a tailored MR imaging assessment that is closely aligned with the ESMO guidelines. MRI protocol should be tailored according to the tumor histology and grade, patient preferences with regard to fertility preservation and the radiologist’s availability to monitor image acquisition (Fig. 1). Briefly, patients with grade 3 endometrioid adenocarcinoma and non-endometrioid histologies (carcinosarcoma, serous carcinoma or clear cell carcinoma) are at high risk of extra-uterine spread including lymph node metastases. In this group, assessment for MI or CSI with MRI is less important, while the detection of extra-uterine disease is critical for treatment planning. DWI through the entire abdomen and pelvis should be performed; contrast-enhanced imaging is recommended but DCE-MRI is not required. In patients with grade 1 or 2 endometrioid adenocarcinoma, MRI should focus on evaluating the depth of MI and presence of CSI. If image acquisition is unsupervised, DWI and contrast-enhanced are recommended to assess MI and CSI. If image acquisition is supervised, DCE-MRI may only be necessary as an adjunct to DWI in challenging cases. In patients of childbearing age who desire fertility preservation and have grade 1 endometrioid cancer, DCE-MRI should be added to T2WI and DWI because the presence of intact subendometrial enhancement is useful to confirm endometrium-confined disease.
Future research directions
DWI
Histologic grade is determined preoperatively using endometrial sampling but is subject to sampling error [68,69,70]. Several studies investigated the role of DWI with ADC for assessing tumor grade, but the results are inconclusive so far [9, 18, 20, 71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. Some found an association between low ADC values and high-grade histology [9, 72, 77, 79], while others did not [71, 73, 74]. FOCUS DWI (field of view (FOV) optimized and constrained undistorted single-shot DWI) [8, 34, 86] is a new approach to DWI acquisition that uses the reduced FOV in the phase encoding direction to minimize artefacts; the results of using FOCUS DWI to assess disease extent in patients with uterus-confined disease are encouraging [8].
PET/CT and PET/MRI
18F-FDG PET/CT is superior to MRI for N and M staging [87]. However, the yield of PET/CT is low in early-stage EC as a result of the low prevalence of lymph node metastases [88]. The present ESUR guidelines endorse the National Comprehensive Cancer Network guidelines that recommend PET/CT for initial staging only in the presence of clinical suspicion for extra-uterine spread [89].
It is hoped that PET/MR will allow the integration of morphologic, functional and metabolic information to facilitate the evaluation of both local and distant extent of disease.
Other new MRI tools
Radiomics is the emerging field that correlates image-based texture features with clinically relevant oncologic outcomes. The role of texture analysis (TA) in EC is an area of active investigation [90]. Preliminary data suggest that quantitative texture features may be useful for evaluating the depth of MI, detecting lymphovascular space invasion and identifying high-grade histology. For evaluating the depth of MI, quantitative texture features show similar accuracy to subjective interpretation by experienced radiologists. Tumor size and tumor volume as determined on MRI are also useful for evaluating the depth of MI and detecting the presence of lymphovascular space invasion [9, 76, 91]. These preliminary findings require further confirmation and external validation. The development of robust auto-segmentation techniques is an active area of research and would facilitate the application of TA in daily clinical practice.
Furthermore, advances in molecular profiling of EC may facilitate new research in radiogenomics. Radiogenomics refers to the study of the relationships between imaging phenotypes and genomic signatures. The Cancer Genome Atlas Research Network has recently improved the characterization of the EC molecular landscape whereby four molecular subtypes have been described: (1) POLE, the smallest group with excellent prognosis, (2) microsatellite unstable tumors, (3) copy-number low microsatellite stable tumors and (4) copy-number high tumors with TP53 mutations. The last group includes serous carcinomas and is associated with a poor prognosis [5, 92,92,93,95]. As such, future radiogenomic studies may lead to path-breaking changes in EC imaging.
Summary
MRI is now widely accepted as the imaging modality of choice for initial staging of EC. Recently, DWI has emerged as a promising tool to facilitate the assessment of local disease extent. These updated ESUR Female Pelvis Imaging working group guidelines build on the 2009 version and the recent ESMO guidelines to reflect new imaging and clinical developments in the field. This update recommends an algorithmic approach to MRI acquisition, addresses patient evaluation prior to fertility-sparing management and proposes a structured MRI report to facilitate effective communication between radiologists and their referring physicians.
Abbreviations
- CSI:
-
Cervical stromal invasion
- DCE-MRI:
-
Dynamic contrast-enhanced MRI
- DWI:
-
Diffusion-weighted imaging
- EC:
-
Endometrial cancer
- ESMO:
-
European Society for Medical Oncology
- ESUR:
-
European Society of Urogenital Radiology
- FOCUS:
-
Field of view (FOV) optimized and constrained undistorted single-shot DWI
- MI:
-
Myometrial invasion
- MRI:
-
Magnetic resonance imaging
- RAM:
-
RAND-UCLA Appropriateness Method
- SI:
-
Signal intensity
- SLN:
-
Sentinel lymph node
- TA:
-
Texture analysis
- T2WI:
-
T2-weighted imaging
References
Kinkel K, Forstner R, Danza FM et al (2009) Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol 19:1565–1574
Colombo N, Preti E, Landoni F et al (2013) Endometrial cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24 Suppl 6:vi33-8
Colombo N, Creutzberg C, Amant F et al (2016) ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer 26:2–30
Colombo N, Creutzberg C, Amant F et al (2016) ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol 27:16–41
Rossi EC, Kowalski LD, Scalici J et al (2017) A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 18:384–392
Frei KA, Kinkel K, Bonel HM, Lu Y, Zaloudek C, Hricak H (2000) Prediction of deep myometrial invasion in patients with endometrial cancer: clinical utility of contrast-enhanced MR imaging-a meta-analysis and Bayesian analysis. Radiology 216:444–449
Cunha TM, Felix A, Cabral I (2001) Preoperative assessment of deep myometrial and cervical invasion in endometrial carcinoma: comparison of magnetic resonance imaging and gross visual inspection. Int J Gynecol Cancer 11:130–136
Bhosale P, Ma J, Iyer R et al (2016) Feasibility of a reduced field-of-view diffusion-weighted (rFOV) sequence in assessment of myometrial invasion in patients with clinical FIGO stage I endometrial cancer. J Magn Reson Imaging 43:316–324
Nougaret S, Reinhold C, Alsharif SS et al (2015) Endometrial cancer: combined MR volumetry and diffusion-weighted imaging for assessment of myometrial and lymphovascular invasion and tumor grade. Radiology 276:797–808
Andreano A, Rechichi G, Rebora P, Sironi S, Valsecchi MG, Galimberti S (2014) MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: a systematic review and meta-analysis. Eur Radiol 24:1327–1338
Seo JM, Kim CK, Choi D, Kwan Park B (2013) Endometrial cancer: utility of diffusion-weighted magnetic resonance imaging with background body signal suppression at 3T. J Magn Reson Imaging 37:1151–1159
Hori M, Kim T, Onishi H et al (2013) Endometrial cancer: preoperative staging using three-dimensional T2-weighted turbo spin-echo and diffusion-weighted MR imaging at 3.0 T: a prospective comparative study. Eur Radiol 23:2296–2305
Beddy P, Moyle P, Kataoka M et al (2012) Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology 262:530–537
Beets-Tan RG, Lambregts DM, Maas M et al (2013) Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 23:2522–2531
Beets-Tan RGH, Lambregts DMJ, Maas M et al (2018) Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 28:1465–1475
Rockall AG, Meroni R, Sohaib SA et al (2007) Evaluation of endometrial carcinoma on magnetic resonance imaging. Int J Gynecol Cancer 17:188–196
Sala E, Crawford R, Senior E et al (2009) Added value of dynamic contrast-enhanced magnetic resonance imaging in predicting advanced stage disease in patients with endometrial carcinoma. Int J Gynecol Cancer 19:141–146
Takeuchi M, Matsuzaki K, Nishitani H (2009) Diffusion-weighted magnetic resonance imaging of endometrial cancer: differentiation from benign endometrial lesions and preoperative assessment of myometrial invasion. Acta Radiol 50:947–953
Emlik D, Kiresi D, Özdemir S, Çelik C, Karaköse S (2010) Preoperative assessment of myometrial and cervical invasion in endometrial carcinoma: comparison of multi-section dynamic MR imaging using a three dimensional FLASH technique and T2-weighted MR imaging. J Med Imaging Radiat Oncol 54:202–210
Rechichi G, Galimberti S, Signorelli M, Perego P, Valsecchi MG, Sironi S (2010) Myometrial invasion in endometrial cancer: diagnostic performance of diffusion-weighted MR imaging at 1.5-T. Eur Radiol 20:754–762
Dogan D, Inan N, Sarisoy HT et al (2013) Preoperative evaluation of myometrial invasion in endometrial carcinoma: diagnostic performance of 3T MRI. Abdom Imaging 38:388–396
Koplay M, Dogan NU, Erdogan H et al (2014) Diagnostic efficacy of diffusion-weighted MRI for pre-operative assessment of myometrial and cervical invasion and pelvic lymph node metastasis in endometrial carcinoma. J Med Imaging Radiat Oncol 58:538–546 quiz 648
Bonatti M, Stuefer J, Oberhofer N et al (2015) MRI for local staging of endometrial carcinoma: Is endovenous contrast medium administration still needed? Eur J Radiol 84:208–214
Teng F, Zhang YF, Wang YM et al (2015) Contrast-enhanced MRI in preoperative assessment of myometrial and cervical invasion, and lymph node metastasis: diagnostic value and error analysis in endometrial carcinoma. Acta Obstet Gynecol Scand 94:266–273
Du L, Li X, Qiu X, Liu X, Wang Y, Yu Y (2016) Application of FLASH-3D dynamic contrast-enhanced imaging for diagnosis of endometrial carcinoma. Br J Radiol 89:20160268
Lin G, Ng KK, Chang CJ et al (2009) Myometrial invasion in endometrial cancer: diagnostic accuracy of diffusion-weighted 3.0-T MR imaging—initial experience. Radiology 250:784–792
Wu LM, Xu JR, Gu HY, Hua J, Haacke EM, Hu J (2013) Predictive value of T2-weighted imaging and contrast-enhanced MR imaging in assessing myometrial invasion in endometrial cancer: a pooled analysis of prospective studies. Eur Radiol 23:435–449
Park SB, Moon MH, Sung CK, Oh S, Lee YH (2014) Dynamic contrast-enhanced MR imaging of endometrial cancer: optimizing the imaging delay for tumour-myometrium contrast. Eur Radiol 24:2795–2799
Manfredi R, Mirk P, Maresca G et al (2004) Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology 231:372–378
Fujii S, Kido A, Baba T et al (2015) Subendometrial enhancement and peritumoral enhancement for assessing endometrial cancer on dynamic contrast enhanced MR imaging. Eur J Radiol 84:581–589
Sadowski EA, Robbins JB, Guite K et al (2015) Preoperative pelvic MRI and serum cancer antigen-125: selecting women with grade 1 endometrial cancer for lymphadenectomy. AJR Am J Roentgenol 205:W556–W564
Shen SH, Chiou YY, Wang JH et al (2008) Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. AJR Am J Roentgenol 190:481–488
Rodriguez-Trujillo A, Martinez-Serrano MJ, Martinez-Roman S et al (2016) Preoperative assessment of myometrial invasion in endometrial cancer by 3D ultrasound and diffusion-weighted magnetic resonance imaging: a comparative study. Int J Gynecol Cancer 26:1105–1110
Takeuchi M, Matsuzaki K, Harada M (2018) Evaluating myometrial invasion in endometrial cancer: comparison of reduced field-of-view diffusion-weighted imaging and dynamic contrast-enhanced MR imaging. Magn Reson Med Sci 17:28–34
Sala E, Rockall A, Rangarajan D, Kubik-Huch RA (2010) The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvis. Eur J Radiol 76:367–385
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Forstner R, Thomassin-Naggara I, Cunha TM et al (2017) ESUR recommendations for MR imaging of the sonographically indeterminate adnexal mass: an update. Eur Radiol 27:2248–2257
Deng L, Wang QP, Chen X, Duan XY, Wang W, Guo YM (2015) The combination of diffusion- and T2-weighted imaging in predicting deep myometrial invasion of endometrial cancer: a systematic review and meta-analysis. J Comput Assist Tomogr 39:661–673
Horta M, Cunha T (2016) Endometrial cancer. In: Forstner R, Hamm B (eds) MRI and CT of the female pelvis. Berlin, Springer, p 1–30
Nougaret S, Lakhman Y, Vargas HA et al (2017) From staging to prognostication: achievements and challenges of MR imaging in the assessment of endometrial cancer. Magn Reson Imaging Clin N Am 25:611–633
Aalders JG, Thomas G (2007) Endometrial cancer—revisiting the importance of pelvic and para aortic lymph nodes. Gynecol Oncol 104:222–231
Muallem MZ, Sehouli J, Almuheimid J, Richter R, Joukhadar R, Plett H (2016) Risk factors of lymph nodes metastases by endometrial cancer: a retrospective one-center study. Anticancer Res 36:4219–4225
Haldorsen IS, Salvesen HB (2012) Staging of endometrial carcinomas with MRI using traditional and novel MRI techniques. Clin Radiol 67:2–12
Thoeny HC, Froehlich JM, Triantafyllou M et al (2014) Metastases in normal-sized pelvic lymph nodes: detection with diffusion-weighted MR imaging. Radiology 273:125–135
Nougaret S, Tirumani SH, Addley H, Pandey H, Sala E, Reinhold C (2013) Pearls and pitfalls in MRI of gynecologic malignancy with diffusion-weighted technique. AJR Am J Roentgenol 200:261–276
Inada Y, Matsuki M, Nakai G et al (2009) Body diffusion-weighted MR imaging of uterine endometrial cancer: is it helpful in the detection of cancer in nonenhanced MR imaging? Eur J Radiol 70:122–127
Roy C, Bierry G, Matau A, Bazille G, Pasquali R (2010) Value of diffusion-weighted imaging to detect small malignant pelvic lymph nodes at 3 T. Eur Radiol 20:1803–1811
Rechichi G, Galimberti S, Oriani M, Perego P, Valsecchi MG, Sironi S (2013) ADC maps in the prediction of pelvic lymph nodal metastatic regions in endometrial cancer. Eur Radiol 23:65–74
Nakai G, Matsuki M, Inada Y et al (2008) Detection and evaluation of pelvic lymph nodes in patients with gynecologic malignancies using body diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr 32:764–768
Boone JM, Mahesh M, Gingold EL, Seibert JA (2016) A call for the structured physicist report. J Am Coll Radiol 13:307–309
Flusberg M, Ganeles J, Ekinci T et al (2017) Impact of a structured report template on the quality of CT and MRI reports for hepatocellular carcinoma diagnosis. J Am Coll Radiol 14:1206–1211
Gassenmaier S, Armbruster M, Haasters F et al (2017) Structured reporting of MRI of the shoulder - improvement of report quality? Eur Radiol 27:4110–4119
Sahni VA, Silveira PC, Sainani NI, Khorasani R (2015) Impact of a structured report template on the quality of MRI reports for rectal cancer staging. AJR Am J Roentgenol 205:584–588
Samartine S, White L, McKeon D, Becker M (2015) Enhancing structured reporting: improving quality by tailoring the report to the clinical scenario. J Am Coll Radiol 12:845–847
Kubik-Huch R, Weston M, Nougaret S et al (2018) European Society of Urogenital Radiology (ESUR) guidelines: MR imaging of leiomyomas. Eur Radiol. https://doi.org/10.1007/s00330-017-5157-5
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E (2016) Endometrial cancer. Lancet 387:1094–1108
Haoula Z, Salman M, Atiomo W (2012) Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod 27:1327–1331
Park JY, Seong SJ, Kim TJ, Kim JW, Bae DS, Nam JH (2017) Significance of body weight change during fertility-sparing progestin therapy in young women with early endometrial cancer. Gynecol Oncol 146:39–43
Yost KJ, Cheville AL, Al-Hilli MM et al (2014) Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol 124:307–315
group As, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK (2009) Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 373:125-136
Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N (2010) Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet 375:1165–1172
Benedetti Panici P, Basile S, Maneschi F et al (2008) Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 100:1707–1716
Frost JA, Webster KE, Bryant A, Morrison J (2015) Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev CD007585. https://doi.org/ https://doi.org/10.1002/14651858.CD007585.pub3
Imai K, Kato H, Katayama K et al (2016) A preoperative risk-scoring system to predict lymph node metastasis in endometrial cancer and stratify patients for lymphadenectomy. Gynecol Oncol 142:273–277
Holloway RW, Gupta S, Stavitzski NM et al (2016) Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol Oncol 141:206–210
Todo Y, Watari H, Kang S, Sakuragi N (2014) Tailoring lymphadenectomy according to the risk of lymph node metastasis in endometrial cancer. J Obstet Gynaecol Res 40:317–321
Phelippeau J, Canlorbe G, Bendifallah S et al (2016) Preoperative diagnosis of tumor grade and type in endometrial cancer by pipelle sampling and hysteroscopy: results of a French study. Surg Oncol 25:370–377
Batista TP, Cavalcanti CL, Tejo AA, Bezerra AL (2016) Accuracy of preoperative endometrial sampling diagnosis for predicting the final pathology grading in uterine endometrioid carcinoma. Eur J Surg Oncol 42:1367–1371
Williams AR, Brechin S, Porter AJ, Warner P, Critchley HO (2008) Factors affecting adequacy of Pipelle and Tao Brush endometrial sampling. BJOG 115:1028–1036
Dijkhuizen FP, Mol BW, Brolmann HA, Heintz AP (2000) The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer 89:1765–1772
Kishimoto K, Tajima S, Maeda I et al (2016) Endometrial cancer: correlation of apparent diffusion coefficient (ADC) with tumor cellularity and tumor grade. Acta Radiol 57:1021–1028
Woo S, Cho JY, Kim SY, Kim SH (2014) Histogram analysis of apparent diffusion coefficient map of diffusion-weighted MRI in endometrial cancer: a preliminary correlation study with histological grade. Acta Radiol 55:1270–1277
Rechichi G, Galimberti S, Signorelli M et al (2011) Endometrial cancer: correlation of apparent diffusion coefficient with tumor grade, depth of myometrial invasion, and presence of lymph node metastases. AJR Am J Roentgenol 197:256-62
Bharwani N, Miquel ME, Sahdev A et al (2011) Diffusion-weighted imaging in the assessment of tumour grade in endometrial cancer. Br J Radiol 84:997–1004
Takahashi M, Kozawa E, Tanisaka M, Hasegawa K, Yasuda M, Sakai F (2016) Utility of histogram analysis of apparent diffusion coefficient maps obtained using 3.0T MRI for distinguishing uterine carcinosarcoma from endometrial carcinoma. J Magn Reson Imaging 43:1301–1307
Mainenti PP, Pizzuti LM, Segreto S et al (2016) Diffusion volume (DV) measurement in endometrial and cervical cancer: a new MRI parameter in the evaluation of the tumor grading and the risk classification. Eur J Radiol 85:113–124
Inoue C, Fujii S, Kaneda S et al (2014) Apparent diffusion coefficient (ADC) measurement in endometrial carcinoma: effect of region of interest methods on ADC values. J Magn Reson Imaging 40:157–161
Fujii S, Matsusue E, Kigawa J et al (2008) Diagnostic accuracy of the apparent diffusion coefficient in differentiating benign from malignant uterine endometrial cavity lesions: initial results. Eur Radiol 18:384–389
Tamai K, Koyama T, Saga T et al (2007) Diffusion-weighted MR imaging of uterine endometrial cancer. J Magn Reson Imaging 26:682–687
Bakir VL, Bakir B, Sanli S et al (2017) Role of diffusion-weighted MRI in the differential diagnosis of endometrioid and non-endometrioid cancer of the uterus. Acta Radiol 58:758–767
Husby JA, Salvesen OO, Magnussen IJ et al (2015) Tumour apparent diffusion coefficient is associated with depth of myometrial invasion and is negatively correlated to tumour volume in endometrial carcinomas. Clin Radiol 70:487–494
Kierans AS, Doshi AM, Dunst D, Popiolek D, Blank SV, Rosenkrantz AB (2016) Retrospective assessment of histogram-based diffusion metrics for differentiating benign and malignant endometrial lesions. J Comput Assist Tomogr 40:723–729
Kilickesmez O, Bayramoglu S, Inci E, Cimilli T, Kayhan A (2009) Quantitative diffusion-weighted magnetic resonance imaging of normal and diseased uterine zones. Acta Radiol 50:340–347
Nakamura K, Imafuku N, Nishida T et al (2012) Measurement of the minimum apparent diffusion coefficient (ADCmin) of the primary tumor and CA125 are predictive of disease recurrence for patients with endometrial cancer. Gynecol Oncol 124:335–339
Wang J, Yu T, Bai R, Sun H, Zhao X, Li Y (2010) The value of the apparent diffusion coefficient in differentiating stage IA endometrial carcinoma from normal endometrium and benign diseases of the endometrium: initial study at 3-T magnetic resonance scanner. J Comput Assist Tomogr 34:332–337
Bhosale P, Ramalingam P, Ma J et al (2017) Can reduced field-of-view diffusion sequence help assess microsatellite instability in FIGO stage 1 endometrial cancer? J Magn Reson Imaging 45:1216–1224
Atri M, Zhang Z, Dehdashti F et al (2017) Utility of PET/CT to evaluate retroperitoneal lymph node metastasis in high-risk endometrial cancer: results of ACRIN 6671/GOG 0233 trial. Radiology 283:450–459
Park JY, Lee JJ, Choi HJ et al (2017) The value of preoperative positron emission tomography/computed tomography in node-negative endometrial cancer on magnetic resonance imaging. Ann Surg Oncol 24:2303–2310
Koh WJ, Abu-Rustum NR, Bean S, et al (2018) Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16:170–199
Ueno Y, Forghani B, Forghani R et al (2017) Endometrial carcinoma: MR imaging-based texture model for preoperative risk stratification-a preliminary analysis. Radiology 284:748–757
Todo Y, Watari H, Okamoto K et al (2013) Tumor volume successively reflects the state of disease progression in endometrial cancer. Gynecol Oncol 129:472–477
MacKay HJ, Levine DA, Bae-Jump VL et al (2017) Moving forward with actionable therapeutic targets and opportunities in endometrial cancer: NCI clinical trials planning meeting report on identifying key genes and molecular pathways for targeted endometrial cancer trials. Oncotarget 8:84579–84594
Suhaimi SS, Ab Mutalib NS, Jamal R (2016) Understanding molecular landscape of endometrial cancer through next generation sequencing: what we have learned so far? Front Pharmacol 7:409
Werner HM, Salvesen HB (2014) Current status of molecular biomarkers in endometrial cancer. Curr Oncol Rep 16:403
Tsikouras P, Bouchlariotou S, Vrachnis N et al (2013) Endometrial cancer: molecular and therapeutic aspects. Eur J Obstet Gynecol Reprod Biol 169:1–9
Acknowledgements
The committee would like to thank Mr Arnold Stipsits, ESUR secretary, for his help with co-ordination of the committee members. The authors would like to acknowledge the contribution of information specialist Dr. Martina Gosteli (Main library - Medicine Careum, University of Zurich) for her valuable assistance with the database searches. We would like to thank the following colleagues: Henrik Leonhardt, Celine Alt, Federico Collettini, Laure Fournier, Diomidis Botsikas, Gavin Stewart, Athina C. Tsili, Theresa Mokry, Riccardo Manfredi, Laura Bunesch Villalba, Sonya Snape, Hebert Alberto Vargas, Maryna Brochwicz-Lewinski, M. Weston, Suzan M. Goldman and Sara Belião for participating to the questionnaire on behalf of the ESUR women’s imaging group. Authors thank Joanne Chin M.F.A. for her editorial assistance with the manuscript.
Funding
Yulia Lakhman's contribution to this work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Rosemarie Forstner
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because no patient data were used.
Ethical approval
Institutional review board approval was not required because no patient data were used.
Methodology
• multicentre study
Electronic supplementary material
ESM 1
(DOCX 183 kb)
Rights and permissions
About this article
Cite this article
Nougaret, S., Horta, M., Sala, E. et al. Endometrial Cancer MRI staging: Updated Guidelines of the European Society of Urogenital Radiology. Eur Radiol 29, 792–805 (2019). https://doi.org/10.1007/s00330-018-5515-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5515-y