Abstract
Purpose
To evaluate the diagnostic accuracy of shear wave elastography (SWE) and transient elastography (TE) in the evaluation of liver fibrosis in chronic hepatitis B (CHB) and C (CHC) patients taking liver biopsy as gold standard.
Methods
Ethics committee approved this prospective cross-sectional study. Between October 2012 and December 2014, consecutive CHB/CHC patients fulfilling the inclusion criteria were included—age more than 18 years, informed written consent, willing and suitable for liver biopsy. SWE, TE, and biopsy were performed the same day. Liver stiffness measurement (LSM) cut-offs for various stages of fibrosis were generated for SWE and TE. AUC, sensitivity, specificity, and positive/negative predictive values were estimated individually or in combination.
Results
CH patients (n = 240, CHB 172, CHC 68), 176 males, 64 females, mean age 32.6 ± 11.6 years were enrolled. Mean LSM of patients with no histological fibrosis (F0) was 5.0 ± 0.7 and 5.1+1.4 kPa on SWE and TE, respectively. For differentiating F2 and F3–4 fibrosis on SWE, at 7.0 kPa cut-off, the sensitivity was 81.3% and specificity 77.6%. For TE, at 8.3 kPa cut-off, sensitivity was 81.8% and specificity 83.1%. For F3 vs. F4, SWE sensitivity was 83.3% and specificity 90.7%. At 14.8 kPa cut-off, TE showed similar sensitivity (83.3%) but specificity increased to 96.5%. Significant correlation between SWE and TE was observed (r = 0.33, p < 0.001). On combining SWE and TE, a drop in sensitivity with increased specificity for all stages of liver fibrosis occured.
Conclusion
SWE is an accurate technique for evaluating liver fibrosis. SWE compares favorably with TE especially for predicting advanced fibrosis/cirrhosis. Combining SWE and TE further improves specificity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Liver fibrosis is the outcome of untreated chronic liver injury occurring due to numerable causes and leads to serious long-term morbidity and mortality. Among the various causes, the important ones include hepatitis B, C infections, alcohol, non-alcoholic fatty liver disease, drugs, and autoimmune liver disease. With the emerging evidence that early fibrosis is reversible with effective treatment, many clinicians believe that patients with at least significant fibrosis should be subjected to therapy [1,2,3,4]. Precise estimation of the degree of liver fibrosis for accurate prognostication, surveillance, and treatment decisions in chronic hepatitis (CH) is thus crucial [5, 6].
Liver biopsy is the “gold standard” for grading liver fibrosis. However, it is an invasive procedure having small but significant risk of morbidity and mortality and results in high patient refusal rate [4, 7, 8]. Additionally, many patients are unsuitable for the procedure due to associated coagulopathy. Liver biopsy has a moderate sensitivity and specificity due to sampling errors and small tissue sample retrieval [9, 10]. Repeating an invasive procedure for monitoring treatment response is also not advisable. Hence, there was a need for a technique which could estimate hepatic fibrosis by non-invasive methods.
Elastography is a technique which has the ability to estimate hepatic fibrosis based on the assessment of tissue stiffness. Among the available armamentarium, transient elastography (TE), was the earliest and most extensively used [11]. TE is difficult to perform in patients with obesity, ascites, shrunken liver, or those with narrow intercostal spaces [12]. Further technological advances led to the emergence of a novel technique of Shear wave elastography (SWE). This uses information of acoustically generated shear wave propagation speed through the liver to provide qualitative (stiffness-based color-coded maps) and quantitative assessment (average value in the region of interest in terms of the Young modulus, kilopascals) of liver fibrosis [13,14,15,16].
How do these techniques of SWE and TE perform with regard to the evaluation of different stages of fibrosis in patients of chronic viral hepatitis, needs comparison. Available literature on elastography has originated from the developed world and largely focused on chronic hepatitis C (CHC) patients. In India, about 50% of patients with chronic liver diseases occur due to hepatitis B and 20% due to hepatitis C [17]. HBV is the most common cause of chronic hepatitis, cirrhosis, and primary liver cell cancer [18].
This study was designed to evaluate chronic hepatitis B (CHB) and CHC patients for the presence of different grades of hepatic fibrosis using SWE and TE taking liver biopsy as the reference method.
Methods
This cross-sectional study was approved by our Institute’s Ethics committee. A written informed consent was obtained. Between October 2012 and December 2014, consecutive patients of CH (CHB and CHC) reporting to our hospital were studied.
Patient work up
A detailed history pertaining to the source of infection, alcohol consumption, and co-morbidities like diabetes, hypertension, and any past history of decompensation was obtained. Liver function tests including serum bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), serum protein, serum albumin, prothrombin time, complete hemogram, renal function tests, blood sugar, ultrasonography, and alpha-fetoprotein levels were done in all patients. Serologies for HBsAg, hepatitis B e antigen (HBeAg), antibodies to HBeAg (anti-HBe), hepatitis C virus (anti-HCV), and human immunodeficiency virus (HIV) were done using standard commercially available enzyme immunoassays (Biorad, Marnes-la-Coquette, France). CHB or CHC was diagnosed based on the positive serology of HBsAg or anti-HCV, respectively. In patients who were HBsAg positive, HBV DNA level was measured using a florescent probe-based detection kit (PG Biotech, Life River, Shanghai, China) on real-time polymerase chain reaction system (Mx 3000 5T) with a range of detection from 100 to 109 copies/mL. In patients who were anti-HCV positive, HCV RNA was detected by reverse transcription-nested polymerase chain reaction.
The inclusion criteria were diagnosed patients of chronic hepatitis B or C of 18 years of age or more, willing to undergo liver biopsy, providing informed written consent, and having normal coagulation profile. Pregnant females, known case of cirrhosis, those with co-morbid illnesses like coronary artery disease, congestive cardiac failure, or coexistent chronic ailments, any contraindication for liver biopsy (coagulopathy, thrombocytopenia, history of bleeding diathesis) were excluded. Enrolled patients were subjected to SWE, TE, and liver biopsy on the same day by different investigators located in different departments who were blinded to the other two investigations of the same patient.
Shear wave elastography: technique
SWE was performed on the ultrasound machine (Aixplorer, Supersonic Imagine). Patients reported after 5 h of fasting, were made to lie supine with right arm in maximum abduction. Breath holding for few seconds was rehearsed (avoiding deep inspiration and expiration) for obtaining liver stiffness measurement.
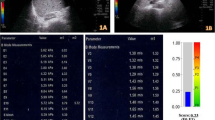
Placing a convex broadband probe in the right intercostal space, a B-Mode ultrasound (US) image of the right lobe of the liver was obtained. SWE mode that had a simultaneous display of B-mode US and SWE, was put on. SWE box was placed onto a vessel-free hepatic parenchyma at a depth of 3–5 cm below the liver capsule. Ensuring image stabilization, while the patient held breath, a color image depicting different grades of stiffness was obtained. Quantitative SWE was performed by choosing a region of interest (ROI) of 10–15 mm diameter in the center of the SWE box displaying a homogeneous color map and the mean, minimum, maximum liver stiffness measurements (LSM) were displayed on the monitor (Fig. 1A–E). Six such LSMs were obtained at different sites in the right lobe the of liver. Subsequently, the mean and standard deviation (SD) of six means with SD were derived depicting the final LSM, which was correlated with the histological grading (metavir score) on biopsy.
A–E Shear wave elastography (SWE) showing different stages of fibrosis based on the metavir stage: A Metavir F0 fibrosis: SWE image showing a rectangular box depicting deep blue homogenous map (qualitative SWE) representing normal soft elasticity of liver. Color code interpretation (displayed on the top right corner of the screen) showing increasing tissue elasticity from deep blue color (soft) to red color (hard). For quantitative SWE, a circular region of interest of 15 mm diameter is marked within the rectangular box and the software displays the measured liver stiffness (LSM) on the screen—mean 3.7 kPa, min (minimum) 3.1 kPa, max (maximum) 4.8 kPa, SD (standard deviation) 0.4 kPa. B Metavir F1 fibrosis: SWE image showing color map of shades of blue with a mean LSM of 6.1 kPa. C Metavir F2 fibrosis: SWE color display of shades of blue, with mean LSM of 7.8 kPa. D Metavir F3 fibrosis: SWE image showing a light blue color map and a mean LSM of 14.8 kPa. E Metavir F4 fibrosis: SWE color map of yellow to orange, mean LSM 27.7 kPa and biopsy confirming metavir F4 stage fibrosis
Transient elastography (TE)
TE was performed on the machine (Echosens, Paris, France) using either M or XL probe. Patients reported after fasting for 5–6 h, were made to lie supine with right arm in full abduction. The probe was placed perpendicular to the skin in the midaxillary line and in the ninth to eleventh intercostal space and on pressing the probe button, ten successful measurements per patient were obtained. TE measures liver stiffness in a volume (1 cm wide, 4 cm long) at 2.5–6.5 cm below the skin surface. If the success rate was <60% or inter-quartile range >30% of the final median liver stiffness, the study was considered unsuccessful.
Liver biopsy: technique
After obtaining informed written consent, the procedure was performed under ultrasound guidance using local anesthesia. Two intercostal spaces above the costal margin, in midaxillary line, the biopsy was done under aseptic precautions using 16 gauge Menghini needle. About 1.5–2.0 cm core of liver tissue was obtained and the punctured site was sealed. The sample was immersed in the neutral buffered formalin vial and sent to the pathology department. The patient was observed for 3 hours for any untoward complication and discharged when stable.
Histological grading of liver biopsy
Biopsies were reviewed by the investigators who were blinded to the SWE and TE findings and the report was conveyed by consensus. Biopsies without adequate number of portal tracts (<10, except in patients with cirrhosis) and/or insufficient length (10 mm) were excluded from the study. In all biopsies hematoxylin-eosin stain was performed, along with reticulin silver stain, Masson’s trichrome stain, and Sirius red stain for the assessment of liver fibrosis. Metavir staging for liver fibrosis was done based on the METAVIR Cooperative Study Group [19]. Five stages ranging from F0 to F4 were assigned—F0, no fibrosis; F1, minimal fibrosis (portal fibrosis with no fibrous septa); F2, significant fibrosis (portal fibrosis with a few porto-portal fibrous septa); F3, advanced fibrosis (numerous porto-portal fibrous septa without cirrhosis); F4, cirrhosis with complete nodule formation.
Statistical analysis
Statistical analysis was performed on Stata, version 14.2. Continuous variables were expressed as mean with standard deviation (SD) or median with inter-quartile range (IQR). All statistical tests were two-sided and statistical significance was taken as p < 0.05. Liver stiffness values determined by the two tests were correlated with Metavir staging using Spearman’s correlation coefficient. The diagnostic accuracy and cut-offs of liver stiffness determined by SWE and TE in detecting histological minimal, significant, and advanced fibrosis and cirrhosis were calculated using the receiver operating characteristics (ROC curves) and the best cut-off value of LSM, area under the curve (AUC), sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) were determined. Subsequently, the diagnostic accuracy was estimated by combining SWE and TE by either/or method.
Results
Baseline demographic and clinical characteristics
Two hundred and fifty-six consecutive patients of chronic viral hepatitis reported to our hospital during the study period (Fig. 2). Liver biopsy could not be done in 14 patients due to associated coagulopathy and in two patients the tissue biopsy sample was inadequate. Hence, 240 patients of CH (CHB-172 and CHC-68) were included who underwent SWE, TE, and liver biopsy.
These patients were 176 males and 64 females, of mean age 32.6 ± 11.6 years (range 15–75 years). Their clinical profile is illustrated in Table 1. All patients were subjected to three investigations of TE, SWE, and liver biopsy on the same day. Both SWE and TE could not be done in one patient, TE could not be done in four and SWE failed in two patients. Thus, the success rate of SWE was 98.7% (237/240) while that of TE was 97.9% (235/240) and there was no difference in the success rate between the two techniques (p = 0.47). Hence, a total of 237 patients underwent SWE while TE could be done in 235 patients.
Liver stiffness measurements according to the fibrosis stage
There was moderately significant correlation between liver biopsy and both SWE (0.533, p < 0.001) as well as TE (0.614, p < 0.001) (Fig. 3). Mean LSM of patients with ‘no fibrosis’ (F0) was 5.0 ± 0.7 and 5.1 ± 1.4 kPa on SWE and TE, respectively. The mean LSM assessed by both SWE and TE increased progressively with the increase in histological fibrosis from F0 to F4 (Table 2).
Cut-offs values of liver stiffness for detecting various stages of fibrosis
For the diagnostic accuracy of SWE and TE with respect to histological fibrosis, the AUC and the best cut-offs were obtained on the ROC curve. The SWE and TE correlated significantly with each other (r = 0.52, p < 0.001).
For SWE
AUC of SWE for the presence of minimal fibrosis (F0 vs. F1–4), significant fibrosis (F0-1 vs. F2–4), advanced fibrosis (F0–2 vs. F3–4), and cirrhosis (F0–3 vs. F4) was 0.81 (95% CI 0.74–0.88), 0.76 (95% CI 0.70–0.82), 0.90 (95% CI 0.85–0.94), and 0.93 (95% CI 0.84–1.00), respectively. The cut-off of 7.0 and 9.7 kPa had a sensitivity and specificity of 81.3% and 77.6%, and 83.3% and 90.7% in discriminating F3–4 from F0 to F2 fibrosis and F4 from F0 to F3 fibrosis, respectively (Table 3).
For TE
AUC of TE for minimal fibrosis (F0 vs. F1–4), significant fibrosis (F0–1 vs. F2–4), advanced fibrosis (F0–2 vs. F3–4), and cirrhosis (F0–3 vs. F4) was 0.72 (95% CI 0.64–0.80), 0.84 (95% CI 0.79–0.89), 0.90 (95% CI 0.85–0.95), and 0.97 (95% CI 0.93–1.00), respectively. The cut-off of 8.3 and 14.8 kPa had a sensitivity and specificity of 81.8% and 83.1%, and 83.3% and 96.5% in discriminating F3–4 from F0 to F2 fibrosis and F4 from F0 to F3 fibrosis, respectively (Table 3).
For both SWE and/ or TE
On using the combination of SWE and TE for all stages of liver fibrosis, there was a drop in the sensitivity whereas the specificity increased markedly in all stages of liver fibrosis (Table 4). The PPV showed improvement for diagnosing F2 and F3 fibrosis. When the elastography techniques were used in either/or method, the reverse was true, that is, increase in sensitivity with drop in specificity (Tables 3 and 4).
Comparison of diagnostic accuracies of SWE and TE (Fig. 4)
The accuracies of SWE and TE were comparable for differentiating F0–2 fibrosis from F3 to F4 [SWE 0.90 (0.85–0.94) vs. TE 0.89 (0.85–0.95), p = 0.87] and for F0–3 from F4 fibrosis [SWE 0.93 (0.84–1.0) vs. TE 0.97 (0.93–1.0), p = 0.23]. However, TE had a better accuracy for differentiating between F0 and F1 from F2 to F4 fibrosis [SWE AUC 0.76 (0.70–0.82) vs. TE AUC 0.84 (0.79–0.89), p = 0.02].
Factors affecting liver stiffness measured by SWE and TE
A univariate analysis of the association of individual factors affecting LSM was undertaken. Factors like age, BMI, ALT, platelet levels, serum albumin, viral marker positivity for HBeAg, HBVDNA, and HCVRNA, duration of disease at the time of evaluation, and presence of steatosis did not show significant association with the mean LSM of individual stages of fibrosis (results not shown).
Discussion
Published literature for estimating liver fibrosis has mainly originated from the developed world [20,21,22] with majority of the studies focused on CHC [23, 24], and scanty literature on CHB patients [25, 26]. Further, limited work comparing SWE with other techniques or serum markers is available [20, 23, 25, 27]. To the best of our knowledge, this is the first study of its kind from South Asia for estimating liver fibrosis in patients with chronic viral hepatitis (CHB and CHC), using SWE and TE on the same cohort with liver biopsy as the reference method.
In our study on 240 patients, SWE could be performed in 237/240 patients (success rate 98.7%) while TE in 235/240 (success rate 97.9%), and there was no difference in the success rate between the two techniques (p = 0.47). These patients in whom elastography failed were either very obese, had BMI >30.5 kg/m2, or had narrow intercostal spaces. For both techniques, obesity posed a significant hurdle (a known limitation), which was faced more with TE than SWE.
Different cut-offs and AUC values for discriminating between different stages of fibrosis by elastography are published. These studies are from different geographical locations and on patients of varied etiologies. Additionally, factors like age, sex, ethnicity, medication, and post prandial status, etc., which are known to cause variability in liver stiffness, are also not known [28]. Hence, the available liver stiffness cut-offs cannot be generalized and data based on our own population are much needed. Further, even the European Federation of Societies for Ultrasound in Medicine and Biology [29] and World Federation for Ultrasound in Medicine and Biology [11] have only specified the guidelines for the use of elastographic techniques but not laid any specific thresholds for diagnosing different stages of fibrosis.
The technique of TE was available at our center earlier than SWE and a significant co-relation between LSM and histological fibrosis (r = 0.58, p < 0.01) was depicted [30]. In this study, we performed SWE and TE in the same cohort and the cut-off values for different stages of fibrosis on SWE were generated for the first time. The mean LSM on SWE in our patients with no histological fibrosis was 5.0 ± 0.7 kPa (range 3.6–6.9 kPa). This value is in good agreement with a retrospective study by Suh et al. (4.4 kPa) who collected LSM data of those patients who underwent SWE and liver biopsy for various other clinical indications and had normal hepatic histology [31]. Additionally, the mean LSM of 4.6 and 4.9 kPa respectively have also been shown in two studies on healthy volunteers without histopathological confirmation [32, 33].
It is difficult to accurately differentiate between adjacent stages of fibrosis due to a substantial overlap in median liver stiffness values. Same was true in our study for both SWE and TE, particularly, for early fibrosis (F1 and F2, Table 2) where we found a modest performance of the two techniques and TE showed an edge over SWE in discriminating between F1 and F2 fibrosis (TE, AUC 0.84 (0.79–0.89) vs. SWE AUC 0.76 (0.70–0.82), p = 0.02) (Table 4).
The degree of liver fibrosis has a positive correlation with the complications of cirrhosis including variceal bleed, ascites and risk of hepatocellular carcinoma, and has been correlated with prognosis [34]. Hence, the differentiation with high precision is clinically important particularly for patients with significant fibrosis and beyond who would require therapy and regular follow-up for the assessment of complications. With increasing fibrosis, a parallel increase in the LSM values as well as in the diagnostic performance of both techniques of SWE and TE was seen (Table 3). SWE and TE had cut-offs of 7.0 kPa (AUC: 0.90) and 9.7 kPa (AUC: 0.93), and 8.3 (AUC: 0.89) and 14.8 kPa (AUC: 0.97) for detecting advanced fibrosis (>F2) and cirrhosis (>F3), respectively. The diagnostic accuracy of TE to detect advanced fibrosis and cirrhosis was almost similar to a study published from our center on CHB patients [30]. However, the accuracy for significant fibrosis in the present study was slightly lower than the previous study which could be because of the difference in the overall number and proportion of patients with different stages of fibrosis. The cut-offs exhibited in the other published studies from different centers were higher than ours for all stages of fibrosis. This difference could be attributed to the heterogeneity in the patients studied and geographic regional variations.
SWE and TE came across as useful non-invasive elastography techniques particularly for evaluation of advanced fibrosis (F3) and cirrhosis (F4). A recent meta-analysis of twelve studies of SWE performed on patients of different etiologies of CH has also illustrated similar sensitivities (89% and 88%) and specificities (84% and 86%) for advanced fibrosis and cirrhosis [35]. Similarly, a meta-analysis of 40 studies on TE for estimating liver fibrosis, concluded that TE had a high sensitivity and specificity for cirrhosis and less for lesser degrees of fibrosis. No optimal stiffness cut-offs for individual fibrosis stages were validated and the wide range of cut-offs with overlap between stages was encountered, the results being quite similar to the observations in the present study. [36]
LSM evaluated by SWE or TE did not have any association with age, BMI, ALT, platelet count, serum albumin, hepatitis B/C viral marker positivity, duration of disease at time of evaluation, and steatosis. However, in the previous study from our center, ALT was shown to be an independent predictor of LSM. This discrepancy could be explained by the difference in the proportion of patients with ALT × 5 times normal in the present and previous study, as in the previous study, it was ALT × 5 times that correlated with LSM rather than all values of ALT. In the previous study 7% (25/352) patients had ALT × 5 times as compared to present study where only 2.5% (6/240) patients had ALT × 5 times normal. Similarly, no factor had any association with LSM in the study by Ferraioli et al. [23] and Samir et al. [14]. However, Poynard et al. [37] on assessing the impact of steatosis and necro-inflammation have shown a less impact of both (steatosis and necro-inflammation) on SWE in comparison to TE in non-advanced fibrosis patients.
We found a modest correlation between SWE and TE (r = 0.52, p < 0.001). Diagnostic accuracy of SWE and TE in discriminating advanced fibrosis and cirrhosis was high. Comparison studies on SWE and TE have documented variable results, some showing superiority of SWE over TE while others depicting comparable outcomes. However, all these studies have been performed on CH patients with different etiologies. Kim et al [38], in a retrospective study showed significant conformity of SWE and TE (p < 0.05) with higher precision of SWE (higher kappa index) in patients of CHB. Leung et al [25] showed that SWE was superior to TE especially for evaluating F2 fibrosis (SWE AUC 0.82 (0.74–0.89), specificity 84.8% and TE AUC was 0.78 (0.69–0.86), specificity 80.9% in chronic B carriers). In our study, for >F2 fibrosis, the AUC of SWE and TE was comparable with similar sensitivities (0.90 (0.85–0.94 vs. 0.89 (0.85–0.95), p = 0.87) but TE depicted a higher specificity of 83.1% compared to that of SWE (77.6%). In contrast, a cohort of 226 CH patients, showed that the performance of SWE and TE was similar for diagnosing fibrosis; however, the SWE applicability was greater than that of TE in patients with ascites [39]. Comparable results of SWE with TE and ARFI (acoustic radiation force impulse) have also been elicited [27].
We also explored the predictive value of fibrosis by combining SWE and TE. Combination resulted in drop in sensitivity with increase in specificity for discriminating between all stages of fibrosis. Hence, SWE and TE combination would be highly specific for predicting fibrosis. Similar findings have been reported by the use of a combination of TE with ARFI. However, the feasibility of using two techniques is rather difficult and would not be cost-effective. This approach may be reserved for cases having high index of suspicion or contraindications for biopsy.
Our study had few limitations. We evaluated patients of chronic hepatitis B and C only, thus, these cut-offs may not be applicable to CH patients of other etiologies in our population. Patients who had definite diagnosis of cirrhosis (clinically or by ultrasound/ CT abdomen or endoscopy) were not included as the identification of these patients can be made on conventional ultrasound. Due to this, we had few patients in the F4 stage on histology and better numbers could have provided us with a more robust data. SWE, by virtue of being a real-time ultrasound technique has the ability to depict the color-coded maps of stiffness (qualitative) and quantitative information of tissue stiffness of the entire liver. We encountered some cases in which both SWE and TE depicted high stiffness values, but their metavir stage was low (Fig. 5). Since fibrosis affects the liver in a heterogenous manner, the liver biopsy is prone to sampling errors. In such cases, liver biopsy performed under SWE guidance (from depicted harder areas) could have helped in obtaining a better represented tissue sample. We could not undertake this due to the logistics and feasibility issues and this perhaps has led to an underestimation of the diagnostic potential of SWE.
Discordance of metavir stage of fibrosis on SWE and TE: SWE image of a 25-year-old CHB patient showing a light blue color map with mean stiffness of 9.1 kPa and the stiffness measured on TE was also high, 11.0 kPa, suggestive of F3 fibrosis on both SWE and TE. However, the biopsy revealed metavir F1 fibrosis
To conclude, SWE is an accurate non-invasive technique for evaluating liver fibrosis especially for predicting advanced fibrosis and cirrhosis and the results compare favorably with TE. Combination of the two techniques results in increased specificity for detecting liver fibrosis. Future studies comparing SWE with liver biopsy performed under SWE guidance may help in clarifying the role of SWE further.
References
Rockey DC (2005) Anti fibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol 3:95–107
Rockey DC, Bissell DM (2006) Noninvasive measures of liver fibrosis. Hepatology 43:S113–S120
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115:209–218
Friedman SL (2003) Liver fibrosis: from bench to bedside. J Hepatol 38:S38–S53
Zeuzem S (2004) Standard treatment in acute and chronic hepatitis C. Z Gastroenterol 42:714–719
Fleig WE, Krummener P, Lesske J, et al. (2004) Diagnosis, progression and therapy of hepatitis C virus infection as well as viral infection in children and adolescents: results of an evidence based consensus conference of the German society for Alimentary Metabolic Disorders and in cooperation with the Hepatitis Competence Network. Z Gastroenterol 42:703–704
Cadranel JF, Rufat P, Degos F (2000) Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32:477–481
Castéra L, Nègre I, Samii K, Buffet C (1999) Pain experienced during percutaneous liver biopsy. Hepatology 30:1529–1530
Maharaj B, Maharaj RJ, Leary WP, et al. (1986) Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet 1:523–525
Regev A, Berho M, Jeffers LJ, et al. (2002) Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 97:2614–2618
Ferraioli G, Filice C, Castera L, et al. (2015) WFUMB guidelines and recommendations for clinical use of ultrasound elastography. Part 3: liver. Ultrasound Med Biol 41:1161–1179
Tatsumi C, Kudo M, Ueshima K, et al. (2008) Noninvasive evaluation of hepatic fibrosis using serum fibrotic markers, transient elastography (FibroScan) and real-time tissue elastography. Intervirology 51:27–33
Friedrich-Rust M, Ong MF, Herrmann E, et al. (2007) Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. Am J Roentgenol 188:758–764
Samir AE, Dhyani M, Vij A, et al. (2015) Shear-wave elastography for the estimation of liver fibrosis in chronic liver disease: determining accuracy and ideal site for measurement. Radiology 274:888–896
Chung JH, Ahn HS, Kim SG, et al. (2013) The usefulness of transient elastography, acoustic-radiation-force impulse elastography, and real-time elastography for the evaluation of liver fibrosis. Clin Mol Hepatol 19:156–164
Li C, Zhang C, Li J, Huo H, Song D (2016) Diagnostic accuracy of real-time shear wave elastography for staging of liver fibrosis: a meta-analysis. Med Sci Monit 22:1349–1359
Acharya SK, Madan K, Dattagupta S, Panda SK (2006) Viral hepatitis in India. Natl Med J India 19:203–217
Batham A, Narula D, Toteja T, Sreenivas V, Puliyel JM (2007) Sytematic review and meta-analysis of prevalence of hepatitis B in India. Indian Pediatr 44:663–674
Boozari B, Potthoff A, Mederacke I, et al. (2010) Evaluation of sound speed for detection of liver fibrosis: prospective comparison with transient dynamic elastography and histology. J Ultrasound Med 29:1581–1588
Jeong JY, Kim TY, Sohn JH, et al. (2014) Real time shear wave elastography in chronic liver diseases: accuracy for predicting liver fibrosis, in comparison with serum markers. World J Gastroenterol 20:13920–13929
Yoon JH, Lee JM, Joo I, et al. (2014) Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology 273:772–782
Zheng J, Guo H, Zeng J, et al. (2015) Two-dimensional shear-wave elastography and conventional US: the optimal evaluation of liver fibrosis and cirrhosis. Radiology 275:290–300
Ferraioli G, Tinelli C, Dal Bello B, et al. (2012) Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology 56:2125–2133
Bavu E, Gennisson JL, Couade M, et al. (2011) Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol 37:1361–1373
Leung VY, Shen J, Wong VW, et al. (2013) Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology 269:910–918
Zeng J, Liu GJ, Huang ZP, et al. (2014) Diagnostic accuracy of two-dimensional shear wave elastography for the non-invasive staging of hepatic fibrosis in chronic hepatitis B: a cohort study with internal validation. Eur Radiol 24:2572–2581
Cassinotto C, Lapuyade B, Mouries A, et al. (2014) Non-invasive assessment of liver fibrosis with impulse elastography: comparison of supersonic shear imaging with ARFI and Fibroscan. J Hepatol 61:550–557
Barr RG, Ferraioli G, Palmeri ML, et al. (2015) Elastography assessment of liver fibrosis: society of radiologists in ultrasound consensus conference statement. Radiology 276:845–861
Cosgrove D, Piscaglia F, Bamber J, et al. (2013) EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: clinical applications. Ultraschall Med 34:238–253
Goyal R, Mallick SR, Mahanta M, et al. (2013) Fibroscan can avoid liver biopsy in Indian patients with chronic hepatitis B. J Gastroenterol Hepatol 28:1738–1745
Suh CH, Kim SY, Kim KW, et al. (2014) Determination of normal hepatic elasticity by using real-time shear-wave elastography. Radiology 271:895–900
Ferraioli G, Tinelli C, Zicchetti M, et al. (2012) Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol 81:3102–3106
Muller M, Gennisson JL, Deffieux T, Tanter M, Fink M (2009) Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med Biol 35:219–229
Strader DB, Wright T, Thomas DL, Seeff LB (2004) American Association for the study of liver diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology 39:1147–1171
Feng JC, Li J, Wu XW, Peng XY (2016) Diagnostic accuracy of supersonic shear imaging for staging of liver fibrosis: a meta-analysis. J Ultrasound Med 35:329–339
Tsochatzis EA, Gurusamy KS, Ntaoula S, et al. (2011) Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 54:650–659
Poynard T, Pham T, Perazzo H, et al. (2016) Real-time shear wave versus transient elastography for predicting fibrosis: applicability, and impact of inflammation and steatosis. A non-invasive comparison. PLoS ONE. doi:10.1371/journal.pone.0163276
Kim HJ, Lee HK, Cho JH, Yang HJ (2015) Quantitative comparison of transient elastography (TE), shear wave elastography (SWE) and liver biopsy results of patients with chronic liver disease. J Phys Ther Sci 26:2465–2468
Poynard T, Munteanu M, Luckina E, et al. (2013) Liver fibrosis evaluation using real-time shear wave elastography: applicability and diagnostic performance using methods without a gold standard. J Hepatol 58:928–935
Acknowledgments
Authors want to acknowledge Mr. Sanjeev Negi and Mr. Dilshad Ali, the research staff, for handling the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors have no conflict of interest.
Ethical approval
The study was performed after approval from the Ethics committee of our institute and all procedures performed on human participants were in accordance with the ethical standards.
Informed consent
A written informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Paul, S.B., Das, P., Mahanta, M. et al. Assessment of liver fibrosis in chronic hepatitis: comparison of shear wave elastography and transient elastography. Abdom Radiol 42, 2864–2873 (2017). https://doi.org/10.1007/s00261-017-1213-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1213-5