Abstract
Objective
The purpose of the study was to examine differences in kidney intravoxel incoherent motion diffusion-weighted imaging (IVIM-DWI) parameters in early-stage diabetic patients versus healthy controls.
Materials and methods
Nineteen type 2 diabetic patients (group A) with a urinary albumin-to-creatinine ratio (ACR) < 30 mg/g and an estimated glomerular filtration rate (eGFR) of 80–120 mL/(min 1.73 m2) and twelve healthy volunteers (group B) were recruited. Kidneys were scanned with 1.5-Tesla IVIM-DWI. Nine b values (0, 50, 100, 150, 200, 300, 400, 600, and 800 s/mm2) were used. The parameters derived from IVIM-DWI were calculated for each kidney by two radiologists and included the perfusion fraction (f), diffusion coefficient (D), and pseudo-diffusion coefficient (D*). The mean values of f, D, and D* were calculated by selecting multiple regions of interest in the kidney. The diagnostic performance of the f, D, and D* values for the diagnosis of early diabetic kidney changes was determined by receiver operating characteristic analysis. Three radiologists independently measured the parameters derived from IVIM-DWI in the two groups by free-hand placing regions of interest, and the interclass coefficients (ICCs) were analyzed by SPSS.16.0 software.
Results
The f values of the kidneys were significantly higher in diabetic patients than in healthy volunteers. The D value of the kidneys was significantly lower in diabetic patients than in healthy volunteers. No significant differences in the D* values of the kidneys were observed between diabetic patients and healthy volunteers. The D values of the right kidneys were significantly higher than those of the left kidneys in both groups. The results of the receiver operating characteristic analysis were as follows: left kidney—f value AUC = 0.650 (cutoff point ≥ 27.49%) and D value AUC = 0.752 (cutoff point ≤ 1.68 × 10−3 mm2/s); and right kidney—f value AUC = 0.650 (cutoff point ≥ 28.24%) and D value AUC = 0.752 (cutoff point ≤ 1.81 × 10−3 mm2/s). The diagnostic performance of the D* value was very low (AUC < 0.6). No significant differences were present between the areas under the curves of the f and D values (P > 0.05). The ICCs of the f value and D value were between 0.637 and 0.827. The ICC of the D* value was less than 0.3.
Conclusion
The results of our study suggest that changes in kidneys detected by IVIM-DWI may serve as indicators of early diabetic kidney disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Diabetic kidney disease (DKD) is a common complication of diabetes, which is the leading cause of end-stage chronic kidney disease. Timely detection and treatment in the early stage of kidney disease may prevent end-stage renal disease, which has important clinical significance [1]. The estimated glomerular filtration rate (eGFR) and albumin-to-creatinine ratio (ACR) are used to grade DKD. Unfortunately, these methods are not sufficiently sensitive to detect early kidney changes, as these indicators are often normal in the early stages of DKD; thus, early clinical diagnosis of such patients is difficult.
We aimed to determine whether structural and functional damage of the kidneys can be detected before these traditional clinical markers of kidney disease become abnormal.
Intravoxel incoherent motion diffusion-weighted imaging (IVIM-DWI) is a method of functional magnetic resonance imaging (MRI) that does not require contrast agents and avoids the risk of contrast nephropathy and nephrogenic systemic fibrosis.
IVIM-DWI can separate the effect of signals of molecular diffusion from those of capillary perfusion using a biexponential model and scan with multiple b values. The parameters derived from IVIM-DWI include the perfusion fraction (f), diffusion coefficient (D), and pseudo-diffusion coefficient (D*). The D value reflects pure molecular diffusion by minimizing the influence of blood flow on tissue diffusion. The D* value reflects the vascular blood and tubular fluid velocities within the kidney. The f value is the ratio of vascular and tubular fluid volume to the total fluid in the tissue.
IVIM-DWI has been used in renal imaging, and several studies have shown that some parameters are sensitive to pathological processes in the kidney, such as renal artery stenosis [2], renal dysfunction [3], allograft rejection [4, 5], and renal tumors [6,7,8]. Therefore, this type of functional MRI may offer an opportunity to noninvasively identify early changes in DKD.
Materials and methods
Study population
The case group (group A) consisted of nineteen type 2 diabetic patients (7 men and 12 women, age range: 34–68 years, mean age: 52.3 years, history of diabetes: 6 months to 5 years, complications include fatty liver, hypertension, and high uric acid) with an ACR < 30 mg/g and eGFR of 80–120 mL/(min 1.73 m2) who were included between July 2016 and May 2017 in our hospital. All patients received a definitive clinical diagnosis of diabetes mellitus. Group B (the control group) consisted of 12 volunteers with no known kidney disease, hypertension, gout disease or heart disease (5 men and 7 women, age range: 32–66 years, mean age: 50.2 years) and normal renal function who were included between July 2016 and May 2017 in our hospital.
The study protocol was approved by the Academic Ethics Committee of the Fifth Hospital affiliated with Guangzhou Medical University. Informed consent was obtained from all patients prior to the examination.
The eGFRs were calculated for all subjects using serum creatinine levels based on the Chronic Kidney Disease (CKD)-Epidemiology Collaboration (EPI) equation [9].
Magnetic resonance examination
MRI was performed using a 1.5-T scanner (MAGNETOM Avanto, Siemens, Germany) with a Total Image Matrix (Tim) coil and coil elements BO1, 2, SP2, and 3.
The routine MRI protocol for the kidneys of the patient and healthy control groups consisted of T1-weighted imaging sequences with the following parameters: axial sections, repetition time/echo time (TR/TE) 139.0/4.78 ms, breath-hold, and slice thickness/gap 4.5 mm/0.9 mm. The field of view (FOV) was 380 mm. Breath-triggered T2-weighted imaging was performed with the following parameters: axial and coronal sections, TR/TE 2200/90 ms, and slice thickness/gap 4 mm/0.8 mm. The FOV was 380 mm.
IVIM-DWI was performed for both the patient and healthy control groups. DWI data were acquired using a single-shot echo-planar imaging pulse sequence in free-breathing conditions with the following scan parameters: TR 3700 ms, TE 80 ms, and matrix size 192 × 153.6. Twenty slices with a slice thickness/gap of 6/1.8 mm, 6 averages, a bandwidth of 1446 Hz/pixel, a k-space-based parallel imaging technique (GRAPPA) acceleration factor of 2, and b values = 0, 50, 100, 150, 200, 300, 400, 600, and 800 s/mm2 were obtained. The echo spacing was 0.8 ms; the FOV was 380 mm; SPAIR fat suppression was used, and the TA was approximately 3 min.
Postprocessing and data analysis
DWI data were post-processed using the Medical Imaging Interaction Toolkit (MITK) 2014.03 Version (German Cancer Research Center) for the IVIM biexponential model analysis.
The values for f, D, and D* were acquired.
IVIM model:
where D represents the slow diffusion coefficient or pure diffusion coefficient; D* represents the fast diffusion coefficient or pseudo-diffusion coefficient, and f represents the perfusion score, which is the proportion of the fast components of the DWI of the structure. Le Bihan et al. first proposed the IVIM-DWI technique [10].
Quantitative analysis of IVIM-DWI data was retrospectively performed by two experienced radiologists using a blind method and consensus; the radiologists were blinded to the patient group and health control group when placing regions of interest. A free-hand region of interest (ROI) was drawn on the b = 0 image of the upper, middle and lower poles of the kidney. The ROI included the renal parenchyma and avoided the renal sinus. The average of three ROI measurements was calculated. In all cases, the mean values of f, D, and D* were derived from the selected ROIs. The kidney cortex and medulla were not considered separately for ROI positioning because they were difficult to distinguish on DWI. Both kidneys were measured in each group, and the size and contour of the kidneys were normal. To estimate the measurement reproducibility of the parameters of intravoxel incoherent motion (IVIM) in the kidneys, 3 radiologists independently measured the parameters derived from IVIM-DWI in the two groups by free-hand placing regions of interest; the interclass coefficients (ICCs) were analyzed by SPSS.16.0 software.
The measured data are expressed as x plus or minus s (x ± s). Statistical analyses were performed using statistical software (SPSS, version 16.0, SPSS/IBM, Chicago, IL, USA), and P < 0.05 was considered statistically significant. Comparisons of IVIM-DWI parameters between the two groups were performed with an independent samples t test. The diagnostic performance of the IVIM-DWI-derived parameters was evaluated with receiver operating characteristic (ROC) analysis using MedCalc 15.8 software (MedCalc Software, Mariakerke, Belgium) and the following method: Fit D and f (high b) and then fit D*. A b-factor cutoff of 200 s/mm2 was used in the IVIM model.
Results
Group A of nineteen patients and group B of twelve healthy volunteers were included in the study. There was no significant difference in renal function between the two groups (eGFR: group A, 102.3 ± 9.6 mL/(min 1.73 m2), group B, eGFR 97.5 ± 7.5 mL/(min 1.73 m2), P = 0.276; ACR: group A, 17.2 ± 3.8 mg/g, group B 12.6 ± 2.9 mg/g, P = 0.328). The gender distribution and age of the two groups were not significantly different (gender, P = 1.00; age, P = 0.306). A total of 62 kidneys had satisfactory image quality (the image was not deformed); moreover, the size of both kidneys was normal, and renal mass lesions were not observed.
The D values of the right kidney were significantly higher than those of the left kidney in both groups (group A: right kidney 1.91 ± 0.19 × 10−3 mm2/s, left kidney 1.78 ± 0.18 × 10−3 mm2/s, P = 0.043; group B: right kidney 1.78 ± 0.15 × 10−3 mm2/s, left kidney 1.66 ± 0.14 × 10− mm2/s, P = 0.038).
No significant differences in the f and D* values of the kidneys were observed between the bilateral kidneys of group A or group B (P > 0.05) (Table 1).
The f values of the kidneys were significantly higher in group B (left kidney 28.54% ± 7.33%, P = 0.042; right kidney 29.85% ± 6.68%, P = 0.009) than in group A (left kidney 23.58% ± 4.78%; right kidney 23.83% ± 6.23%).
The D values of the kidneys were significantly lower in group B (left kidney 1.66 ± 0.14 × 10−3 mm2/s, P = 0.033; right kidney 1.78 ± 0.15 × 10−3 mm2/s, P = 0.034) than in group A (left kidney 1.78 ± 0.18 × 10−3 mm2/s; right kidney 1.91 ± 0.19 × 10−3 mm2/s).
No significant differences in the D* values of the kidneys were observed between group A (left kidney 15.97 ± 11.02 × 10−3 mm2/s, right kidney 13.08 ± 6.34 × 10−3 mm2/s) and group B (left kidney 12.61 ± 4.9 × 10−3 mm2/s, P = 0.252; right kidney 12.80 ± 10.73 × 10−3 mm2/s, P = 0.924) (Table 2). Table 3 shows the measurement repeatability of parameters.
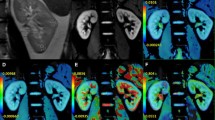
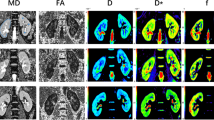
The diagnostic efficacies of IVIM-derived parameters for detecting diabetic kidney changes were as follows: (Fig. 1) Left kidney: f value AUC = 0.650 (cutoff point ≥ 27.49%), sensitivity (95% CI) = 61.1% (35.7–82.0), specificity (95% CI) = 77.8% (52.4–93.6); D value AUC = 0.752 (cutoff point ≤ 1.68 × 10−3 mm2/s), sensitivity (95% CI) = 61.1% (35.7–82.0), specificity (95% CI) = 88.9% (65.3–98.6); and Right kidney: f value AUC = 0.650 (cutoff point ≥ 28.24%), sensitivity (95% CI) = 72.2% (46.5–90.0), specificity (95% CI) = 72.2% (46.5–90.3); D value AUC = 0.752 (cutoff point ≤ 1.81 × 10−3 mm2/s), sensitivity (95% CI) = 44.4% (25.9–69.1), specificity (95% CI) = 83.3% (58.6–96.4). The diagnostic performance of the D* values was very low (AUC < 0.6). No significant difference was present between the areas under the curves of the f and D values (P > 0.05). Figure 2 shows parameters in a healthy volunteer and a patient.
The diagnostic efficacy of IVIM-derived parameters for detecting diabetic kidney changes. A Left kidney: f value AUC = 0.650 (cutoff point ≥ 27.49), sensitivity (95% CI) = 61.1% (35.7–82.7), specificity (95% CI) = 77.8% (52.4–93.6); and D value AUC = 0.752 (cutoff point ≤ 1.68 × 10–3 mm2/s), sensitivity (95% CI) = 61.1%(35.7–82.7), specificity (95% CI) = 88.9% (65.3–98.6). B Right kidney: f value AUC = 0.650 (cutoff point ≥ 28.24), sensitivity (95% CI) = 72.2% (46.5–90.3), specificity (95% CI) = 72.2% (46.5–90.3); and D value AUC = 0.752 (cutoff point ≤ 1.81 × 10–3 mm2/s), sensitivity (95% CI) = 44.4%(25.9–69.2), specificity (95% CI) = 83.3% (58.6–96.4). No significant differences were observed between the diagnostic efficacies of the f and D values (P < 0.05). The diagnostic performance of the D* value was very low. No significant difference was present between the areas under the curves for the f and D values (P > 0.05)
A1–3 a healthy volunteer, eGFR = 96 mL/(min 1.73 m2); A2 left kidney f = 26.58%, D = 1.70, D* = 14.41; and A3 right kidney f = 23.95%, D = 1.84, D* = 14.41. B1–3 a patient, eGFR = 102 mL/(min 1.73 m2); B2 left kidney f = 29.96%, D = 1.65, D* = 11.43; and B3, right kidney f = 32.00% D = 1.75, D* = 20.37. Compared to those in healthy volunteers, the signal-decay curve, plotted as a function of the b values, was steeper for low b values in patients
Discussion
Previous diffusion-weighted MRI studies have shown that kidney apparent diffusion coefficient (ADC) values and IVIM-DWI-derived parameters were reduced in patients with a variety of acute and chronic kidney dysfunctions [2, 11,12,13,14,15,16,17,18].
In contrast to previous studies, however, the current study utilized IVIM-DWI techniques to focus on diabetic subjects with relatively intact renal function (eGFR 80–120 mL/(min 1.73 m2)) and normal albuminuria (NAU). In clinical practice, some patients may have a decreased GFR despite having normal albuminuria. A previous study showed that type 2 diabetes mellitus patients at the NAU stage generally had significantly lower average ADC values in the renal parenchyma than healthy patients [19].
IVIM-DWI is a possible technique for the early detection of renal changes that can guide treatment strategies for diabetes. Management of hyperglycemia, hypertension and dyslipidemia, dietary adjustments, and a healthy lifestyle can help improve the prognosis of early DKD patients and prevent or delay their progression into clinical diabetic nephropathy and end-stage renal disease. IVIM-DWI can separate the true diffusion movement of water molecules in tissues from the pseudo-diffusion that is mainly caused by microcirculation perfusion.
In this study, we identified significantly increased f values and decreased D values among diabetic patients with relatively intact renal function (eGFR 80–120 mL/(min 1.73 m2)) when compared to healthy control subjects using quantitative IVIM-DWI techniques. The D* values were not significantly different between the two groups. The IVIM-derived D value was calculated with b factors larger than 200 s/mm2 and was relatively insensitive to blood flow [20]. In our study, the high b-factor cutoff point was 200 s/mm2.
Our findings suggested that both f and D values may help identify early changes in diabetic patients and could also indicate early DKD.
The pathophysiological process underlying the observed changes in f and D values in the diabetic subjects is not well understood. The possible mechanism is as follows: Microcirculation of the glomerulus and the renal tubule fluid and the movement of water molecules are present in the kidney. The f value responds to the water load of the kidneys and does not simply reflect the microcirculation of the blood [21]. In the early stages of DKD, the blood volume increases, and the fluid load in the kidneys is increased, including the expansion of the renal tubules, which leads to an increase in the f value.
The results show that the D value change has high specificity, which indicates that the D value is a useful imaging indicator in the early stage of kidney injury in diabetic patients. The decrease in the D value reflects the limited movement of renal water molecules, possibly due to the thickening of the glomerular basement membrane.
The D values of the right kidney were significantly higher than those of the left kidney in both groups, but the cause is unclear. A possible reason may be left gastrointestinal motility rather than right bowel motility, thus affecting the D value of the kidney. Previous studies have shown that the D value of the right kidney in normal adults is higher than that in the left kidney [22].
The results indicate that the left and right kidneys should be evaluated separately when analyzing IVIM-DWI parameters. The D* value reflects the density of local capillaries [8]. In the early stage of diabetes, changes in the capillary density may not be obvious; therefore, no significant difference in the D* value was observed between the two groups.
The free-breathing technique is suitable for patients with renal dysfunction. A previous study showed that ADC and IVIM parameters from free-breathing and navigator-controlled acquisition strategies were not significantly different and that free-breathing acquisition is highly efficient and results in higher image quality [23].
Our study showed that the repeatability of f and D measurements was good, whereas that of D* measurement was poor. Previous studies have shown that in normal kidney parenchyma, the reproducibility of D was excellent; f was good and D* was poor when derived from IVIM-DWI [24, 25].
Our study has some limitations. First, the number of subjects in the study was relatively small; however, statistically significant differences in f and D values were demonstrated. Second, the histological results of renal biopsy were not available in the diabetic group. However, DKD mainly relies on clinical diagnosis, which does not require renal biopsy. Moreover, due to medical ethics, kidney biopsies are not performed on healthy volunteers.
In conclusion, we demonstrated that the f value and D value derived from IVIM-DWI may be potential imaging biomarkers for the detection of early changes in DKD.
References
National Kidney Foundation (2012) KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 60(5):850–886
Herrmann J, Ittrich H, Kaul MG, et al. (2017) Functional assessment of the kidneys in a 10 month-old child with renal artery stenosis by intravoxel incoherent motion. Nephrology 22:257–260
Notohamiprodjo M, Chandarana H, Mikheev A, et al. (2015) Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magnetic Resonance in Medicine 73(4):1526–1532
Poynton CB, Lee MM, Li Y, et al. (2017) Intravoxel incoherent motion analysis of renal allograft diffusion with clinical and histopathological correlation in pediatric kidney transplant patients: a preliminary cross-sectional observational study. Pediatr Transplant 26(6):e12996
Ren T, C-l Wen, L-h Chen, et al. (2016) Evaluation of renal allografts function early after transplantation using intravoxel incoherent motion and arterial spin labeling MRI. Magn Reson Imaging 34(7):908–914
Ding Y, Zeng M, Rao S, et al. (2016) Comparison of biexponential and monoexponential model of diffusion-weighted imaging for distinguishing between common renal cell carcinoma and fat poor angiomyolipoma. Korean J Radiol 17(6):853–863
Rheinheimer S, Stieltjes B, Schneider F, et al. (2012) Investigation of renal lesions by diffusion-weighted magnetic resonance imaging applying intravoxel incoherent motion-derived parameters–initial experience. Eur J Radiol 81(3):e310–e316
Chandarana H, Kang SK, Wong S, et al. (2012) Diffusion-weighted intravoxel incoherent motion imaging of renal tumors with histopathologic correlation. Invest Radiol 47(12):688–696
Pugliese G, Solini A, Bonora E, et al. (2011) The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provides a better definition of cardiovascular burden associated with CKD than the Modification of Diet in Renal Disease (MDRD) Study formula in subjects with type 2 diabetes. Atherosclerosis 218(1):194–199
Le Bihan DBE, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Cakmak P, Yagci AB, Dursun B, Herek D, Fenkci SM (2014) Renal diffusion-weighted imaging in diabetic nephropathy: correlation with clinical stages of disease. Diagn Interv Radiol 20(5):374–378
Bane O, Wagner M, Zhang JL, et al. (2016) Assessment of renal function using intravoxel incoherent motion diffusion-weighted imaging and dynamic contrast-enhanced MRI. J Magn Reson Imaging 44(2):317–326
Carbone SF, Gaggioli E, Ricci V, et al. (2007) Diffusion-weighted magnetic resonance imaging in the evaluation of renal function: a preliminary study. Radiol med 112:1201–1210
Emre T, Kilickesmez O, Buker A, et al. (2016) Renal function and diffusion-weighted imaging: a new method to diagnose kidney failure before losing half function. Radiol Med 121(3):163–172
Heusch P, Wittsack H-J, Pentang G, et al. (2013) Biexponential analysis of diffusion-weighted imaging: comparison of three different calculation methods in transplanted kidneys. Acta Radiol 54(10):1210–1217
Ichikawa S, Motosugi U, Ichikawa T, et al. (2013) Intravoxel incoherent motion imaging of the kidney: alterations in diffusion and perfusion in patients with renal dysfunction. Magn Reson Imaging 31(3):414–417
Li Q, Li J, Zhang L, et al. (2014) Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: a preliminary clinical study. Eur J Radiol 83:756–762
Thoeny HC, De Keyzer F, Oyen RH, Peeters RR (2005) Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 235(3):911–917
Chen X, Xiao W, Li X, et al. (2014) In vivo evaluation of renal function using diffusion weighted imaging and diffusion tensor imaging in type 2 diabetics with normoalbuminuria versus microalbuminuria. Front Med 8(4):471–476
Le Bihan D (2008) Intravoxel incoherent motion perfusion mr imaging: a wake-up call. Radiology 249(3):748–752
Sigmund EE, Vivier PH, Sui D, et al. (2012) Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology 263(3):758–769
Ding Y, Miao X, Li R, et al. (2016) Intravoxel incoherent motion diffusion weighted imaging of normal adult kidney. J PractRadiol 32(4):71–78
Jerome NP, Orton MR, D’Arcy JA, et al. (2014) Comparison of free-breathing with navigator-controlled acquisition regimes in abdominal diffusion-weighted magnetic resonance images: effect on ADC and IVIM statistics. J Magn Reson Imaging 39(1):235–240
Pan J, Zhang H, Man F, et al. (2017) Measurement and scan reproducibility of parameters of intravoxel incoherent motion in renal tumor and normal renal parenchyma: a preliminary research at 3.0T MR. Abdom Radiol
Barbieri S, Donati OF, Froehlich JM, Thoeny HC (2016) Comparison of intravoxel incoherent motion parameters across MR imagers and field strengths: evaluation in upper abdominal organs. Radiology 279(3):784–794
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
All authors declare that they have no conflicts of interest.
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Deng, Y., Yang, B., Peng, Y. et al. Use of intravoxel incoherent motion diffusion-weighted imaging to detect early changes in diabetic kidneys. Abdom Radiol 43, 2728–2733 (2018). https://doi.org/10.1007/s00261-018-1521-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1521-4