Abstract
Background
The clinical significance of performing computed tomography (CT) for acute lower gastrointestinal bleeding (LGIB) remains unknown. This study aimed to evaluate the role of urgent CT in acute LGIB settings.
Methods
The cohort comprised 223 patients emergently hospitalized for LGIB who underwent early colonoscopy within 24 h of arriving at the hospital, including 126 who underwent CT within 3 h of arrival. We compared the bleeding source rate between two strategies: early colonoscopy following urgent CT or early colonoscopy alone.
Results
No significant differences in age, sex, comorbidities, vital signs, or laboratory data were observed between the strategies. The detection rate was higher with colonoscopy following CT for vascular lesions (35.7 vs. 20.6 %, p = 0.01), leading to more endoscopic therapies (34.9 vs. 13.4 %, p < 0.01). Of the 126 who underwent colonoscopy following CT, 26 (20.6 %) had extravasation and 34 (27.0 %) had nonvascular findings. The sensitivity and specificity of CT extravasation and nonvascular findings for predicting vascular lesions and inflammation or tumors were 37.8 and 88.9 and 81.3 and 80.9 %, respectively. A high κ agreement (0.83, p < 0.01) for active bleeding locations was found between CT and subsequent colonoscopy. There were no cases of contrast-induced nephropathy after 1 week of CT.
Conclusions
Urgent CT before colonoscopy had about 15 % additional value for detecting vascular lesion compared to colonoscopy alone and thus enabled subsequent endoscopic therapies. Contrast-enhanced CT in acute LGIB settings was safe and correctly identified the presence and location of active bleeding, as well as severe inflammation or tumor stenosis, facilitating decision making.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the diagnostic strategies for the initial management of overt lower gastrointestinal bleeding (LGIB), colonoscopy is optimal for confirming the source of bleeding [1, 2]. In particular, early colonoscopy within 24 h of admission, rather than elective colonoscopy, leads to better diagnostic and therapeutic opportunities, which decreases the need for surgical intervention, rate of rebleeding, and length of hospital stay [3, 4]. However, compared with radiographic interventions, early colonoscopy requires more colon preparation and experienced staff, as well as access to endoscopy facilities [1].
Contrast-enhanced multidetector computed tomography (MDCT), on the other hand, which is readily available in many hospitals [5], has several advantages: it can be rapidly performed, it does not require bowel preparation, and it has the potential to identify bleeding sources to guide optimal interventions. MDCT may also provide information on the underlying etiologies of vascular and nonvascular diseases such as inflammatory or neoplastic lesions [6]. Thus, MDCT is helpful for determining appropriate therapeutic approaches while also reducing the rate of unnecessary examinations. Data on the accuracy of MDCT in cases of LGIB are available from small case series studies (n < 52) [7–9], but whether or not incorporating MDCT will increase the rate of detecting bleeding sources and improve subsequent clinical outcomes remains unknown. In addition, prior studies on the gold standard diagnosis have produced unclear findings or were based on elective colonoscopy without preparation [7–9], which can influence the detection rate of bleeding sources on colonoscopy.
Against this background, we conducted a comparative study of a relatively large cohort of patients hospitalized for acute LGIB, who underwent one of two strategies: early colonoscopy following urgent MDCT or early colonoscopy alone. The specific objectives of this study were (1) to determine the added value of urgent MDCT in the workup of patients with LGIB, (2) to identify the accuracy of MDCT findings for predicting colonoscopic diagnosis, and (3) to determine the short-term safety of urgent MDCT.
Materials and methods
Study design, setting, and participants
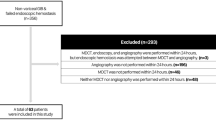
This study was approved by the institutional review board (no. 1579) of the National Center for Global Health and Medicine (NCGM). We retrospectively studied 404 patients emergently admitted for acute, continuous, or frequent overt LGIB who underwent colonoscopy at NCGM between August 2008 and December 2013 (Fig. 1). All patients had outpatient onset LGIB. The NCGM has 900 beds and is one of the largest emergency hospitals in the Tokyo metropolitan area. After excluding patients with upper gastrointestinal bleeding (n = 3) identified on upper endoscopy after colonoscopy, those who had undergone colonoscopy with over 24 h of hospitalization (n = 140), those who had undergone MDCT without contrast (n = 16), those with a history of colonic resection (n = 9), and those who had undergone barium impaction therapy (n = 27) in a randomized controlled trial (approval no. 765), the remaining 223 patients were included in the analysis (Fig. 1).
Clinical factors
Past history, comorbidities, medication use, physical examination findings, initial vital signs, and emergency room laboratory data were assessed. The use of nonsteroidal antiinflammatory drugs (NSAIDs), low-dose aspirin (81 mg buffered aspirin or 100 mg enteric-coated aspirin), non-aspirin antiplatelet drugs (clopidogrel, ticlopidine, cilostazol, and dipyridamole), or warfarin was defined as oral administration within 1 month before admission. Serum creatinine on the day of pre-contrast MDCT exposure and 1 week after MDCT was collected. Contrast-induced nephropathy was defined as an increase in creatinine ≥0.5 mg/dl before and after contrast medium exposure [10].
MDCT procedures
All MDCT scans were done at our center and could be performed within 3 h of arriving at the emergency room because the CT machine was situated in a medical examination room adjacent to the emergency department. A total of 90 ml iopamidol (Oypalomin 300; Konica Minolta, Tokyo, Japan) was power-injected intravenously. Patients were assessed with a 64-data acquisition system multidetector raw CT scanner (Aquilion CX; Toshiba Medical Systems, Japan) while in the supine position. All CT examinations were performed using helical scanning with the following parameters: 64 × 0.5 mm collimation, 120 kV, auto exposure control set with standard deviation 10; beam pitch 0.83 (table feed per rotation, 25.6 mm; collimation beam width, 32 mm), 0.5-s exposure time per rotation, 512 × 512 matrix, and 350–500-mm field of view. All images were reconstructed using a standard reconstruction algorithm. MDCT images were interpreted by two expert radiologists (≥15 years' experience).
Detection of the bleeding source on MDCT was defined as follows: (1) visualization of the extravasation of contrast medium into the intestinal wall (Fig. 2a, b), which is suggestive of vascular findings; (2) visualization of intestinal wall thickening (Fig. 2c), edema, mass lesion (Fig. 2d), or stenosis (Fig. 2d), which are suggestive of nonvascular findings.
Contrast-enhanced multidetector computed tomography (MDCT) and colonoscopy findings. a Vascular findings on MDCT showing extravasation and pooling of contrast medium into the rectum (axial image). A case of rectal ulcer. b Extravasation of contrast medium into the colonic lumen (coronal image). A case of diverticular bleeding. c Intestinal wall thickening and edema (coronal image). A case of ulcerative colitis. d Intestinal wall thickening, mass lesion, and stenosis (axial image). A case of colonic cancer. e Active bleeding in rectal ulcerous mucosa. f Visible vessel in the colonic diverticulum
Colonoscopy procedures
An electronic high-resolution video endoscope (model CFH260; Olympus Optical, Tokyo, Japan) was used to diagnose bleeding sources. Intestinal lavage for colonoscopy was performed using sodium picosulfate on the day before the assessment and 2 l polyethylene glycol solution on the day of assessment [11]. Patients who were unable to take an oral preparation were given an enema. If colonoscopy showed insufficient bowel preparation, we used a water-jet scope (Olympus Flushing Pump; Olympus Optical, Tokyo, Japan) because 2 l preparation may be insufficient for adequately clearing the bowel in the acute care setting [12]. Before colonoscopy, upper gastrointestinal sources of bleeding were ruled out in all patients by nasogastric lavage and/or on upper endoscopy. The timing of colonoscopy differed according to the attending physician’s decision or the time of visiting our institution. One physician may have performed early colonoscopy when the bleeding persisted, whereas another may have tended to wait and perform elective colonoscopy after spontaneous cessation of bleeding, especially when LGIB patients visited at night or on the weekend. However, the timing of colonoscopy is important because it can significantly influence the detection rate of bleeding sources on colonoscopy [12]. Therefore, we included patients who underwent early colonoscopy within 24 h of arriving at the hospital [13]. When the bleeding source could not be identified on colonoscopy, repeated upper endoscopy, capsule endoscopy, and/or double-balloon endoscopy were attempted whenever possible. The gold standard for detecting bleeding sources is based on colonoscopy and defined as follows. (1) Vascular lesions included active bleeding (Fig. 2e), an adherent clot, or visible vessel (Fig. 2f) [4, 14]. A vascular lesion was identified in diverticular bleeding, radiation telangiectasia, angioectasia, rectal ulcer, hemorrhoid, post-polypectomy/endoscopic mucosal resection, and small bowel bleeding. (2) A nonvascular lesion was defined as inflammation or tumor such as colitis, inflammatory bowel disease, and colorectal cancer. Patients in whom the bleeding source was identified received endoscopic treatment such as clipping or endoscopic ligation. At our institution, angiography or surgery is indicated for patients with persistent or severe bleeding who do not respond to endoscopic treatment.
The diagnostic criteria of diverticular bleeding are divided into those concerning definite and presumptive bleeding [4, 14]. A definite diagnosis is based on colonoscopic visualization of a colonic diverticulum with vascular lesions such as active bleeding, a visible vessel, or an adherent clot [4, 14]. On the other hand, a presumptive diagnosis is based on the following criteria: (1) fresh blood localized to the colonic diverticula in the presence of a potential bleeding source on total colonoscopy, (2) bright red blood in the rectum confirmed by objective color testing and colonoscopy demonstrating a single potential bleeding source in the colon complemented by negative upper endoscopy or negative capsule endoscopy, or (3) negative nasogastric lavage findings [4, 14].
Outcomes
Clinical outcomes were the detection rate of the bleeding source, need for endoscopic therapies, need for transfusion, and rebleeding. Units of packed red blood cells were evaluated after colonoscopy. During hospitalization, blood transfusion was indicated for patients when the hemoglobin level fell below 7.0 g/dl, or below 8.0 g/dl in those with unstable vital signs. Rebleeding was defined as significant fresh bloody or wine-colored stool accompanied by unstable vital signs, systolic blood pressure ≤90 mmHg or pulse ≥110 beats/min, and non-response to ≥2 units of transfused blood after colonoscopy [15]. In such cases, colonoscopy was performed in addition to anoscopy or MDCT to evaluate the bleeding source whenever possible.
Statistical analysis
We compared the baseline characteristics and clinical outcomes between two strategies: early colonoscopy following MDCT or early colonoscopy alone, using the Mann-Whitney U test or Pearson’s chi-squared test.
Of the 126 patients who underwent early colonoscopy following MDCT, we identified the accuracy of MDCT findings (extravasation or nonvascular findings) for predicting colonoscopic diagnosis (vascular lesion or inflammation or tumor). We calculated the sensitivity, specificity, and positive and negative likelihood ratios. The interobserver agreement for colonic location between MDCT and colonoscopy was measured using kappa statistics. In addition, interobserver agreement of CT extravasation between two experts was analyzed. κ > 0.80 denoted excellent agreement, >0.60–0.80 good, >0.40–0.60 moderate, >0.20–0.40 fair, and ≤0.20 poor [16].
To identify the predictors for extravasation identification on MDCT, we conducted multiple logistic regression analysis using the backward elimination method on factors found to be significant (p < 0.10) in univariate analysis, and we calculated the estimated odds ratios (OR) and 95 % confidence intervals (CI). Wilcoxon matched-pairs signed-ranks test was used to identify the change in creatinine levels before and after the MDCT. All statistical analysis was performed using Stata version 13 software (StataCorp, College Station, TX), and p < 0.05 was considered significant.
Results
Patients
The baseline characteristics of the 223 patients are shown in Table 1: 126 underwent early colonoscopy following urgent MDCT and 97 underwent early colonoscopy alone. No significant differences were observed in theage, sex, past history, all comorbidities, initial vital signs, laboratory data, enema, water-jet scope usage, or cecal intubation rate between the two strategies. The use of NSAIDs and non-aspirin antiplatelets as well as abdominal pain were significantly higher in the MDCT group. Bleeding sources of the lower GI tract are shown in Table 2.
Clinical outcomes between the two strategies
Clinical outcomes of the 223 subjects are shown in Table 3. Between the two strategies, colonoscopy following MDCT had a significantly (p = 0.014) higher vascular lesion detection rate. Vascular lesions in the ascending colon were more (p = 0.035) detectable than those in other colonic locations by colonoscopy following MDCT. The colonoscopy following MDCT group had a significantly (p < 0.001) higher rate of endoscopic therapy. No significant differences in rebleeding and transfusion need were observed between the two strategies.
Accuracy of MDCT findings for predicting colonoscopic vascular lesion
Interobserver agreement of extravasation findings between two experts was excellent (κ: 0.96, p < 0.001). Of the 126 patients, MDCT revealed 26 (20.6 %) patients with extravasation, and subsequent colonoscopy revealed 45 (35.7 %) patients with vascular lesion (Table 3). The sensitivity and specificity of extravasation findings on MDCT for detecting colonoscopic vascular lesions were 37.8 and 88.9 %, respectively (Table 4). The agreement rate and kappa of the 17 cases with both extravasation on MDCT and vascular lesions on colonoscopy were 88.2 % and 0.83 (p < 0.001), respectively.
In patients with diverticular bleeding (n = 77), extravasation on MDCT was identified in 24 (31.2 %) cases, and a vascular lesion on subsequent colonoscopy was identified in 35 (45.5 %) cases. The sensitivity and specificity of extravasation findings on MDCT for detecting colonoscopic vascular lesions were 42.9 and 78.6 %, respectively (Table 4).
Accuracy of MDCT findings for predicting colonoscopic inflammation or tumor (nonvascular lesion)
MDCT revealed 34 (27.0 %) patients with nonvascular findings, and subsequent colonoscopy revealed 16 (12.7 %) patients with nonvascular lesions (inflammation or tumor) (Table 3). The sensitivity and specificity of nonvascular findings on MDCT for detecting colonoscopic inflammation or tumor were 81.3 and 80.9 %, respectively (Table 4).
Predictors for extravasation identification on MDCT and its effect on clinical outcomes
Univariate analysis revealed that positive extravasation on MDCT was significantly associated with a history of diverticular bleeding (p < 0.001) and marginally associated with hypertension (p = 0.064), cardiovascular disease (p = 0.088), non-tender abdominal examination (p = 0.081), and low platelet levels (p = 0.087) (Table 5). Multivariate analysis shown that past history of diverticular bleeding (OR 6.3; 95 % CI 2.4–16.1; p < 0.001) and low platelet levels (OR 0.94; 95 % CI 0.88–1.01; p = 0.09) were associated with positive extravasation on MDCT.
Endoscopic therapy was conducted more frequently in patients with extravasation on MDCT than in those without (Table 5). There were no significant differences in rebleeding and transfusion need between the two groups.
Contrast-induced nephropathy in acute LGIB
Of the 126 patients who underwent MDCT, 6 with a mean creatinine level of 5.2 mg/dl had undergone hemodialysis after MDCT. The creatinine level before and 1 week after MDCT in the remaining 120 patients without hemodialysis is shown in Fig. 3. Of them, 35 (29.2 %) had a creatinine level >1 mg/dl before MDCT. There were no cases of contrast-induced nephropathy. A significant (p < 0.001) decrease was noted in the creatinine level before and after MDCT. One patient developed an increased level of creatinine after 10 days, not because of contrast enhancement, but due to rebleeding, frequent transfusions, and heart failure.
Discussion
We showed that early colonoscopy following MDCT was significantly more effective than early colonoscopy alone for detecting vascular lesions and therefore enables specific endoscopic treatment to be performed. In regard to diagnostic accuracy, MDCT had high specificity but low sensitivity for predicting vascular lesions and high sensitivity and specificity for predicting inflammation or tumor. Positive extravasation on MDCT was found to be associated with clinically suspected diverticular bleeding. Finally, in terms of the safety of MDCT, the possibility of exacerbating creatinine levels after contrast medium enhancement in MDCT was low.
Although no studies have compared the bleeding source rate and outcomes between two strategies (colonoscopy following CT or colonoscopy alone), several studies have indicated that radiographic interventions are useful for acute LGIB diagnosis. When Jensen et al. [17] evaluated 22 patients with severe bleeding on emergency colonoscopy and upper endoscopy, as well as emergency angiography, the diagnostic yield of colonoscopy was 82 % (vs. 12 % for angiography) [17]. In a retrospective study by Strate et al. [18], initial colonoscopy within 24 h of admission provided a diagnostic yield of 85 % (vs. 45 % for initial scintigraphy and angiography). Chau and Ridley [19] conducted a systematic review of eight studies comparing the use of CT angiography to standard endoscopy, angiography, and surgery and showed that CT angiography had a pooled sensitivity of 86 % and specificity of 95 %. They concluded that CT is an effective modality for revealing the precise location and etiology of bleeding. In a retrospective study by Amarteifio et al. [6], 55 % of acute GI bleeding cases, including tumors, were detected on multislice CT, and CT proved useful for detecting nonvascular lesions such as swelling of the intestinal wall, edema, and stenosis.
We consider CT useful for deciding whether or when colonoscopy should be performed before making a definite diagnosis of LGIB. Three points warrant mentioning with regard to performing additional CT prior to colonoscopy. First, our study revealed no CT findings in 66 patients in whom subsequent colonoscopy identified 38 as having no bleeding source (specificity, 57.6 %). These data are useful for physicians to determine whether colonoscopy should be performed, especially in patients who cannot undergo bowel preparation, and particularly in those with comorbidities, advanced age, or low performance status, as well as for institutions where early colonoscopy cannot be performed because of lack of sufficiently experienced staff or endoscopy facilities. Second, the vascular lesion detection rate on colonoscopy following CT was 1.7-fold higher than that on colonoscopy alone. In addition, a high kappa agreement (0.83) for active bleeding locations between the groups and the high specificity for predicting vascular lesions on colonoscopy mean that CT is useful for identifying bleeding sources in LGIB and can also be used as an indicator for subsequent endoscopic procedures, which is an additional benefit. These findings will be useful for physicians to determine whether patients with LGIB should undergo colonoscopy immediately or later. Third, CT had a high sensitivity (81.3 %) for inflammation and tumors due to inflammatory bowel disease, other colitis, or colorectal cancer, suggesting that CT can be used to predict severe inflammation or stenosis in LGIB patients.
Although there are differences in LGIB management between institutions, the consensus view seems to be to perform early colonoscopy or transcatheter arterial embolization (TAE) when extravasation is identified on MDCT in acute LGIB. When an experienced endoscopist and experienced medical staff are available, we propose early colonoscopy should be performed within 24 h of admission, because early colonoscopy can identify the bleeding site and help determine which of the various endoscopic therapies to perform, such as band ligation, endo-clip, and heater probe or argon plasma coagulation [1, 2]. Colonoscopy, instead of angiography, may be also indicated for patients with chronic kidney disease or bleeding tendencies, including antithrombotic users. However, colonoscopy requires colon preparation as well as available experienced staff and endoscopy facilities. Therefore, for patients who cannot undergo bowel preparation or are at high risk of aspiration, when TAE specialists are available, we recommend TAE as first-line treatment in acute LGIB. Several studies have shown that TAE or superselective embolization is a safe and effective treatment for acute LGIB, including diverticular bleeding, and it is likely to be more detectable and successful in performing TAE when extravasation is identified on MDCT [20–22].
Although we demonstrated that urgent CT before colonoscopy had about 15 % additional value for detecting vascular lesions and a higher rate of endoscopic therapy compared to colonoscopy alone, it did not improve rebleeding or transfusion need. Foutch et al. [23] showed that patients with vascular lesions and patients taking NSAIDs experienced a greater number of bleeding episodes and an increased need for transfusion. We suggest that a higher rate of NSAID use or vascular lesions in the CT group could have some effect on the rate of adverse outcomes in this study.
Because the low sensitivity of extravasation on CT was an issue, we explored which LGIB patients were indicated for urgent CT. We found that positive CT extravasation was associated with a history of diverticular bleeding, hypertension, coronary artery disease, non-tender abdomen, and low platelet level. Thus, our analysis indicated that urgent CT was most beneficial for patients with clinically suspected diverticular bleeding.
In the acute LGIB setting, we may hesitate to perform contrast-enhanced MDCT for fear of contrast-induced nephropathy. We found that the creatinine level in most patients (97 %) with a high level (>1 mg/dl) did not increase after contrast enhancement, and no patients developed contrast-induced nephropathy, suggesting the method is a safe modality for LGIB. However, as elderly patients with hypertension, pre-existing renal disease, anemia, and concomitant use of NSAIDs [10] have an increased risk of contrast-induced nephropathy, which are also risks for GI bleeding [24], caution should be exercised in such patients in the initial management of LGIB.
The strength of the present study is that all participants underwent early colonoscopy with full preparation and a water-jet scope, which maximized the detection rate of bleeding sources. Moreover, the relatively large number of cases of MDCT and early colonoscopy enabled us to analyze the accuracy of CT findings in comparison with previous studies. However, this study also has several limitations. First, the retrospective design included a selection bias, especially the indication for MDCT. Although the patient characteristics between the two strategies were similar in this study, a further prospective or randomized controlled study with investigators blinded to the MDCT findings is needed. Second, we did not collect information on stool color, stool frequency, and hours from onset of bleeding to presentation, which might be significant predictors of extravasation on MDCT because they are associated with severe LGIB [25]. Third, it is a reality in clinical practice that some patients with acute LGIB do have to wait before undergoing endoscopy or do not undergo endoscopy at all. However, it is unclear from our data whether this applies to patients with acute LGIB who do not undergo colonoscopy or who undergo elective colonoscopy.
In conclusion, urgent CT before colonoscopy had about 15 % additional value for detecting vascular lesions compared to colonoscopy alone and thus enabled subsequent endoscopic therapies. Contrast-enhanced CT in the acute LGIB setting was safe and correctly identified the presence and location of active bleeding as well as severe inflammation or tumor stenosis. These findings will facilitate decision making for acute LGIB.
References
Lhewa DY, Strate LL. Pros and cons of colonoscopy in management of acute lower gastrointestinal bleeding. World J Gastroenterol. 2012;18:1185–90.
ASGE Standards of Practice Committee, Pasha SF, Shergill A, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc. 2014;79:875–85.
Kaltenbach T, Watson R, Shah J, et al. Colonoscopy with clipping is useful in the diagnosis and treatment of diverticular bleeding. Clin Gastroenterol Hepatol. 2012;10:131–7.
Jensen DM, Machicado GA, Jutabha R, et al. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78–82.
Yamaguchi T, Yoshikawa K. Enhanced CT for initial localization of active lower gastrointestinal bleeding. Abdom Imaging. 2003;28:634–6.
Amarteifio E, Sohns C, Heuser M, et al. Detection of gastrointestinal bleeding by using multislice computed tomography—acute and chronic hemorrhages. Clin Imaging. 2008;32:1–5.
Jaeckle T, Stuber G, Hoffmann MH, et al. Detection and localization of acute upper and lower gastrointestinal (GI) bleeding with arterial phase multi-detector row helical CT. Eur Radiol. 2008;18:1406–13.
Yoon W, Jeong YY, Shin SS, et al. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006;239:160–7.
Obana T, Fujita N, Sugita R, et al. Prospective evaluation of contrast-enhanced computed tomography for the detection of colonic diverticular bleeding. Dig Dis Sci. 2013;58:1985–90.
Barrett BJ, Parfrey PS. Clinical practice. preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379–86.
Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. 2008;103:883–93.
Niikura R, Nagata N, Aoki T, et al. Predictors for identification of stigmata of recent hemorrhage on colonic diverticula in lower gastrointestinal bleeding. Gastroenterol: J Clin; 2014.
Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34:643–64.
Zuckerman GR, Prakash C. Acute lower intestinal bleeding. Part II: etiology, therapy, and outcomes. Gastrointest Endosc. 1999;49:228–38.
Green BT, Rockey DC, Portwood G, et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100:2395–402.
Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85:257–68.
Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia. the role of urgent colonoscopy after purge. Gastroenterology. 1988;95:1569–74.
Strate LL, Syngal S. Predictors of utilization of early colonoscopy vs. radiography for severe lower intestinal bleeding. Gastrointest Endosc. 2005;61:46–52.
Chua AE, Ridley LJ. Diagnostic accuracy of CT angiography in acute gastrointestinal bleeding. J Med Imaging Radiat Oncol. 2008;52:333–8.
Hur S, Jae HJ, Lee M, et al. Safety and efficacy of transcatheter arterial embolization for lower gastrointestinal bleeding: a single-center experience with 112 patients. J Vasc Interv Radiol. 2014;25:10–9.
Gady JS, Reynolds H, Blum A. Selective arterial embolization for control of lower gastrointestinal bleeding: recommendations for a clinical management pathway. Curr Surg. 2003;60:344–7.
Tan KK, Nallathamby V, Wong D, et al. Can superselective embolization be definitive for colonic diverticular hemorrhage? An institution’s experience over 9 years. J Gastrointest Surg. 2010;14:112–8.
Foutch PG. Diverticular bleeding: are nonsteroidal anti-inflammatory drugs risk factors for hemorrhage and can colonoscopy predict outcome for patients? Am J Gastroenterol. 1995;90:1779–84.
Nagata N, Niikura R, Aoki T, et al. Lower GI bleeding risk of nonsteroidal anti-inflammatory drugs and antiplatelet drug use alone and the effect of combined therapy. Endosc: Gastrointest; 2014.
Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch Intern Med. 2003;163:838–43.
Acknowledgments
We thank the clinical research coordinators Ms. Hisae Kawashiro, Sawako Iijima, Yoko Tanigawa, Aiko Gotannda, and Yaeko Sawada for help with data collection. This study was partly supported by a grant for research and development from the National Center for Global Health and Medicine (26A-201).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagata, N., Niikura, R., Aoki, T. et al. Role of urgent contrast-enhanced multidetector computed tomography for acute lower gastrointestinal bleeding in patients undergoing early colonoscopy. J Gastroenterol 50, 1162–1172 (2015). https://doi.org/10.1007/s00535-015-1069-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1069-9