Abstract
Polypoid endometriosis is a benign, rare variant of endometriosis which forms multiple polypoid nodules in the female pelvis mimicking malignant tumors; however, it may rarely cause malignant transformation. We report magnetic resonance imaging findings of a case of polypoid endometriosis with malignant transformation. Multiple high-signal intensity polypoid nodules in the cul-de-sac surrounded by low-signal intensity rim-like fibrous adhesion protruding to the posterior wall of the uterine body were demonstrated on T2-weighted images. The polypoid nodules showed weak contrast enhancement compared with that of uterine myometrium on post-contrast T1-weighted images, and slight high signal intensity on diffusion-weighted images with relatively high mean apparent diffusion coefficient. Reported cases of polypoid endometriosis showed intense contrast enhancement similar to that of uterine myometrium, and weak contrast enhancement similar to that of endometrial carcinoma may be suggestive for malignant transformation of polypoid endometriosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polypoid endometriosis is a benign, rare variant of endometriosis which forms multiple polypoid nodules mimicking malignant tumors on imaging examination, at operation, and on gross pathologic examination [1–8]. Polypoid endometriosis may often show areas of hyperplasia or atypical hyperplasia, and rarely transformation to a malignant epithelial neoplasm may occur [1, 2]. We report magnetic resonance (MR) imaging findings of a case of polypoid endometriosis with malignant transformation.
Case report
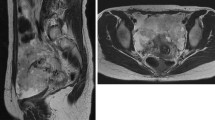
A 52-year-old woman (gravida 2, para 2) with menstrual irregularity was referred to our hospital for further examination of the uterus because endometrial cancer screening test revealed class III cytology and the histological diagnosis by subsequent endometrial biopsy was complex endometrial hyperplasia. No genital bleeding was observed. On admission, biochemistry revealed elevations of serum carbohydrate antigen 19-9 up to 482 IU/mL (normal level, <37 IU/mL) and cancer antigen 125 up to 123 IU/mL (normal level, <42 IU/mL). A pelvic MRI was obtained on a system with a 1.5-T superconducting unit (Signa Excite; General Electric). Multiple high-signal intensity polypoid nodules in the cul-de-sac surrounded by low-signal intensity rim-like fibrous adhesion protruding to the posterior wall of the uterine body were demonstrated on T2-weighted images (Fig. 1A–C). The nodules showed low signal intensity on T1-weighted images and weak contrast enhancement compared with that of uterine myometrium during any phase of dynamic contrast-enhanced MRI (Fig. 1D) and on post-contrast T1-weighted images (Fig. 1E, F). The polypoid nodules showed slight high signal intensity on diffusion-weighted images (DWI) (b = 800 s/mm2) (Fig. 1G), and relatively high mean apparent diffusion coefficient (ADC) (1.77 × 10−3 mm2/s, measured in the largest nodule) (Fig. 1H). Adenomyosis was observed on T2-weighted images (Fig. 1A–C). Endometrial thickening exhibiting high signal intensity containing small cystic areas on T2-weighted images (Fig. 1B) showed weak contrast enhancement on post-contrast T1-weighted images (Fig. 1F). Bilateral, multiple high-signal intensity ovarian cysts on T1-weighted images (multiplicity) suggested endometriomas. Polypoid endometriosis was suspected, and the patient underwent total hysterectomy, together with bilateral salpingo-oophorectomy and resection of the nodules. At surgery, severe adhesions associated with endometriosis were found around the pelvic cavity involving the polypoid nodules, the posterior wall of the uterus, and the bilateral ovarian endometriomas. Intraoperative frozen section diagnosis of the largest nodule located at left posterior to the uterus was atypical endometrial hyperplasia to well-differentiated endometrioid carcinoma, and intrapelvic lymph node dissection was also performed. Histological examination of the removed nodules confirmed them to be atypical endometrial hyperplasia to well-differentiated endometrioid carcinoma and considered as malignant transformation of polypoid endometriosis. Thickened endometrium was also diagnosed as atypical endometrial hyperplasia to well-differentiated endometrioid carcinoma, and ovarian endometriomas contained atypical endometriosis. No pelvic lymph node metastasis was revealed.
A, B Sagittal fast spin-echo T2-weighted images (repetition time/echo time (TR/TE) = 4000/102.4) and C Axial fast spin-echo T2-weighted image (TR/TE = 4000/102.4) show the retroflexed uterine body with adenomyosis as ill-defined low-signal intensity areas in the posterior uterine wall with swelling, and high-signal intensity polypoid nodules with surrounding low-signal intensity rim-like structures (arrowheads) in the cul-de-sac protruding to the posterior wall of the uterine body. Endometrial thickening (arrow) exhibiting high signal intensity containing small cystic areas. Right ovarian endometriomas are also demonstrated. D Dynamic contrast-enhanced MR images (3D fast spoiled gradient-recalled echo sequence with fat suppression; TR/TE, 4.6/2.1; slice thickness, 3 mm/1.5 mm overlap) with intravenous administration of 0.1 mmol/kg of gadopentetate dimeglumine at 2 mL/s show weak contrast enhancement of the nodules (arrowheads) compared with that of uterine myometrium during any phase of the dynamic contrast-enhanced study. E Post-contrast axial gradient-echo T1-weighted image (TR/TE = 6.6/3.1) with fat suppression shows weak contrast enhancement of the nodules (arrowheads) compared with that of uterine myometrium, and intense contrast enhancement of surrounding rim-like structures similar to the adjacent uterus. F Post-contrast sagittal gradient-echo T1-weighted image (TR/TE = 6.6/3.1) with fat suppression shows weak contrast enhancement of the thickened endometrium (arrow) similar to that of the nodules (arrowhead). G Axial echo-planar diffusion-weighted image (TR/TE = 4000/72.4, b = 800 s/mm2), and H corresponding apparent diffusion coefficient (ADC) map, which was generated from b values of 0 and 800 s/mm2 shows the polypoid nodules (arrowheads) as slight high signal intensity, and high ADC value (measured in the largest nodule: 1.77 × 10−3 mm2/s), respectively. I Photomicrograph of the largest nodule (hematoxylin and eosin staining) revealed the admixture of benign endometrial hyperplasia, atypical endometrial hyperplasia, and well-differentiated endometrioid carcinoma
Discussion
Polypoid endometriosis is benign endometriosis with histological features that resemble benign endometrial polyps of the uterus. Polypoid endometriosis frequently affects peri- to postmenopausal women when compared with usual endometriosis, and hormonal factors such as tamoxifen use or unopposed estrogen therapy can play a role in its pathogenesis [1, 5]. In our case, the patient had not received hormonal therapy, and the mechanism of malignant transformation of polypoid endometriosis is not clear; however, multicentric atypical endometrial hyperplasia to well-differentiated endometrioid carcinomas in uterine endometrium and peritoneal polypoid endometriosis, and the presence of atypical endometriosis in endometriomas may be associated with some sort of increased local estrogenic stimulation [5, 8]. Polypoid endometriosis may appear as polypoid masses arising within an ovarian endometrioma mimicking ovarian cancer, or existing in the pelvic cavity protruding to adjacent structures simulating peritoneal dissemination or malignant tumors arising from pelvic endometriosis [3–8]. Takeuchi et al. reported that high-signal intensity masses with the presence of surrounding fibrous tissue showing low signal intensity on T2-weighted images and intense contrast enhancement similar to that of the adjacent uterus on post-contrast T1-weighted images may be diagnostic clues to this rare entity [3]. Kozawa et al. reported a case of polypoid endometriosis of the ovary, which showed slight high signal intensity on DWI with relatively high ADC (1.69 × 10−3 mm2/s), and concluded that DWI findings may contribute to the diagnosis [4]. The MR imaging findings of our case on T2-weighted images were considered as compatible with those of typical polypoid endometriosis, and DWI findings (slight high signal intensity with relatively high mean ADC) were suggestive for benign to low-grade malignant lesion. Several investigators reported that the ADC values in endometrial cancers were lower than those in benign endometrial lesions [9–11]. Their results may reflect the cellularity of endometrial cancer and benign endometrial lesions: increased cellularity in endometrial cancers may restrict water diffusion and decrease the ADC compared with that in benign endometrial lesions such as endometrial hyperplasia or polyps in which edematous tissue and abundant cystic components may widen the extracellular space and increase the ADC [9]. However, contrast enhancement pattern (weak contrast enhancement compared with that of the myometrium) was different from that of reported polypoid endometriosis cases as intense contrast enhancement [3–7]. Malignant areas were admixed with hyperplasia in our case and may not be sufficient for significant mean ADC decrease of the whole lesion. Weak contrast enhancement is suggestive for malignant endometrial pathologies and might be a suggestive finding for atypical hyperplasia to well-differentiated endometrioid carcinoma in our case, possibly reflecting the relatively decreased stroma [12, 13]. In our case, MR imaging findings of thickened endometrium and nodules in the cul-de-sac were almost identical: high signal intensity on T2-weighted images and weak contrast enhancement on post-contrast T1-weighted images. Histological diagnosis of both thickened endometrium and nodules was atypical endometrial hyperplasia to well-differentiated endometrioid carcinoma, and considered as multicentric malignant transformation.
We conclude that polypoid endometriosis is a benign variant of endometriosis and should be differentiated from malignant tumors; however, polypoid endometriosis may rarely cause malignant transformation, and diagnosis in early stage is considered to be feasible for the better prognosis of patients. Weak contrast enhancement pattern similar to that of endometrial carcinoma may be suggestive for the malignant transformation of polypoid endometriosis.
References
Parker RL, Dadmanesh F, Young RH, Clement PB (2004) Polypoid endometriosis: a clinicopathologic analysis of 24 cases and a review of the literature. Am J Surg Pathol 28:285–297
Hansen K, Simon RA, Lawrence WD, Quddus MR (2012) Unilateral pelvic mass presenting in postmenopausal patients: report of two unusual cases. Ann Diagn Pathol 16:298–301
Takeuchi M, Matsuzaki K, Furumoto H, Nishitani H (2008) Case report: a case of polypoid endometriosis: MR pathological correlation. Br J Radiol 81:e118–e119. doi:10.1259/bjr/23847518
Kozawa E, Inoue K, Iwasa N, et al. (2012) MR imaging of polypoid endometriosis of the ovary. Magn Reson Med Sci 11:201–204
Kraft JK, Hughes T (2006) Polypoid endometriosis and other benign gynaecological complications associated with tamoxifen therapy—a case to illustrate features on magnetic resonance imaging. Clin Radiol 61:198–201
Marugami N, Hirohashi S, Kitano S, et al. (2008) Polypoid endometriosis of the ureter mimicking fibroepithelial polyps. Radiat Med 26:42–45. doi:10.1007/s11604-007-0188-5
Yamada Y, Miyamoto T, Horiuchi A, Ohya A, Shiozawa T (2014) Polypoid endometriosis of the ovary mimicking ovarian carcinoma dissemination: a case report and literature review. J Obstet Gynaecol Res 40:1426–1430. doi:10.1111/jog.12358
Syrcle SM, Pelch KE, Schroder AL, et al. (2011) Altered gene expression profile in vaginal polypoid endometriosis resembles peritoneal endometriosis and is consistent with increased local estrogen production. Gynecol Obstet Invest 71:77–86. doi:10.1159/000320736
Takeuchi M, Matsuzaki K, Nishitani H (2009) Diffusion-weighted magnetic resonance imaging of endometrial cancer: differentiation from benign endometrial lesions and preoperative assessment of myometrial invasion. Acta Radiol 50:947–953. doi:10.1080/02841850903099981
Wang J, Yu T, Bai R, et al. (2010) The value of the apparent diffusion coefficient in differentiating stage IA endometrial carcinoma from normal endometrium and benign diseases of the endometrium: initial study at 3-T magnetic resonance scanner. J Comput Assist Tomogr 34:332–337. doi:10.1097/RCT.0b013e3181d0f666
Kierans AS, Bennett GL, Haghighi M, Rosenkrantz AB (2014) Utility of conventional and diffusion-weighted MRI features in distinguishing benign from malignant endometrial lesions. Eur J Radiol 83:726–732. doi:10.1016/j.ejrad.2013.11.030
Imaoka I, Sugimura K, Masui T, et al. (1999) Abnormal uterine cavity: differential diagnosis with MR imaging. Magn Reson Imaging 17:1445–1455
Park BK, Kim B, Park JM, et al. (2006) Differentiation of the various lesions causing an abnormality of the endometrial cavity using MR imaging: emphasis on enhancement patterns on dynamic studies and late contrast-enhanced T1-weighted images. Eur Radiol 16:1591–1598
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
For this type of study, formal consent is not required, and the written informed consent of the patient was obtained for the MR examination.
Rights and permissions
About this article
Cite this article
Takeuchi, M., Matsuzaki, K., Bando, Y. et al. A case of polypoid endometriosis with malignant transformation. Abdom Radiol 41, 1699–1702 (2016). https://doi.org/10.1007/s00261-016-0696-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0696-9