Abstract
Purpose
The purpose of this study was to evaluate the utility of intravoxel incoherent motion diffusion-weighted imaging (IVIM DWI) parameters in identifying early renal function changes in diabetics.
Methods
A total of 40 patients with type 2 diabetes mellitus and 20 healthy control subjects underwent multiple b value DWI. The diabetic patients were stratified into two groups based on albuminuria category: NAU (normal to mildly increased albuminuria; ACR < 30 mg/g) and MAU (moderately increased albuminuria; 30 ≤ ACR < 300 mg/g). The mean cortical and medullary IVIM parameters (D, D*, f, and ADC) were calculated and compared among the different groups, and the correlation of ACR and eGFR was also calculated.
Results
The present study revealed the limited water molecule diffusion and hyperperfusion of renal cortex and medulla in diabetic patients before proteinuria detection. Mean cortical and medullary D values negatively correlated with the ACR values in diabetics with 30 ≤ ACR < 300 mg/g, whereas no correlation was found between ACR values and other IVIM parameters.
Conclusion
IVIM DWI might be helpful in noninvasively identifying early-stage DN. The IVIM parametric values are more sensitive than the ACR in detecting early-stage kidney changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Diabetic nephropathy (DN) is the leading cause of chronic renal disease [1]. It is also one of the most significant long-term complications in terms of morbidity and mortality for diabetic patients. Primary prevention of DN is feasible if it can be identified and treated in its early stage. Albuminuria has been used as the first clinical indicator of DN in real practice [2]. However, its accuracy, sensitivity, and specificity are still controversial [3,4,5]. Renal biopsy currently remains the diagnostic gold standard for DN. However, it is an invasive procedure and has to consider the observing and sampling errors [6]. Biopsy is rarely performed for ethical reasons either. Thus, developing a new noninvasive modality to allow an early identification of DN is a task of great importance for an efficacious management of DN.
DN is associated with structural and hemodynamic changes in its early stage [7]. However, functional damage often precedes anatomical abnormality. The intravoxel incoherent motion diffusion-weighted imaging (IVIM DWI) can separate the estimation of tissue pseudodiffusion and diffusivity of protons in abdominal organs [8]. Several studies have demonstrated the utility of IVIM DWI in many kidney diseases [9,10,11,12,13]. The IVIM parameter changes were assessed in the renal cortex and medulla with the progression of unilateral ureter obstruction (UUO) and correlated with the histopathological alterations using a well-established rat model of UUO [13]. IVIM DWI is believed to have a great potential in the noninvasive assessment of renal fibrosis induced by UUO. However, it is unclear whether IVIM parameters could identify the early presence of DN in diabetic patients and contribute to the early diagnosis of DN.
Therefore, the objective of this study was to explore the feasibility of IVIM parameters to identify early changes in renal diffusion and perfusion in diabetic patients.
Methods
Ethics statement
This study was approved by the ethics committee of our Hospital. Each subject provided written informed consent prior to participation. All methods were performed in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Subjects
A total of 44 patients with confirmed type 2 diabetes mellitus (T2DM) were enrolled from the in-patient department of endocrinology in the our Hospital between August 2014 and December 2016. Our volunteers were fasting for 4 h before the examination, and 3 h of relative water prohibition (no more than 300 ml of water was drinkable within 3 h of the test). The diagnosis of T2DM was based on the established criteria according to the American Diabetes Association recommendations [14]. Detailed information about family history, clinical symptoms, and complications was collected. Laboratory tests including urine albumin concentration, urine creatinine, and blood creatinine were performed. The ACR and eGFR were then calculated [15, 16]. The exclusion criteria were as follows: (1) primary hypertension before or when diagnosed with T2DM; (2) presence of other kidney diseases; (3) other severe systemic or organic diseases; (4) intolerance to magnetic resonance imaging (MRI) examination; and (5) age less than 20 years or more than 80 years. In addition to the aforementioned criteria, four patients were excluded from the data analysis because of multiple renal cysts (n = 2), excessive motion during MRI (n = 1), and massive hepatic cyst compressing the kidney (n = 1).
The 40 patients with T2DM were eventually divided into two groups based on albuminuria category [17]: the NAU group (normal to mildly increased albuminuria; ACR < 30 mg/g) and MAU group (moderately increased albuminuria; ACR = 30–300 mg/g). The NAU group consisted of 20 patients, 12 males and 8 females, aged 35–78 years, with a median age of 52.40 ± 8.73 years. The MAU group consisted of 20 patients, 7 males and 13 females, aged 29–78 years, with a median age of 53.65 ± 12.14 years.
A group of 20 sex- and age-matched individuals (6 men and 14 women; mean age, 54.3 ± 6.9 years; range 39–65 years) from the check-up center in our Hospital were enrolled as control subjects. The subjects had no renal or renal vascular diseases or hypertension. All controls had normal serum creatinine levels, and they showed no morphological abnormalities in both kidneys on conventional MRI.
MRI protocols
MRI examinations of both kidneys were performed on a 3.0-T MRI system (MR750, GE Healthcare, WI, USA) with an eight-channel body array coil. Conventional MRI and IVIM DWI with 11 b values were performed on the coronal plane.
Conventional MRI sequences included T1-weighted gradient echo (TR/TE, 3.7 ms/1.1 ms; matrix size, 256 × 200; field of view, 40 × 40 cm [2]; number of excitation, 0.69; slice thickness, 6.0 mm; gap, 3.0 mm), T2-weighted imaging with fat-saturated fast recovery fast spin echo (FRFSE/Prop) with respiratory triggering (TR/TE, 6000 ms/73.9 ms; matrix size, 320 × 320; field of view, 40 × 40 cm2; number of excitation, 2.5; slice thickness, 5 mm; gap, 1.0 mm).
The IVIM DWI was acquired using respiratory triggering spin echo–echo planar imaging with the following parameters: slice thickness, 5 mm; gap, 1.5 mm; TR/TE, 4247 ms/93 ms; matrix size, 128 × 160; field of view, 40 × 40 cm2; number of excitation, 1; and 11 b values (b = 0, 30, 50, 80, 100, 150, 200, 300, 500, 800, and 1000 s/mm2). The b values were chosen to cover both the molecular diffusion decay (b ≥ 200 s/mm2) and initial pseudodiffusion decay (b < 200 s/mm2). Total acquisition time was about 4 min and 40 s.
Data analysis
According to the IVIM theory, the parameters were calculated based on the following Eq. (1)[18]:
where S0 and Sb are the signal intensities without and at a given b value, respectively; D is the pure diffusion coefficient that reflects the random motion of intracellular and intercellular water molecules (slow component of diffusion); f is the pseduodiffusion fraction; and D* is the perfusion-related diffusion (representing incoherent microcirculation within the voxel or fast component of diffusion). The apparent diffusion coefficient (ADC) was also calculated using two b values: 0 and 800 s/mm2.
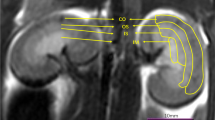
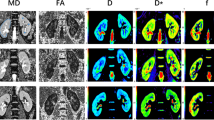
IVIM parameter measurements were conducted by two experienced abdominal radiologists [with 5 and 22 years of experience in abdominal imaging, respectively (Z.Y.C. and X.R.C.)] blinded to clinical information. Six circular regions of interest (ROIs) were positioned at the upper, middle, and lower poles in the cortex and medulla of each kidney at the level of renal hilum. ROIs were first drawn in both kidneys on the coronal T2-weighted images or DWI images (b = 0 s/mm2). They were then copied and transferred to the same positions of IVIM parametric maps. Each cortical and medullary ROI was set with a size of 4–10 mm2 and ≥ 22 mm2, respectively (Fig. 1). The mean value of each measurement was used for later statistical analysis.
Example of ROI measurement on the coronal T2-weighted image in a 38-year-old male patient (A), IVIM diffusion-weighted image obtained with b = 0 s/mm2 (B) and pseudocolor images (C: D; D: D*; E: f; F: ADC). ROIs were positioned in the medulla (1–3) and cortex (4–6) in the upper, middle, and lower parts of each kidney on coronal T2WI (A). And the ROI had an area of 4–30 mm2 (medulla: 4–10 mm2; cortex: ≥ 22 mm2). ROI, region of interest; IVIM, intravoxel incoherent motion; D, slow diffusion coefficient; D*, fast diffusion coefficient; f, pseduodiffusion fraction; ADC, apparent diffusion coefficient
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 13.0 software (SPSS Inc, Illinois, USA). Parameters were expressed as mean ± standard deviation. Male-to-Female ratio of three groups was compared using χ2 test. Duration, mean age, ACR, and eGFR between the groups were compared using the One-way ANOVA test. Pearson correlation analysis was used to see the correlation of ROIs between the two readers. The mean renal cortical and medullary IVIM DWI parameters were compared among the groups using the single-factor analysis of variance including the least significant difference and Bonferroni or Tamhane’s T2 test. A paired t test was used to compare data between the left and right kidneys. The Spearman correlation test was used to evaluate the relationship between mean IVIM parameter values and ACR or eGFR. A p value less than 0.05 was considered statistically significant for all analyses.
Results
Basic information
The basic information [duration, gender distribution, age, albumin–creatinine ratio (ACR), and estimated glomerular filtration rate (eGFR) values] of all subjects is shown in Table 1. No significant differences were found in gender distribution and age among the groups. The MAU group had a significantly higher ACR value compared with the NAU and control groups (p < 0.001). The NAU and MAU groups had significantly higher eGFR values compared with the control group (p < 0.05). However, no significant difference was observed between the NAU and MAU groups (p = 0.960).
Pearson correlation analysis of ROIs between the two readers
Strong correlations of upper, middle, and lower ROIs of kidney between the two readers in all IVIM parameters of Control subjects were found (Table 2). Scatter plots for ROIs in the upper (a–d), middle (e–h), and lower (i–l) parts of the kidney between the two readers in Control group (Fig. 2).
Comparison of the IVIM parameters and ADC value between the left and right kidneys in the three groups
The mean medullary and cortical IVIM parameters of bilateral kidneys in the three groups are shown in Supplementary materials table. The mean medullary or cortical D, D*, f, and ADC values between the left and right kidneys were similar and had no significant differences in the patient and control groups.
Comparison of the IVIM parameters and ADC value among the three groups
The mean medullary and cortical D, D*, f, and ADC values in the control, NAU, and MAU groups are shown in Table 3. Comparison of the IVIM parameters and ADC value among the three groups is shown in Fig. 3.
The mean medullary and cortical D values in the NAU and MAU groups were statistically lower than those in the control group (p < 0.05). Similarly, those values in the MAU group were significantly lower than those in the NAU group (p < 0.05).
The mean medullary and cortical f and cortical D* values in the NAU group were significantly higher than those in the control group (p < 0.05). But no significant difference was observed in the medullary D* value between the two groups (p = 0.080). Also, the medullary D*and cortical f values in the MAU group were significantly higher than those in the control group (p < 0.05). However, no significant difference was observed in the cortical D* or medullary f value between the MAU and control groups (D*: Pc = 0.995; f: Pm = 0.791). Compared with the NAU group, the mean medullary D* value significantly decreased in the MAU group (Pm < 0.001). No significant differences were observed in the cortical D* and medullary and cortical f values between the two groups (D*: Pc = 0.115; f: Pm = 0.332, Pc = 0.810).
Compared with the control group, the medullary and cortical ADC values in the NAU group show no statistical significance (Pm = 0.407, Pc = 0.298); the medullary and cortical ADC values significantly decreased in the MAU group (Pm = 0.010, Pc = 0.015). No significant differences were observed in medullary and cortical ADC values between NAU and MAU groups (Pm = 0.392, Pc = 0.656).
Correlations between IVIM parametric values and eGFR or ACR
Correlations between IVIM parametric values and eGFR or ACR are shown in Fig. 4. A negative correlation was found between the ACR and medullary or cortical ADC and D value in diabetics with 30 ≤ ACR < 300 mg/g. Weak correlation was shown between ACR and cortical ADC in diabetics with ACR < 30 mg/g. Weak correlation was shown between eGFR and medullary ADC or cortical D value. No correlations existed between any other cortical and medullary parameters and ACR or eGFR (p > 0.05).
Correlations between ACR and D (A, E), D* (B, F), f (C, G), and ADC (D, H) (A–D: 30 ≤ ACR < 300 mg/g; E–H: ACR < 30 mg/g). Correlations between eGFR and D (I), D* (j), f (K), and ADC (L). Depicted were significant correlations between ACR and medullary and cortical D values in diabetics with 30 ≤ ACR < 300 mg/g (rm = − 0.894, p < 0.001; rc = − 0.895, p < 0.001) (A). Moderate correlation was shown between ACR and cortical and medullary ADC values (rm = − 0.554, p = 0.011; rc = − 0.485, p = 0.030) (A). Weak correlations were shown between ACR (patients with ACR < 30 mg/g) and cortical ADC (ADC: r = − 0.340, p = 0.032) (H), eGFR and medullary ADC values (ADC: r = − 0.331, p = 0.010) (l). No correlations were found between any other cortical and medullary parameters and ACR or eGFR (p > 0.05). ACR, albumin–creatinine ratio; D, slow diffusion coefficient; D*, fast diffusion coefficient; eGFR, estimated glomerular filtration rate; f, pseduodiffusion fraction; ADC, apparent diffusion coefficient
Discussion
In the present study, IVIM DWI was used to noninvasively assess the early changes of renal diffusion and perfusion in diabetic patients without or with moderately increased albuminuria. The results demonstrated lower diffusion and higher pseudodiffusion values of the renal cortex and medulla in diabetic patients with normal proteinuria. This finding suggested that the abnormally low water molecule diffusion and high blood perfusion in the cortex and medulla occurred prior to albuminuria detection. Some prior studies have shown diffusion tensor imaging (DTI) [19] and blood oxygenation level-dependent (BOLD) technique [20] might be more sensitive to assess the renal function at the early stage of DN. Thus, IVIM DWI could be another noninvasive technique, which has the great potential in detecting the early renal damage when DN is still in the insidious stage, and then monitor intervention effects to avoid the development of DN.
The present study results revealed a progressively decreasing trend in the renal cortical and medullary D values from the control to the NAU and MAU groups. Significantly lower cortical and medullary D values were found in the NAU and MAU groups compared with the control group, indicating limited water molecule diffusion in both the cortex and medulla. This might be related to a series of early histological characters, including glomerular basement membrane thickening, tubular epithelial cell swelling, mesangial expansion, accumulation of transparent material in the glomerulus basement membrane epithelium, capillary and capsular adherence, and compromised energy metabolism [3, 21,22,23,24,25]. These histological changes might reduce the extracellular space and renal water content and consequently limit the diffusion of water molecules [26]. Furthermore, slight infiltration of inflammatory cell in the early course was found [21, 27], which could increase cell density [13]. So it also contributed to the limited diffusion. The present study found that the early changes of diffusion occurred in both the renal cortex and medulla in the diabetic patients. A recent study demonstrated that metabolites representing renal interstitial and tubular injury have been detected in the early stage of DN [28], which might indirectly support the findings of this study. In addition, Lu et al. [29]. and Chen et al. [19]. demonstrated the changes of fractional anisotropy values in the renal medulla in diabetic patients with normal renal function using diffusion tensor imaging. But Peng et al. [23]. employed BOLD imaging and fat quantitative sequence in diabetic rats and found higher fat and lower oxygen levels mainly in the cortex, which was consistent with the vulnerable areas displayed by pathology (glomeruli and proximal convoluted tubules). Luo et al. [21]. also used superparamagnetic iron oxide nanoparticles on early DN rats to find out early DN changes mainly in glomeruli. In the current study, statistical differences were also found in the cortical and medullary D values between NAU and MAU groups, suggesting that water molecule diffusions in the cortex and medulla were further limited with the progression of DN. Yin et al. [20]. found no statistical difference in R2* of the cortex and medulla between the two groups. Chen et al. [19]. also obtained the similar result in the cortical and medullary FA values between the two groups. Whether D value is more sensitive than R2* and FA in detecting early renal function changes is still to be determined. Further comparative studies need to be conducted to confirm it.
The present study found a significant increase in the mean renal cortical and medullary f and cortical D* values in the NAU group, suggesting an abnormally high microcirculation perfusion in diabetic patients prior to abnormal albuminuria detection. This might be related to high blood sugar, high-protein diet, increased renal tubular flow, and ultrafiltration volume caused by the renin–angiotensin–aldosterone system (RAAS), increased blood vessels, and the relatively broader diameter of the renal tubules [3, 30]. A study from Luik et al. [31]. found normal or mildly increased renal plasma flow before albuminuria in DN patients with type I diabetes. Furthermore, increased renal tubular flow can also increase the D* value [32]. The mean renal cortical f value in the MAU group still showed a significant increase in the present study. However, the mean medullary f value and the cortical or medullary D* value decreased from the NAU to the MAU group and nonsignificant changes were observed in medullary f and cortical D* between the MAU and control groups. This finding suggested that cortical high perfusion was persistent as the disease progressed, whereas medullary high perfusion decreased to nearly normal level when moderately increased albuminuria was present. A possible explanation for this is that the renal cortex has some reserve capacity for the damage, whereas the medulla is susceptible to injuries from ischemic hypoxia, toxic substance accumulation [18, 23, 33]. Whether the changes in medullary perfusion levels from high to nearly normal levels are indicative of reversible to irreversible disease progression remains unclear and requires further research.
Both water molecular diffusion and perfusion contribute toward ADC value [34]. In the present study, the mean cortical and medullary ADC values statistically decreased in the MAU group, which was consistent with some previous studies [2]. It was speculated that water molecule diffusion might outweigh perfusion contribution for the decreased ADC values due to the persistent decline in the D value. Interestingly, no change was found in the mean cortical and medullary ADC values in the NAU group, which was consistent with the findings of Cakmak et al. [2]. This finding suggested that IVIM DWI might be more sensitive than the conventional DWI in detecting the early changes in water molecular diffusion in diabetic patients.
This study demonstrated that the mean cortical and medullary D values negatively correlated with the ACR values in diabetics with 30 ≤ ACR < 300 mg/g, whereas no correlation was found between ACR values and other IVIM parameters. Albuminuria with ACR between 30 and 300 mg/g is commonly considered as the forerunner of DN in the clinical practice, which reflects both glomerular and tubulointerstitial damage [35]. Also, lower cortical and medullary D values in the MAU group in our study might indicate the injured cortical and medullary function. Despite all this, the further research is still needed to confirm whether the D values might better reflect the early-stage renal injury compared with other IVIM parameters. In our study, weak or no correlations were shown between IVIM parameters and eGFR values, although an increasing trend was noted in the eGFR values from control to NAU to MAU groups. It was consistent with the findings of Xu et al. [36]. Furthermore, some other previous studies showed a statistically significant correlation between them in diabetic patients with mid-end stage [2].
Limitations
This study had several limitations. First, the overall sample size was small. Larger number of diabetic patients may provide more precise values. Second, no biopsy evidence was obtained in this study. Accordingly, IVIM parameter changes could not be supported by the histopathological results from this study. Our future study will focus on IVIM DWI for evaluating the histopathological changes in a rat model of early DN. Third, follow-up data were not collected because of limited research period. Finally, renal medulla could not be further divided into inner and outer medulla to measure ROIs due to the limited spatial resolution of the IVIM images.
Conclusions
In conclusion, the present study revealed the limited water molecule diffusion and hyperperfusion of renal cortex and medulla in diabetic patients before proteinuria detection. Renal IVIM DWI might have a great potential in noninvasively identifying early-stage DN. The IVIM parametric values are more sensitive than the ACR in detecting the early-stage renal changes. In the future, a large sample of research may be needed to determine the critical value of IVIM parameters.
References
Reutens AT, Atkins RC. (2011) Epidemiology of diabetic nephropathy. Contrib Nephrol 1–7.
Cakmak P, Yagci AB, Dursun B, Herek D, Fenkci SM (2014) Renal diffusion-weighted imaging in diabetic nephropathy: correlation with clinical stages of disease. Diagn Interv Radiol. 20(5):374–378
Caramori ML, Fioretto P, Mauer M (2003) Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 52(4):1036–1040
Fioretto P, Steffes MW, Mauer M (1994) Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes. 43(11):1358–1364
Caramori ML, Fioretto P, Mauer M (2000) The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes. 49(9):1399–1408
Parrish AE (1992) Complications of percutaneous renal biopsy: a review of 37 years’ experience. Clin Nephrol 38(3):135–141
Schrijvers BF, De Vriese AS, Flyvbjerg A (2004) From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev 25(6):971–1010
Dyvorne HA, Galea N, Nevers T, et al. (2013) Diffusion-weighted imaging of the liver with multiple b values: effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters–a pilot study. Radiology. 266(3):920–929
Kim JW, Chang HL, Yoo KH, et al. (2016) Intravoxel incoherent motion magnetic resonance imaging to predict vesicoureteral reflux in children with urinary tract infection. Eur. Radiol 26(6):1–8
Rheinheimer S, Schneider F, Stieltjes B, et al. (2012) IVIM-DWI of transplanted kidneys: reduced diffusion and perfusion dependent on cold ischemia time. Eur J Radiol 81(9):951–956
Rheinheimer S, Stieltjes B, Schneider F, et al. (2012) Investigation of renal lesions by diffusion-weighted magnetic resonance imaging applying intravoxel incoherent motion-derived parameters–initial experience. Eur J Radiol 81(3):e310–316
Liang L, Chen WB, Chan KW, et al. (2016) Using intravoxel incoherent motion MR imaging to study the renal pathophysiological process of contrast-induced acute kidney injury in rats: Comparison with conventional DWI and arterial spin labelling. Eur Radiol 26(6):1597–1605
Cai XR, Yu J, Zhou QC, et al. (2016) Use of intravoxel incoherent motion MRI to assess renal fibrosis in a rat model of unilateral ureteral obstruction. J Magn Reson Imaging 44(3):698–706
Executive summary: standards of medical care in diabetes–2014. Diabetes Care 2014;37 Suppl 1:S5–13.
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15(7):539–553
Kong X, Ma Y, Chen J, et al. (2013) Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant 28(3):641–651
Stevens PE, Levin A (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Internal Med 158(11):825–830
Welch WJ (2006) Intrarenal oxygen and hypertension. Clin Exp Pharmacol Physiol 33(10):1002–1005
Chen X, Xiao W, Li X, et al. (2014) In vivo evaluation of renal function using diffusion weighted imaging and diffusion tensor imaging in type 2 diabetics with normoalbuminuria versus microalbuminuria. Front Med 8(4):471–476
Yin WJ, Liu F, Li XM, et al. (2012) Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur J Radiol 81(7):1426–1431
Luo B, Wen S, Chen YC, et al. (2015) LOX-1-Targeted iron oxide nanoparticles detect early diabetic nephropathy in db/db mice. Mol Imaging Biol 17(5):652–660
Bakris GL (1996) Microalbuminuria: prognostic implications. Curr Opin Nephrol Hypertens 5(3):219–223
Peng XG, Bai YY, Fang F, et al. (2013) Renal lipids and oxygenation in diabetic mice: noninvasive quantification with MR imaging. Radiology. 269(3):748–757
Soininen P, Kangas AJ, Wurtz P, Suna T, Ala-Korpela M (2015) Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet 8(1):192–206
Xiao L, Zhu X, Yang S, et al. (2014) Rap1 ameliorates renal tubular injury in diabetic nephropathy. Diabetes. 63(4):1366–1380
Li Q, Li J, Zhang L, et al. (2014) Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: a preliminary clinical study. Eur J Radiol 83(5):756–762
Tervaert TW, Mooyaart AL, Amann K, et al. (2010) Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21(4):556–563
Mise K, Hoshino J, Ueno T, et al. (2016) Prognostic value of tubulointerstitial lesions, urinary N-acetyl-beta-d-glucosaminidase, and urinary beta2-microglobulin in patients with type 2 diabetes and biopsy-proven diabetic nephropathy. Clin J Am Soc Nephrol 11(4):593–601
Lu L, Sedor JR, Gulani V, et al. (2011) Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol 34(5):476–482
Su J, Li SJ, Chen ZH, et al. (2010) Evaluation of podocyte lesion in patients with diabetic nephropathy: Wilms’ tumor-1 protein used as a podocyte marker. Diabet Res Clin Pract 87(2):167–175
Luik PT, Hoogenberg K, Van Der Kleij FG, et al. (2002) Short-term moderate sodium restriction induces relative hyperfiltration in normotensive normoalbuminuric Type I diabetes mellitus. Diabetologia. 45(4):535–541
Le Bihan D (2008) Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology. 249(3):748–752
Cai XR, Zhou QC, Yu J, et al. (2015) Assessment of renal function in patients with unilateral ureteral obstruction using whole-organ perfusion imaging with 320-detector row computed tomography. PLoS ONE 10(4):e0122454
Notohamiprodjo M, Chandarana H, Mikheev A, et al. (2015) Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magn Reson Med 73(4):1526–1532
Nauta FL, Boertien WE, Bakker SJL, et al. (2011) Glomerular and tubular damage markers are elevated in patients with diabetes. Diabet Care 34(4):975–981
Xu X, Fang W, Ling H, Chai W, Chen K (2010) Diffusion-weighted MR imaging of kidneys in patients with chronic kidney disease: initial study. Eur Radiol 20(4):978–983
Acknowledgments
The authors wish to thank Zhong-Ping Zhang, advanced application specialist at GE Healthcare MR research China, Guangzhou, for his technical consultation for practicing the IVIM protocol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Guangdong Science and Technology project in China (Grant No. 2014A020212442).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, YZ., Chen, XQ., Yu, J. et al. Intravoxel incoherent motion (IVIM) at 3.0 T: evaluation of early renal function changes in type 2 diabetic patients. Abdom Radiol 43, 2764–2773 (2018). https://doi.org/10.1007/s00261-018-1555-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1555-7