Abstract
Purpose
The purpose of this study was to determine whether the measured size of active gastrointestinal hemorrhage was useful in predicting subsequent positive findings at catheter angiography.
Materials and methods
This was a single-institution retrospective study consisting of 32 patients with computed tomography angiography (CTA) positive for gastrointestinal bleeding who went on to receive catheter angiography. Each CTA was reviewed, with axial measurements of the anterior–posterior and transverse dimensions of the largest foci of hemorrhage recorded. Volumetric analysis was used to measure the volume of hemorrhage. These measurements were performed for both the arterial and portal venous phases. Additionally, the interval growth between the arterial and portal venous phase was also calculated.
Results
There was a statistically significant difference in the absolute size of the maximum transverse dimension on portal venous phase imaging (mean = 19.8 mm, p < 0.001), as well as an interval increase in transverse (mean = 8.5 mm, p < 0.001) and anteriorposterior (mean = 5.4 mm, p = 0.027) size between arterial and portal venous phases in patients with positive catheter angiography versus negative catheter angiography. There was a statistically significant difference in the volume of hemorrhage on arterial (mean = 1.72 cm3, p = 0.020) and portal venous phases (mean = 5.89 cm3, p = 0.016), as well as an interval change in the size of hemorrhage between the two phases (mean = 4.17 cm3, p = 0.020) in patients with positive catheter angiography versus patients in the negative catheter angiography group.
Conclusions
The absolute axial size and volume of hemorrhage, as well as the interval change between the arterial and portal venous phases of CTA imaging is predictive of subsequent positive catheter angiography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Catheter angiography is the mainstay of image-guided treatment for acute lower gastrointestinal (GI) bleeding. Owing to the technical advancements in multidetector computed tomography (MDCT) and its superior spatial resolution for localizing hemorrhage, catheter angiography has been utilized to evaluate patients with lower GI bleeding [1,2,3,4,5,6]. Prior diagnostic paradigms have focused on binary analysis of hemorrhage versus no hemorrhage as well as localizing that hemorrhage [7,8,9]. Given that computed tomography angiography (CTA) has a higher sensitivity than catheter angiography, many hemorrhages detected by CTA will be occult on catheter angiography [10, 11].
In the trauma literature, smaller CTA size of pelvic arterial extravasation predicts which patients are more likely to be successfully managed conservatively (without the use of catheter angiography) [12]. In the neuroimaging literature, interval increase in the CTA size of acute intracranial hemorrhage between two phases using a single contrast bolus, multi-phase acquisition technique also predicts subsequent hematoma expansion and in-hospital mortality [13]. Therefore, identifying which patients are most likely to benefit from catheter angiography based on the size of lower GI hemorrhage seen on CTA could be advantageous for pre-procedure work-up, triaging, and predicting outcomes. The purpose of this study was to determine whether the measured size of the active hemorrhage was useful in predicting subsequent positive findings at catheter angiography.
Materials and methods
Patient selection
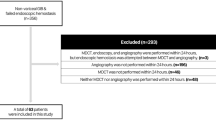
This was a HIPAA-compliant, IRB-approved single-institution retrospective study; informed consent was waived. The initial study population consisted of 40 consecutive patients over a 3-year period who presented to the emergency department with lower GI bleeding, who were found to have active arterial contrast extravasation on CTA done per protocol for initial imaging work-up. All patients found to have active arterial contrast extravasation subsequently went on to receive catheter angiography. Eight patients were excluded due to technical limitations of the diagnostic study that precluded the use of volume analysis software (incompatibility of raw data with volume analysis software). This yielded an effective study population of 32 patients.
Seventeen patients had positive findings at catheter angiography consisting of active arterial extravasation. Fifteen patients had negative findings at catheter angiography defined as no active arterial extravasation arising from branches of the celiac, superior mesenteric, and inferior mesenteric arteries. Additional patient characteristics were gathered including gender, age, anticoagulation, time to catheter angiography after CTA diagnosis, blood pressure, heart rate, clinical concern for repeat hemorrhage after catheter angiography, and need for repeat CTA or lower endoscopy after catheter angiography. In addition, we collected data on creatinine prior to CTA, following CTA, and following catheter angiography to determine the risk of contrast-induced nephropathy. Anatomic vascular territory of hemorrhage was also recorded.
Data acquisition
The CTA protocol included a non-contrast phase, an arterial phase to detect extravasation, and a portal venous phase to evaluate for bleeding expansion. The index CTA for each of these patients was analyzed and the axial slice with the largest focus of hemorrhage was selected. The maximum transverse and anterior–posterior dimensions of the focus of extravasation was recorded for both the arterial and portal venous phases of the exam. Subsequently, a calculation was performed to measure the difference between these measurements in the arterial and portal venous phases.
Next, the index CTA was input into dedicated volumetric software to calculate the three-dimensional volume of hemorrhage. Similarly, the volume of hemorrhage of the focus of hemorrhage was calculated and recorded. This was performed for both the arterial and portal venous phases of the exam. Subsequently, a calculation was performed to measure the difference between these measurements in the arterial and portal venous phases.
Technical parameters of image acquisition and analysis
All CTAs were performed using 64 MDCT scanners (Lightspeed VCT, GE Medical Systems, Milwaukee, WI). The following parameters were used: reconstruction thickness, 1.25 and 3.75 mm; 120 kVp; noise index, 23 (automatic dose modulation); pitch, 1:0.984; and gantry rotation time, 0.5 s. As per protocol, an initial non-contrast phase was acquired from the diaphragm to the greater trochanters. Subsequently, a timing bolus using 20 mL of intravenous contrast (ioversol, Optiray 350 mg/mL Mallinckrodt Inc., Hazelwood, MO) was injected at 4 mL/sec and with a 10 s scan delay. A timing bolus was used rather than an aortic enhancement trigger as part of the investigating institution’s diagnostic radiology protocol, which was not altered for the purposes of this study. The second acquisition was obtained after 80 ml contrast injection at 4 ml/s at an interval determined by the timing bolus peak plus 15 s. Finally, a portal venous phase acquisition was obtained at 40 s following the arterial phase acquisition. Axial 1.25 mm and 3.75 mm reconstructions, along with reconstructed multi-planar reformations (coronal and sagittal, 2.5 × 2.5 mm, reconstruction thickness x interval) were available on the picture archiving and communication system (PACS) workstations. Additional post-processing options were readily available using the PACS-integrated Advantage Windows suite (General Electric Medical Systems, Milwauke, WI).
Volumetric analysis was performed via a semi-automatic segmentation method using dedicated software (Fig. 1): MIM Vista (MIM Software Inc, Cleveland, OH). The PET edge function was used for automated measurement and calculation of hemorrhage size. All diagnostic imaging studies were interpreted by fellowship-trained diagnostic radiologists with varying levels of post-fellowship experience.
All catheter angiograms were performed in one of the two angiography rooms (Allura Xper FD20, Philips) by one of the four fellowship-trained interventional radiologists. When possible, all patients had celiac, superior mesenteric, and inferior mesenteric angiography performed as well as selective catheterization of vessels which were identified or suspected based on the CTA. All catheter angiography were performed via common femoral artery access using 6 French sheath and 5 French base catheters (which itself varied based on operator preference). Further sub-selective angiography based on the results of the CTA and findings of initial mesenteric angiograms was at the discretion of the operating Interventional Radiologist. All sub-selective angiography was performed using microcatheter systems, with specific microcatheter and wire selection based on individual operator preference. Contrast agent type, total contrast dose, injection rate, and use of a power injector were determined by the individual operator based on patient characteristics such as size of vessels, age, and pre-procedure glomerular filtration rate.
Statistical analysis
In comparing patient characteristics, T tests were performed for continuous variables such as heart rate, blood pressure, and creatinine, whereas χ2 tests were performed for categorical variables such as gender and repeat hemorrhage rates.
T tests were performed to compare the size of hemorrhage between the positive catheter angiography group and the negative catheter angiography group:
Axial measurements
Maximum axial transverse size of hemorrhage (arterial and portal venous phase)
Maximum axial anterior–posterior size of hemorrhage (arterial and portal venous phase)
Growth of transverse size of hemorrhage (arterial to portal venous phase)
Growth of anterior–posterior size of hemorrhage (arterial to portal venous phase)
Volume measurements
Volume of hemorrhage (arterial phase)
Volume of hemorrhage (portal venous phase)
Growth of volume of hemorrhage (arterial to portal venous phase)
Differences in the measurements of the anterior–posterior and transverse dimensions on both these phases, as well as the interval increase between these two phases were compared between these two groups using a p value of 0.05.
Results
Patient characteristics and clinical course
There were no significant differences in the baseline characteristics of the two patient groups or their subsequent clinical course, as summarized in Table 1. Most notably, there was no significant difference in the clinical suspicion of repeat hemorrhage (i.e., continued episodes of bloody bowel movements, drop in hematocrit, or changes in vitals), repeat imaging, or use of subsequent endoscopy. Anatomic sites of hemorrhage found at catheter angiography are listed in Table 2.
Three patients in the positive catheter angiography group had colonoscopy before catheter angiography, all of which were negative. Four patients in the negative catheter angiography group had colonoscopy before catheter angiography, all of which were negative. One patient in the negative catheter angiography group underwent capsule endoscopy which was positive for hemorrhage, but which failed to identify a site of bleeding.
Of the patients with positive catheter angiography, fourteen patients underwent successful coil embolization. One patient underwent partial coil embolization as it was determined that further embolization would result in severe bowel ischemia or infarction. A second patient was determined to have an arteriovenous malformation that was too distal to embolize. Both these patients were treated by colonoscopy with clipping resulting in resolution of bleeding. All remaining endoscopic/colonoscopic interventions were non-diagnostic. One patient could not undergo embolization due to the failure of super-selective access given its proximity to a larger vessel and bleeding was spontaneously self-resolved.
Of the patients with negative catheter angiography, none had repeat catheter angiography that demonstrated recurrence of hemorrhage. Six patients with negative catheter angiography later underwent lower endoscopy, all of which were negative. One of these patients was diagnosed with hereditary hemorrhagic telangiectasia and an arteriovenous malformation was discovered on upper endoscopy.
There was no significant difference in creatinine prior to CTA and following CTA in the two patient groups. There was no significant difference in creatinine prior to CTA and following catheter angiography in the two patient groups. Results are summarized in Table 3.
Axial measurements
The average maximum transverse size of hemorrhage on the arterial phase was 11.2 mm (range 1–22 mm) for the positive catheter angiography group as compared to 7.7 mm (range 3–17 mm) for the negative catheter angiography group. These were not statistically significantly different (p = 0.067).
The average maximum anterior–posterior size of hemorrhage on the arterial phase was 8.6 mm (range 1–26 mm) for the positive catheter angiography group as compared to 7.3 mm (range 1–22 mm) for the negative catheter angiography group. These were not statistically significantly different (p = 0.263).
The average maximum transverse size of hemorrhage on the portal venous phase was 19.8 mm (range 7–38 mm) for the positive catheter angiography group as compared to 8.8 mm (range 3–24 mm) for the negative catheter angiography group. This was statistically significantly different (p < 0.001).
The average maximum anterior–posterior size of hemorrhage on the portal venous phase was 14.0 mm (range 2–64 mm) for the positive catheter angiography group as compared to 8.0 mm (range 1–34 mm) for the negative catheter angiography group. These were not statistically significantly different (p = 0.057).
The average increase in maximum transverse size of hemorrhage between the arterial and portal venous phases was 8.5 mm (range 0–24 mm) for the positive catheter angiography group as compared to 1.2 mm (range 0–9 mm) for the negative angiography group. This was statistically significantly different (p < 0.001).
The average increase in maximum anterior–posterior size of hemorrhage between the arterial and portal venous phases was 5.4 mm (range 0–38 mm) for the positive catheter angiography group as compared to 0.5 mm (range 0–12 mm) for the negative angiography group. This was statistically significantly different (p = 0.027).
These findings are summarized in Table 4 with representative cases of patients presenting with gastrointestinal bleeding (Figs. 2, 3, 4).
Volume measurements
The average volume of hemorrhage on the arterial phase was 1.72 cm3 (range 0.06–8.01 cm3) for the positive catheter angiography group as compared to 0.37 cm3 (range 0.06–0.93 cm3) for the negative catheter angiography group. This was statistically significantly different (p = 0.020).
The average volume of hemorrhage on the portal venous phase was 5.89 cm3 (range 0.06–25.82 cm3) for the positive catheter angiography group as compared to 1.00 cm3 (range 0.06–6.93 cm3) for the negative catheter angiography group. This was statistically significantly different (p = 0.016).
The average increase in the volume of hemorrhage between the arterial and portal phases was 4.17 cm3 (range 0.01–21.43 cm3) for the positive catheter angiography group as compared to 0.63 cm3 (range 0–1.62 cm3) for the negative catheter angiography group. This was statistically significantly different (p = 0.020).
Discussion
These results show that larger absolute volume of hemorrhage as well as the interval change in that volume between the arterial and portal venous phases are correlated with positive findings at catheter angiography. Furthermore, the maximum transverse dimension of the focus of hemorrhage seen on an axial slice in the portal venous phase is a practical at-the-workstation marker which correlates with positive findings at catheter angiography.
The finding that this difference was only apparent in the portal venous phase (rather than the arterial phase) is possibly due to the fact that the growth of the hemorrhage over time is a better predictor of positive catheter angiography than the initial arterial size of the hemorrhage. The finding that the other dimensions of hemorrhage, notably in the anterior–posterior dimension, do not correlate with positive findings at catheter angiography likely owes to the effects of gravity in patients evaluated with CTA. The authors suspect that a patient scanned in the supine position will pool blood preferentially in the transverse (dependent) direction rather than anterior–posterior dimension.
The diagnostic data provided the time interval between the arterial and portal venous phases, which affords crucial information about the rate of hemorrhage. This is a distinct advantage of the single-bolus technique used to evaluate hemorrhage where the patient receives a single bolus of contrast and is scanned at multiple points in time.
These results show that objective imaging characteristics can be used to predict likelihood of positive catheter angiography. These results do not suggest that catheter angiography is unnecessary for smaller size hemorrhage, but instead assist in triage and risk stratification. Nonetheless, while there remains a role for prophylactic embolization in patients with lower GI hemorrhage regardless of positive findings at catheter angiography, improving patient selection by better understanding which candidates stand to benefit the most from catheter intervention can only help to minimize risk and maximize diagnostic and therapeutic yield.
A major limitation of this study is that there was no clear threshold size for hemorrhage below which the hemorrhage could not be identified at catheter angiography. This could be due to individual patient factors affecting the rate of hemorrhage, image quality, as well as change in the rate of hemorrhage between CTA and catheter angiography. Future controlled studies with higher power may eventually identify a threshold at which small hemorrhages need not receive catheter angiography and instead be managed conservatively. Another major limitation of this study is the heterogeneity among individual operators. To name a few examples, contrast dose, injection rate, use of power injector, degree of angiographic subselection, and selection of catheters/wires were all based on operator preference. Furthermore, it is possible that the exhaustiveness of the search for an active site of hemorrhage may have been influenced by patient factors such as hemodynamic status, clinical condition, and human factors such as patient tolerance of the procedure and operator fatigue.
Contrast-induced nephropathy (CIN) is important to consider in the older patient population, especially in patients subject to repeated studies using iodinated contrast. The data on CIN specifically associated with evaluating gastrointestinal bleeding is limited. Few studies have produced mixed results: A systematic review of CIN in the setting of acute stroke, which found that CTA did not significantly increase the risk of acute kidney injury, even in patients with chronic kidney disease [14]. A meta-analysis of patients with elevated myocardial infarction undergoing percutaneous coronary intervention demonstrated a pooled CIN incidence of 13.3% and a close association with hypertension and diabetes [15]. In our patient population, there was no significant difference in creatinine in either groups when comparing values measured before CTA, after CTA, and after angiography. Furthermore, while each patient should be considered on a case-by-case basis, the urgency of the gastrointestinal bleed outweighed the risk of CIN in our sample. Our evaluation of CIN in patients with gastrointestinal bleeding is limited, and a dedicated study with larger sample size and more stringent criteria would be beneficial.
Finally, there was a notable difference in the time to catheter angiography after CTA (2:52 versus 9:07). While this difference did not reach statistical significance (p = 0.08), this could have been due to a type 2 error owing to the low power resulting from the small sample size. However, upon review of the raw data, this was more likely due to the two outliers in the negative angiography group. The two longest intervals between CTA and catheter angiography in this group were 55 and 22 h; in both cases, clinical improvement in the form of hemodynamic status, response to transfusion, and clinical decrease in hemorrhage resulted in deferral of catheter angiography until a later time when it was determined that the patient could no longer be medically managed. Catheter angiography was performed within 5 h of positive CTA in 93% of the remainder of cases.
In an evolving field where CTA benefits not only catheter angiography, but also endoscopists, further extrapolating the characteristics of CTA studies is even more important for the multidisciplinary approach to care for these critically ill patients [16, 17].
Conclusion
The absolute axial size and volume of hemorrhage, as well as the interval change between the arterial and portal venous phases of CTA imaging is predictive of subsequent positive catheter angiography.
References
Scheffel H, Pfammatter T, Wildi S, Bauerfeind P, Marincek B, Alkadhi H. Acute gastrointestinal bleeding: detection of source and etiology with multi-detector-row CT. Eur Radiol. 2007;17(6):1555-65.
Yoon W, Jeong YY, Shin SS, et al. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006;239(1):160-7
Ernst O, Bulois P, Saint-drenant S, Leroy C, Paris JC, Sergent G. Helical CT in acute lower gastrointestinal bleeding. Eur Radiol. 2003;13(1):114-7
Tew K, Davies RP, Jadun CK, Kew J. MDCT of acute lower gastrointestinal bleeding. AJR Am J Roentgenol. 2004;182(2):427-30
Martí M, Artigas JM, Garzón G, Alvarez-sala R, Soto JA. Acute lower intestinal bleeding: feasibility and diagnostic performance of CT angiography. Radiology. 2012;262(1):109-16
García-blázquez V, Vicente-bártulos A, Olavarria-delgado A, et al. Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: systematic review and meta-analysis. Eur Radiol. 2013;23(5):1181-90
Jacovides CL, Nadolski G, Allen SR, Martin ND, Holena DN, Reilly PM, Trerotola S, Braslow BM, Kaplan LJ, Pascual JL. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization.
Shukla PA, Zybulewski A, Kolber MK, Berkowitz E, Silberzweig J, Hayim M. No catheter angiography is needed in patients with an obscure acute gastrointestinal bleed and negative CTA. Clin Imaging. 2017;43:106-109
He B, Yang J, Xiao J, et al. Diagnosis of lower gastrointestinal bleeding by multi-slice CT angiography: A meta-analysis. Eur J Radiol. 2017;93:40-45
Kuhle WG, Sheiman RG. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology. 2003;228(3):743-52
Wildgruber M, Wrede CE, Zorger N, et al. Computed tomography versus digital subtraction angiography for the diagnosis of obscure gastrointestinal bleeding. Eur J Radiol. 2017;88:8-14
Anderson SW, Soto JA, Lucey BC, Burke PA, Hirsch EF, Rhea JT. Blunt trauma: feasibility and clinical utility of pelvic CT angiography performed with 64-detector row CT. Radiology. 2008;246(2):410-9
Wu TC, Chen TY, Shiue YL, et al. Added value of delayed computed tomography angiography in primary intracranial hemorrhage and hematoma size for predicting spot sign. Acta Radiol. 2018;59(4):485-490
Brinjikji W, Demchuck AM, et al. Neurons over nephrons: systematic review and meta-analysis of contrast-induced nephropathy in patients with acute stroke. Stroke. 2017 Jul; 48(7): 1862-68
He H, Chen XR, et al. Elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI): a meta-analysis. Journal of Interventional Cardiology. 2019 Aug 25: 2750173
Oakland K, Isherwood J, et al. Diagnostic and therapeutic tyreatment modalities for acute lower gastrointestinal bleeding: a systematic review. Endoscopy International Open. 2017 Oct; 5(10): 959-73
Nagata N, Niikura R, Aoki T et al. Role of urgent contrast-enhanced multidetector computed tomography for acute lower gastrointestinal bleeding in patients undergoing early colonoscopy. J Gastroenterol. 2015;50:1162–1172
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no relevant relationships.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hsu, M., Shah, N., Bernal-Fernandez, M. et al. CTA measurements of acute lower gastrointestinal bleeding size predict subsequent positive catheter angiography. Abdom Radiol 45, 615–622 (2020). https://doi.org/10.1007/s00261-019-02386-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02386-5