Abstract
Prostate cancer is the most commonly diagnosed noncutaneous cancer and second-leading cause of death in men. Many patients with clinically organ-confined prostate cancer undergo definitive treatment of the whole gland including radical prostatectomy, radiation therapy, and cryosurgery. Active surveillance is a growing alternative option for patients with documented low-volume, low-grade prostate cancer. With recent advances in software and hardware of MRI, multiparametric MRI of the prostate has been shown to improve the accuracy in detecting and characterizing clinically significant prostate cancer. Targeted biopsy is increasingly utilized to improve the yield of MR-detected, clinically significant prostate cancer and to decrease in detection of indolent prostate cancer. MR-guided targeted biopsy techniques include cognitive MR fusion TRUS biopsy, in-bore transrectal targeted biopsy using robotic transrectal device, and in-bore direct MR-guided transperineal biopsy with a software-based transperineal grid template. In addition, advances in MR compatible thermal ablation technology allow accurate focal or regional delivery of optimal thermal energy to the biopsy-proved, MRI-detected tumor, utilizing cryoablation, laser ablation, high-intensity focused ultrasound ablation under MR guidance and real-time or near simultaneous monitoring of the ablation zone. Herein we present a contemporary review of MR-guided targeted biopsy techniques of MR-detected lesions as well as MR-guided focal or regional thermal ablative therapies for localized naïve and recurrent cancerous foci of the prostate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In 2015, the American Cancer Society (ACS) estimates that there will be 220,800 new diagnosed cases of prostate cancer with an estimated 27,540 deaths. Prostate cancer is the most commonly diagnosed noncutaneous cancer and second-leading cause of death in men [1]. As MR imaging for prostate cancer increases there is a growing number of patients with organ-confined prostate cancer that are raising the question of imaging directed biopsy and/or the possibility of focal ablation [2–4].
Unfortunately, it is unknown at which time an aggressive prostate cancer should be detected to attain the highest chance of cure. However, it seems intuitive that very early detection may play a role in preventing the development of metastatic disease [5]. In view of the significant disparity on recommendations for early detection and prostate cancer screening among various scientific organizations (i.e., American Urological Association, American Society of Clinical Oncology, National Comprehensive Cancer Network, ACS, US Preventative Task Force, and the European Association of Urology), and the data uncertainty of the harm/benefit balance of screening, this overview will not delve into this controversy. Our focus will be on the current state of the art of prostate biopsy techniques once the decision has been made to obtain tissue. The significance of precise tissue collection is further amplified by Level 1 evidence supporting detection and subsequent aggressive treatment of intermediate and high-risk prostate cancer [6]. Therefore accurate ascription of cancer risk (i.e., grade and stage) using the biopsy and imaging is critical. Advances in prostate imaging and image guidance play a crucial role in providing this information.
Historically, transrectal ultrasonography (TRUS) of the prostate has been integral to extend the capabilities of the physical exam of this organ. TRUS is most frequently performed in conjunction with needle biopsy of the prostate and was first popularized by Cooner and associates in early 1990 [7]. In the past, the indication for prostate TRUS was either an abnormality of the digital rectal exam (DRE) or an elevation in serum prostate-specific antigen (PSA). TRUS allows for precise direction of the biopsy needle into regional prostate anatomy and regions of interest seen on ultrasound (US). However, studies have confirmed the inability of standard gray-scale TRUS alone to localize early prostate cancer with targeting of sonographically suspicious areas (i.e., hypo- or hyper-echoic lesions) [8]. Contemporary use of prostate TRUS has evolved from conventional gray-scale ultrasonography to the use of color and power Doppler, contrast media-enhanced US, strain or shear wave elastography to better identify regions and targets of interest [9]. The added benefit of these other US modalities have not been thoroughly vetted when used as multiparametric package analogous to the series of sequences used with multiparametric MRI (mpMRI).
The TRUS prostate biopsy has remained the cornerstone for prostate cancer diagnosis dating back to the systematic ‘sextant’ biopsy protocol with 3 cores per side [10]. A meta-analysis of 68 studies led to a recommendation of a more laterally directed schema with 12 cores improving prostate cancer detection rates by a factor of 1.3 [11]. Using this systematic 12-core TRUS sampling for men undergoing initial biopsy with elevated PSA yields cancer detection rates between 30% and 55% [12]. The false-negative rate for this 12-core schema is on the order of 20%–24% [13] and repeated 12-core or saturation biopsies show detection rates of 11%–47% [14]. This is particularly true for men with anteriorly located tumors [15]. To improve the accuracy of the sampling, some experts advocate the use of template, transperineal-mapping biopsies to systematically sample all quadrants of the prostate [16]. This has been criticized for oversampling of insignificant tumors with risk of additional morbidity and need for general anesthesia. In the last decade, increasing evidence supports the use of pre-biopsy multiparametric magnetic resonance imaging for significant disease localization, with intent of addressing the limitations of the systematic biopsy, reducing the detection of low-risk cancers, and improving the detection of clinically significant prostate cancer [16–18]. A variety of approaches have evolved to capitalize on the accuracy of the multiplanar, high soft-tissue contrast, high resolution, and ability to image functional and anatomic parameters with mpMRI.
Using the ubiquitous nature of TRUS in clinical practice of prostate disease and combining the superior image characteristics of mpMRI one can combine these advantages by image fusion. The coupling of these two technologies by either software-based techniques or cognitive/visual methods promises to increase the diagnostic accuracy and may detect the same number of men with clinically significant disease using fewer cores as compared with standard biopsy [19]. “In-bore” direct MRI-guided prostate biopsies using transperineal grids, MRI-compatible robot-assisted devices, and transrectal equipment have also evolved to exploit the superb lesion conspicuity of the mpMRI [20].
The ideal prostate biopsy strategy would be to identify clinically significant prostate cancer and minimize the detection of indolent disease. Screening patients using mpMRI may avoid morbidity associated with biopsy if no lesions are seen [21] and restriction of biopsy to a targeted approach could reduce over diagnosis of low-risk disease [22]. Using MRI and targeted biopsy strategies is a promising approach. Finally, targeted biopsies have implications for active surveillance protocols with the promise of improved detection of intermediate- and high-risk disease facilitating better selection of candidates for initial biopsy [23]. The following sections will explore the evolution of MRI for cancer diagnosis, biopsy targeting, and the advancement of MRI-targeted ablative therapies for the treatment of prostate cancer.
MRI for prostate cancer imaging
Prostate cancer has traditionally been diagnosed by PSA screening and DRE followed by DRE-directed biopsy. Use of US imaging has helped direct the biopsies further but has fallen short of being sensitive enough to find all the prostate cancer within the gland. Furthermore, random sampling the entire organ has provided some answers but may also miss or undersample small volume but clinically significant disease. The recent introduction and maturation of multiparametric magnetic resonance imaging (mpMRI) now allows for imaging-based identification of prostate cancer, which may improve diagnostic accuracy for higher-risk tumors [24]. In 2015, a consensus panel agreed to PI-RADS v2 (an organized reporting system for mpMRI findings in the prostate) which is designed to improve detection, localization, characterization, and risk stratification in patients with suspected cancer in treatment naïve prostate glands [25]. Targeted biopsy of suspected cancer lesions detected by MRI is associated with increased detection of high-risk prostate cancer and decreased detection of low-risk prostate cancer particularly with the aid of MRI/US fusion platforms [26]. The use of mpMRI has expanded beyond staging to detection, characterization, monitoring for active surveillance, and cases of suspected recurrence after failed definitive therapy.
The use of MRI for recurrent prostate cancer continues to evolve and has potential to evaluate both local recurrence and distant bony and nodal metastases [27]. In 2013, a consensus panel chaired by Professor Michael Marberger endorsed utilization mpMRI to identify patients for focal therapy [28]. mpMRI is capable of localizing small tumors for focal therapy. While mpMRI plays an established, critical role in native and recurrent prostate cancer imaging, functional, metabolic imaging for prostate cancer is in its formative years. 11C-choline PET/CT has an advantage to reveal both local recurrent and distant metastatic prostate cancers. 11C-choline PET/CT had a sensitivity of 73%, a specificity of 88%, a positive predictive value (PPV) of 92%, a negative predictive value (NPV) of 61%, and an accuracy of 78% for the detection of clinically suspected recurrent prostate cancer in postsurgical patients [29]. In a study of post-prostatectomy patients with rising PSA, mpMRI is superior for the detection of local recurrence, 11C-choline PET/CT is superior for pelvic nodal metastasis, and both are equally excellent for pelvic bone metastasis. 11C-choline PET/CT and mp-MR imaging are complementary for restaging prostatectomy patients with suspected recurrent disease [27]. However, 11C-choline PET/CT is not widely available.
With the limitations of US and PET/CT imaging, MRI remains preeminent for detection and staging of prostate tumors within the pelvis. MRI provides superior soft-tissue contrast resolution, high spatial resolution, direct multiplanar imaging capabilities, and a large field of view.
If the focal treatment is being considered for potential treatment, it is very important to assure that there is no distant disease with whole body imaging. Unfortunately, no imaging modality is perfect, and appropriate selection of image staging is unique to each patient.
MRI-targeted prostate biopsy techniques
Cognitive/visual: directed MRI-targeted biopsy
During TRUS-guided biopsy procedures the operator can estimate the location of MRI-suspicious lesion based on prior review of prostate MRI. With appropriate experience this is straightforward and easy to implement without an upfront equipment investment. However, this cognitive fusion (or visual estimation) based targeted biopsy method is highly operator dependent. This method is prone to error in reliably mapping the MRI-suspicious lesion on real-time TRUS and the confirmation of TRUS-guided targeted biopsy needle location over the MRI-suspicious lesion is not feasible. In a study of 555 patients by systematic biopsy as well as cognitive fusion-guided targeted biopsy, overall 54% (302/555) of patients were found to have cancer; 82% of them were clinically significant. Systematic biopsy and cognitive fusion-guided targeted biopsy detected 88% and 98% of clinically significant cancers, respectively [30]. Cognitive fusion targeted biopsy showed 16% more high-grade cancers and higher mean cancer core lengths than standard systematic biopsy. Cognitive targeted biopsy would only avoid 13% of insignificant tumors. Using a similar visually directed approach for the transperineal biopsy, Valerio et al. compare this to a software-based targeted biopsy. The software-based, targeted transperineal approach found more clinically significant disease than visually directed biopsy although this was not statistically significant (51.9% vs. 44.3%, p = 0.24) [31]. The current diagnostic ability of visually/cognitively targeted and software-based biopsies seem to be nearly comparable. Ongoing prospective trials hope to confirm these findings, albeit based on those with significant operator experience.
Software-based ultrasound: MRI fusion targeted biopsy
Software-based MRI/TRUS fusion-guided biopsy allows combining the advantages of real-time TRUS including better temporal resolution and cost-effectiveness with those of MRI including better spatial resolution and more accurate cancer detection, and has the potential to improve detection of clinically significant cancers and for re-biopsy of patients with elevated PSA despite prior benign biopsy results. There are three key tracking methods including (1) image organ-based tracking, (2) electromagnetic sensor-based tracking, and (3) mechanical arm, sensor-based tracking.
Image organ-based tracking method fuses prior MRI with real-time US using a surface-based registration and elastic organ-based deformation algorithm (Urostation, Koelis). MRI-suspicious lesions will be brought into the aiming mechanism of the biopsy gun attached to the US probe. This is relatively inexpensive and allows systemic biopsy. However, confirmation of targeted needle biopsy tracts is retrospective [32].
Electromagnetic sensor-based tracking using non-rigid registration algorithm allows real-time spatial tracking of targets and needle location. This system is less operator dependent and allows a free-hand scanning during procedures (UroNav, InVivo; Real-time Virtual Sonography [RVS], Hitachi-Aloka). In a recent prospective study of 1003 men undergoing a MR/US fusion targeted biopsy and concurrent standard biopsy, targeted MR/US fusion biopsy was shown to diagnose 30% more high-risk prostate cancer (defined as Gleason score 4 + 3 or greater) while a combination of standard and targeted biopsies revealed 22% more prostate cancer, mostly (83%) low-risk prostate cancer (defined as Gleason score 3 + 3 and low-volume 3 + 4) [26].
Mechanical arm, sensor-based tracking system, in which a mechanical tracking arm is attached to a conventional US probe, allows real-time spatial tracking of targets and needle location (Artemis, Eigen). This system is less operator dependent but relatively expensive. In a recent retrospective review of 601 men who underwent both MRI-US fusion targeted biopsy and systematic biopsy, targeted MRI-US fusion biopsy detected fewer Gleason score 6 prostate cancers (75 vs. 121; p < 0.001) and more Gleason score ≥ 7 prostate cancers (158 vs. 117; p < 0.001) when compared with systemic biopsy [33]. In a review of 105 patients with prior negative biopsies and elevated PSA, MRI-US fusion targeted biopsy improved detection of clinically significant prostate cancer when compared with systemic biopsy [34].
In-bore direct MRI-targeted biopsy
Direct in-bore MRI-guided biopsy allows imaging confirmation of targeting without need for sophisticated image registration and fusion with live US images. Additionally, the bacterial transmission risk of a transrectal approach for biopsy is avoided. In a study of 265 patients with rising PSA despite prior negative TRUS-guided biopsies, direct in-bore MRI-guided biopsy detect cancer in 41% (108/265) of cases; 87% (94/108) were clinically significant [35]. Reporting results from a prospective clinical observational study, Penszkofer et al. showed the utility of in-bore prostate biopsies with at least one MRI-detected lesion in men with no prior prostate cancer, those undergoing active surveillance monitoring and in men with suspected recurrent cancer following treatment [20]. Overall cancer was detected in 56.7% with 48.1% with no prior cancer, 72% of those under active surveillance, and 72% of those in whom recurrence was expected. The accuracy of the transperineal in-bore biopsy appears good as demonstrated by analysis of biopsy and post-prostatectomy histopathology [36]. MRI-detected targets located in the anterior gland had the highest cancer yield (62.5%). Although these advantages are attractive, this approach is not widely used because it requires upfront investment including MRI-compatible equipment, use of scanner time for a longer procedure, higher costs/procedure, and collaboration with the urologist, radiologist, and anesthesiologist.
MRI-specialized techniques
MR guidance technologies
Interventional MR techniques have utilized needle guidance devices which have integrated varying amounts of software interface from with robotic guidance systems to live imaging during free-hand placement. Several robotic systems have been developed to guide needle placement into the prostate [37–39]. Other, systems from Invivo (Invivo Corp, Gainesville, FL) and Sentenelle (Hologic, Toronto, CA) have integrated CAD diagnostic imaging with needle guidance systems. These systems benefit from the power of separate diagnostic imaging performed pre-procedurally which can be fused with intraprocedural imaging to guide needle/probe placement. Many of the MR vendors offer live-imaging packages for aiding needle placement with live imaging using an in-room monitor.
MRI thermometry
One of the major advantages associated with MR guidance of thermal ablations is the MRI thermometry and subsequent dose estimations, which are performed in near real-time and allow for adjustments of treatment parameters and tumor targeting. The thermometry to monitor local temperatures most commonly is accomplished using the known linear dependence of proton resonance frequency (PRF) as a function of temperature. During delivery of ablative energy (generated by US transducer or laser applicator a series of 2D phase sensitive T1-weighted fast spoiled gradient-recalled echo MR images are acquired on MRI scanner [40–42]. Based on temperature changes, a thermal dose can be calculated to predict a tissue lethal dose [43].
Urethral protection catheter
The Galil Medical Urethral Warming Set (Galil Medical Ltd, Minneapolis, MN) is a disposable component used to protect and warm urethral tissue when performing cryogenic destruction of prostatic tissue. The Urethral Warming System is designed to circulate a warm saline solution through a warming double lumen catheter to maintain urethral tissue near body temperatures while the surrounding prostate tissue is being frozen. During cryoablation therapies of prostate cancer the urethral warming catheter constitutes an external heat-source, which may counter the effects of cryoablations at the lesion site. Although the isotherms around cryoneedles have been investigated [44], little is still known about the interactions between these isotherms and the urethral warmer. Recent numerical simulations [45] and phantom experiments [46], using single- and multi-needle configurations in the presence of the warmer, suggest that while the warmer indeed provides sufficient tissue protection, this comes at a detrimental cost to both, temperatures inside the cryoablation ice, and dimensions of the critical isotherms [46]. Further investigations are needed and could prove to be critical to pre-operative planning and treatment.
Focal thermal ablative therapy options
Once prostate cancer is found, localized, and imaged, it is necessary to determine a potential treatment plan. Standard therapies include radiotherapy, surgery, or androgen deprivation [47]. However, these therapies have significant risk and morbidity to the patient’s health related quality of life with potential impact on sexual, urinary, and bowel function [3]. Active screening programs for prostate cancer have enabled earlier identification of low-risk prostate cancer because of related morbidity from standard therapies and are using active surveillance to delay treatment until cancer progression [2]. Due to the morbidities of standard therapies, patients and physicians are beginning to examine focal therapies for early treatment. Focal therapy is still controversial due to the potential multifocality of prostate cancer, limitations of current biopsy strategies, suboptimal staging by accepted imaging modalities and less than robust prediction models for indolent prostate cancers. In spite of these restrictions focal therapy continues to confront the current paradigm of therapy for low-risk disease [4]. Furthermore, prostate cancer recurrence rates after established forms of therapy range from 20% to 60% [48]. In this review, we describe some of the recent advances in focal therapy for native prostate cancer and recurrent prostate cancer where MR imaging is used to direct focal therapy.
Selection of patients with native prostate cancer for MR-guided focal therapy
In selecting the appropriate patient for focal therapy of the native prostate gland, it is critical to determine that the patient has localized low-risk disease. With low-risk disease, there is level 1 evidence that implies a lack of benefit from radical therapy [49–51]. Patients are many times targeted for cancer workup due to rising PSA or nodule on DRE. Patients are further evaluated with a mapping biopsy and/or mpMRI with targeted biopsy. Patients are classified to have low or intermediate prostate cancer with a focal positive lesion on mpMRI, Gleason ≤ 4 + 3, and PSA < 20 ng/mL. For consideration for focal therapy, the target lesion should be confined to one lobe of the prostate [52]. Furthermore, the target should be visible with the imaging modality which will be used to guide the focal ablation treatment.
Selection of patients with localized recurrent prostate cancer for MR-guided focal therapy
The first issue is to determine whether the rising PSA represents local recurrence, systemic recurrence, or both [53]. The second issue in managing patients with biochemical recurrence (BCR) of prostate cancer is assessing the risk of cancer treatment vs. the risk of further intervention. Overall, rapid PSA rise, short disease-free interval, and high-grade disease are all poor prognostic indicators with a higher likelihood of systemic recurrence, while slow PSA rise, long disease-free interval, and low-grade disease are better prognostic indicators with a higher likelihood of local recurrence [53, 54].
Suggested criteria for MR-guided focal ablative treatment in recurrent prostate cancer are as follows: (1) biopsy-proven local recurrent tumor that can be visualized by MRI, (2) absence of distant metastasis confirmed with chest, abdomen, pelvis CT, and/or MRI plus bone scintigraphy, and/or 11C choline PET/CT scan [55]. Although these selection criteria are not perfect, they are helpful in avoiding treatment of what is thought to be a local recurrence which is really systemic.
Focal therapy treatments for native prostate cancer
Although radical prostatectomy and radiation therapy remain preferred definitive therapy of choice for men with newly diagnosed prostate cancer and with a life expectancy >10 years [56, 57], there is increasing interest in less radical focal methodologies for treatment especially in the watchful waiting population. For this population of low- and intermediate-risk prostate cancer patients, active surveillance may be undesirable from the patient standpoint, yet the complications and co-morbidities associated with standard therapies may seem too great for the patient. This patient-driven interest for a more minimally invasive approach is driving focal therapies for prostate carcinoma in low-risk patients [58]. As a result, several minimally invasive thermal ablation methods under direct MR guidance, most prominently cryotherapy [59], laser ablation [60], and high-intensity focused ultrasound (HIFU) [61], have been developed and are currently being evaluated.
MR-guided cryoablation
MR-guided percutaneous cryoablation combines excellent soft-tissue resolution and ice ball monitoring. Early experience combining cryoablation with MRI has shown a high degree of accuracy in defining normal and frozen tissue on all MR imaging sequences [62, 63]. Additionally, MR imaging allows visualization of the ice ball in multiple planes which becomes critically important in the pelvis where there is limited safety for non-target ice ball growth. The final advantage is that this procedure does not appreciably limited by the prior surgical or radiation to the treated area [64, 65].
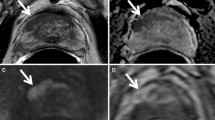
There is limited data using MR-guided cryoablation within the native prostate has limited data. Two published canine studies demonstrated feasibility and overall safety [66, 67]. These studies did bring out one limitation of cryoablation which is that the visualized edge of the ice (0 °C) does not represent the ablation margin. The actual ablation margin is best demonstrated with contrast enhancement post-procedure and is actually at the −20 °C isotherm, which is just inside the edge of the ice ball. There are two published reports of MR-guided cryoablation in native prostate glands, each with relatively small numbers (Fig. 1) [64, 65]. Gangi et al. performed MRI-guided prostate cryoablation in eleven patients on 1.5T MRI [65]. They had some minor complications of hematuria, dysuria, and urine retention. Additionally, they had one major complication of rectal fistula with spontaneous closure after 3 months. The other study examined 18 patients with two slightly different methodologies [64]. The group treated with a more aggressive freezing regimen had better results over time [64]. These studies confirm that MR-guided cryoablation is technically feasible with relative safety, however, more short- and long-term data is needed to assess overall efficacy.
MR-guided cryoablation images from preprocedure diagnostic 3.0T MRI (A and B) and intraprocedural MRI 1.5T. A Axial 3T T2-weighted FRFSE image preprocedure demonstrating a small area of marked focal low T2 signal (white arrow) in the peripheral zone of the right posterior prostate gland with some diffuse indeterminate low signal throughout the posterior prostate gland on both sides. B Axial dynamic contrast-enhanced T1-weighted LAVA-flex image at 3T demonstrating hyperenhancement corresponding to the focal area of low T2 signaling (white arrow). C Axial unenhanced T1-weighted Vibe image at 1.5T performed for examination of needle position (small signal voids) relative to the urethra which has the urethral warming catheter (asterisk) within it. D Subsequent axial Vibe image demonstrating the extent of the ice ball as a large signal void (blue arrows). E Contrast enhanced imaging immediately after cryoablation demonstrates a lack of enhancement in the mid and posterior prostate gland with some enhancement in the anterior prostate. F Contrast enhanced imaging 6 months after cryoablation again demonstrates a lack of enhancement in the mid and posterior prostate gland with some enhancement in the anterior prostate
MR-guided laser ablation
Laser-induced interstitial thermal therapy (LITT) uses a locally placed laser fiber to deliver targeted thermal ablation. LITT is inherently magnetic resonance (MR) compatible making it an obvious choice of ablation technologies to couple with MR imaging. MR-based temperature monitoring allows real-time feedback during LITT treatment as both deposition of light energy and MR signal acquisition can be performed simultaneously without degradation in the MR image [68]. Performance of the ablation within the MRI allows use of post-treatment imaging to verify treatment delivery. Because MR images clearly depict the prostate anatomy and the surrounding critical structures, MR imaging is critical for monitoring ablation growth to prevent encroachment onto adjacent critical structures.
Two publications demonstrated feasibility in canine prostate as well [69, 70]. These were beneficial due to showing technical feasibility but also demonstrated correlation of the MR temperature map with contrast-enhanced T1-weighted images. A subsequent study in cadavers demonstrated technical feasibility in the human prostate within a 3T MRI scanner [71]. Lee et al. published early results on 23 patients treated with focal laser ablation demonstrating promising results [71]. Raz et al. described using laser ablation for treatment of 2 prostate cancer patients at 1.5T with discharge 3 h after the procedure [72]. These studies demonstrate the potential utility of laser ablation in the prostate. However, more data is needed to determine short- and long-term effects.
MR-guided focused ultrasound ablation
Treatment of the prostate with focused US ablation is not new although MRI-guided version of procedure has not been approved by FDA in the United States. This has been performed with transrectal US imaging guidance with success in Europe for many years [61, 73]. However, a major limitation of US imaging guidance is the difficulty in visualizing the focus of cancer, especially if the focus is small. Therefore, the treatment strategy used with US-guided HIFU is to ablate the entire prostate, or relatively large region where the site of biopsy-proven cancer was found using a mapping biopsy and/or mpMRI. This often results in inadequate tumor control or over-ablation of unnecessary normal/neural tissue with potential subsequent morbidity. An early study, using US-guided focused US ablation in prostate by Gelet et al., treated 82 patients who were treated and followed up for 24 months duration. These patients also received subsequent radiation treatment [74]. Among these patients, 68% were cancer free at the time of follow-up. Due to relatively high complication rates, the treatment device underwent multiple iterations and improvements. A subsequent study, by Gelet et al., demonstrated incontinence and impotency rates around 14% and 61% respectively at 19 months post-treatment. In both studies, major limitations were identified as total procedure time due to need to cover the entire prostate and inability to monitor temperatures or ablation zone expansion [75]. MR thermal monitoring and localization of lesions/zones within prostate should allow optimization of ablation treatment zone while ablation temperature monitoring should allow improved safety margin in regard to vital adjacent tissues. Currently, there are two MRI integrated systems using transrectal (Exablate, InSightec, Haifa, Israel) or transurethral (Profound Medical Inc., Toronto, Canada) transmission routes for treatment of prostate lesions with focused US technology. The system is fully integrated with the MRI console with temperature feedback control to adjust power, frequency, and rotation rate. Both systems are currently being used in patient trials assessing safety and efficacy for evidence to get FDA approval.
Recurrent prostate cancer
Standard therapies for treatment of recurrent prostate cancer
Traditional curative therapy for prostate cancer has been either surgical resection or radiotherapy. Patients are roughly split with half choosing surgery and half choosing radiotherapy. Recurrences after surgical resection can range from 25% to 40%, which is usually manifest by rising serum level of PSA [53, 76, 77]. Approximately 30,000 men will develop BCR with rising PSA after radical prostatectomy each year in the USA [77]. In one study examining where recurrences happen found 81% had a local prostate bed recurrence which could be demonstrated with an MRI using an endorectal coil [78]. For those that undergo radiotherapy, BCR can range widely from 33% to 63% over 10 years, and contributes another 45,000 men/year with recurrent cancer in the USA from radiotherapy [79, 80]. Although 5-year disease-free survival from prostate cancer approach 100% in the United States, including good outcomes from primary therapies, these figures clearly demonstrate a significant number of men develop recurrent cancer each year. Salvage treatments currently available for recurrent prostate cancer include; salvage radical prostatectomy, salvage radiotherapy, salvage US (U/S)-guided HIFU, salvage U/S-guided cryoablation, and newly described salvage MRI-guided laser and cryoablation.
MR-guided cryoablation
MR-guided cryoablation for recurrent prostate cancer has also been shown feasible and successful in a number of small limited studies. Woodrum et al. published on 18 patients treated with MR-guided cryoablation for locally recurrent prostate cancer. They broke the cohort into two groups of nine patients with alteration of the cryoablation technique between the groups [64]. They demonstrated that a tight (5 mm) spacing, three freeze–thaw cycles, and sometimes decreasing the urethral warmer temperature produced better short-term recurrence free intervals. Additionally, Gangi et al. demonstrated successful MR-guided cryoablation treatment of several patients with recurrent prostate cancer as well [65]. Using MR guidance, cryoablation treatment can be tailored to the desired region (Fig. 2) or focal area (Fig. 3).
Multiparametric 3.0T MRI (A and B) with endorectal coil in place. Axial dynamic contrast-enhanced LAVA-FLEX images demonstrating abnormal T2 signal (arrow) in the left peripheral zone (A) and corresponding abnormal tumor enhancement on axial dynamic contrast images (arrow) (B). Intraprocedural 1.5T MRI axial image demonstrates iceball coverage (blue arrowheads) within the left half of the prostate extending across the posterior midline with the urethral warmer preventing encroachment on the urethra (C). Post-ablation contrast enhancement demonstrating the ablation defect covering the previously enhancing lesion (blue arrowheads) (D)
Axial CT PET/C 11 choline image demonstrates increased activity in the right seminal vesicle remnant consistent with recurrent prostate cancer (arrows, A). Multiparametric 3.0T MRI with endorectal coil in place demonstrates axial T1-weighted images showing focal thickening of the right seminal vesicle remnant (arrows, B). Axial (C) and sagittal (D) TSE images obtained during treatment showing the final extent of the ice ball (arrowhead) encompassing the seminal vesicle prostate cancer recurrence (biopsy proven) and saline displacement of the rectum (asterisk)
MR-guided laser interstitial therapy (LITT)
Using Laser interstitial thermal therapy (LITT) for recurrent prostate cancer is a relatively recent development. A feasibility study and a case report using a 3T MRI with Visualase 980 nm diode laser system (Medtronic, Minneapolis, MN, USA) to treat a prostate cancer [81]. A small case series was also presented by the same group which demonstrated feasibility of treating recurrent prostate cancer with laser ablation (Fig. 4) [81]. One complicating factor with MR-guided laser ablation in patients with prior surgical resection is the presence of surgical clips, which cause susceptibility artifacts and disrupt the MR-based temperature mapping. Therefore, recurrences within the surgical clips present would be a relative contraindication for this method of treatment.
MR images of the 15W visualase laser ablation with double applicator (A–D) activation. Preprocedure axial DCE MR image (A) demonstrating hyperenhancement (white arrow) consistent with biopsy-proven recurrent prostate cancer in the left posterior gland. Intra-ablation MRI monitoring with corresponding temperature sensitive phase imaging (B) displaying color-coded temperature changes, and calculated damage map (red arrows) (C). The calculated damage map takes into account the temperature and time variables estimating ablation damage using Arrhenius model of thermal tissue damage, projected back onto the magnitude images as solid orange (red arrows) (C). Post-ablation contrast-enhanced imaging demonstrates the ablation zone (red arrows) (D)
Post-procedural imaging following MR directed treatments
To appropriately assess the ablative zone following ablative treatment, imaging immediately post-ablation includes T1/T2-weighted images in axial, sagittal, and coronal planes. Diffusion weighted images in the axial plane can also be acquired. Finally, DCE MR images are critical with images acquired in the axial plane and also potentially in the sagittal and coronal planes. The contrast-enhanced images give the best immediate ablation zone examination. However, it is important be aware with cryoablation that there can be residual vessel enhancement immediately after ablation and also for several months which is normal and does not indicate a failed ablation [82].
Follow-up imaging
After MR-guided thermal ablation, the best way to monitor the patient is a combination of MR imaging and serum PSA. For the salvage patients, PSA levels are expected to be undetectable within several weeks of salvage procedure. However, for the focal gland ablation within the native gland, the PSA levels should lower and then plateau at a new baseline. MR imaging becomes important post-ablation for monitoring the involution of the ablation zone with repeat biopsies as needed. A rise in a previously undetectable or stable postoperative PSA levels during post-treatment follow-up raise concerns about recurrent or new prostate cancer. Careful assessment of PSA velocities at 3 and 6 months may trigger concern for systemic disease.
Challenges to MR guidance
Limitations of MRI thermometry
PRF temperature mapping utilizes the phenomenon of linear change of resonance frequency of water protons with temperature. PRF temperature mapping is a powerful tool in MRI, however, there are certain limitations to the technique due mainly to the fact that these types of thermometry measures phase changes between an initial baseline image and all subsequent images acquired in real-time. Ideally, all these images should be in perfect alignment with no motion between them. As a consequence, motion is a large problem where the baseline image alignment is disrupted causing phase registration artifacts. A method that has been proposed to alleviate this is the reference-less temperature mapping [83, 84]. Another issue is metallic artifact causing signal drop-out with resulting artifact. In the native prostate, this is less of an issue, but in the postsurgical prostate bed surgical clip artifact becomes a real problem for phase change-based temperature imaging. The final issue with temperature mapping is problem with tissue/fat interface. The PRF temperature mapping method is not much less sensitive in fat as in glandular tissue due to differing water content.
Limitations to MRI visualization of iceball temperature isotherms
A major limitation for MR-guided cryoablation is that the iceball isotherms are not readily visualized. The leading edge of the iceball is well visualized, but this corresponds to 0 °C and may not be lethal. Therefore, it is necessary to carry the edge of the ice beyond the tumor margin by at least 5 mm. However, this assumes that iceball lethal isotherms of −40 °C are less than 5 mm from the leading edge of the iceball [85]. When other factors such as major vessels or urethral warmers are added to the treatment scenario, this assumption may or may not be true [86]. Additionally, there is currently no reliable MR compatible temperature monitoring devices to confirm lethal temperatures. Confounding the need for good margin coverage is the problem of very restrictive space in and around the prostate bed with close proximity to the rectum, bladder, and external striated urethral sphincter with very little margin for error.
Conclusions
As the most common cancer in men, prostate cancer diagnosis and treatment for new or recurrent disease will demand considerable resources and effort for years to come. MRI is playing a seminal role in the management of this disease. MRI and US fusion for prostate biopsy guidance appears to represent the next step in timely diagnosis and navigation to clinically significant cancers. While MR-guided focal ablation of native or recurrent prostate cancer is feasible and rapidly becoming a viable treatment alternative, all focal therapy treatment series suffer from relatively small patient numbers and need for comparison to established therapies. Additionally, it is critically important that good prospective clinical trials for each treatment modality be performed to assess the advantage of each treatment modality and to determine long-term efficacy.
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics. CA Cancer J Clin 65(1):5–29
Jemal A, Siegel R, Ward E, Murray T, et al. (2006) Cancer statistics. CA Cancer J Clin 56(2):106–130
Potosky AL, Davis WW, Hoffman RM, et al. (2004) Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst 96(18):1358–1367
Onik G, Vaughan D, Lotenfoe R, et al. (2007) “Male lumpectomy”: focal therapy for prostate cancer using cryoablation. Urology 70(6 Suppl):16–21
Vickers J, Ulmert D, Sjoberg D, et al. (2013) Strategy for detection of prostate cancer based on relation between prostate specific antigen at 40-55 and long term risk of metastasis: case-control study. BMJ 346:f2023
Bill-Axelson A, Holmberg I, Garmo H, et al. (2014) Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 370:932–942
Cooner W, Mosley R, Rutherford D, et al. (1990) Prostate cancer detection in a clinical urological practice by ultrasonography, digital rectal examination and prostate specific antigen. J Urol 143:1146–1152
Flanigan R, Catalona W, Richie J, et al. (1994) Accuracy of digital rectal examination and transrectal ultrasonography in localizing prostate cancer. J Urol 152:1506–1509
Maxeiner A, Stephan C, Durmus T, et al. (2015) Added value of multiparametric ultrasonography in magnetic resonance imaging and ultrasonography fusion-guided biopsy of the prostate in patients with suspicion for prostate cancer. Urol 86(1):108–114
Hodge K, McNeal S, Terris M, et al. (1989) Random systematic versus directed ultrasound-guided transrectal core biopsies of the prostate. J Urol 142:71–74
Eichler K, Hempel S, Wilby J, et al. (2006) Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol 175:1605–1612
Jones J (2007) Saturation biopsy for detecting and characterizing prostate cancer. BJU Int 99:1340–1344
Lane B, Zippe C, Abouassaly R, et al. (2008) Saturation technique does not decrease cancer detection during follow-up after initial prostate biopsy. J Urol 179:1746–1750
Nelson A, Harvey R, Parker R, et al. (2013) Repeat prostate biopsy strategies after initial negative biopsy: meta-regression comparing cancer detection of transperineal, transrectal saturation and MRI guided biopsy. PloS One 8:e57480
Ahmed H, Emberton M, Kepmer G, et al. (2012) A biomedical engineering approach to mitigate the errors of prostate biopsy. Nat Rev Urol 9:227–231
Salami S, Ben-Levi E, Yaskiv O, et al. (2015) In patients with a previous negative prostate biopsy and a suspicious lesion on MRI, is a 12 core biopsy still necessary in addition to a targeted biopsy? BJU Int 115:562–570
Arumainayagam N, Ahmed H, Moore C, et al. (2013) Multiparametric MR imaging for detection of clinically significant prostate cancer: a validation cohort study with transperineal template prostate mapping as the reference standard. Radiology 268:761–769
Ahmed H, Kirkham A, Arya M, et al. (2009) Is it time to consider a role for MRI before prostate biopsy? Nav Rev Clin Oncol 6:197–206
Moore C, Robertson N, Arsanious N, et al. (2013) Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 63:125–140
Penskofer T, Tuncall K, Fedorov A, et al. (2015) Transperineal in-bore 3-T MRI imaging-guided prostate biopsy: a prospective clinical observational study. Radiology 274(1):170–180
Hamoen E, de Rooij M, Witjes J, et al. (2015) Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol 67:1112–1121
Futterer J, Briganti A, De Visschere P, et al. (2015) Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 68:1045–1053
Schoots I, Petrides N, Giganti F, et al. (2015) Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol 67:627–636
Turkbey B, Mani H, Shah V, et al. (2011) Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 186(5):1818–1824
Radiology ACo (2015) MR prostate imaging reporting and data system version 2.0. http://www.acrorg/Quality-Safety/Resources/PIRADS/
Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. (2015) Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama 313(4):390–397
Kitajima K, Murphy RC, Nathan MA, et al. (2014) Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med 55(2):223–232
Futterer JJ, Gupta RT, Katz A, et al. (2014) The role of magnetic resonance imaging (MRI) in focal therapy for prostate cancer: recommendations from a consensus panel. BJU Int 113(2):218–227
Reske SN, Blumstein NM, Glatting G (2008) [11C] choline PET/CT imaging in occult local relapse of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging 35(1):9–17
Haffner J, Lemaitre L, Puech P, et al. (2011) Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int 108:E171–E178
Valerio M, McCartan N, Freeman A, et al. (2015) Visually directed vs. software-based biopsy compared to transperineal template mapping biopsy in the detection of clinically significant prostate cancer. Urol Oncol 33:424.e9-424.e16
Mozer P, Rouprêt M, Le Cossec C, et al. (2015) First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int 115(1):50–57
Meng X, Rosenkrantz AB, Mendhiratta N, et al. (2015) Relationship between prebiopsy multiparametric magnetic resonance imaging (MRI), biopsy indication, and MRI-ultrasound fusion-targeted prostate biopsy outcomes. Eur Urol. doi:10.1016/j.eururo.2015.06.005
Sonn GA, Chang E, Natarajan S, et al. (2014) Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol 65:809–815
Hoeks CMA, Schouten MG, Bomers JGR, et al. (2012) Three-Tesla magnetic resonance–guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol 62:902–909
Garmer M, Busch M, Mateiscu S, et al. (2015) Accuracy of MRI-targeted in-bore prostate biopsy according to the Gleason score with postprostatecomy histopathologic control—a targeted biopsy-only strategy with limited number of cores. Acad Radiol 22(11):1409–1418
Elhawary H, Zivanovic A, Rea M, et al. (2006) The feasibility of MR-image guided prostate biopsy using piezoceramic motors inside or near to the magnet isocentre. Med Image Comput Comput Assist Interv Int Conf 9(Pt 1):519–526
Lagerburg V, Moerland MA, van Vulpen M, et al. (2006) A new robotic needle insertion method to minimise attendant prostate motion. Radiother Oncol 80(1):73–77
van den Bosch MR, Moman MR, van Vulpen M, et al. (2010) MRI-guided robotic system for transperineal prostate interventions: proof of principle. Phys Med Biol 55(5):N133–N140
Vitkin IA, Moriarty JA, Peters RD, et al. (1997) Magnetic resonance imaging of temperature changes during interstitial microwave heating: a phantom study. Med Phys 24(2):269–277
Hynynen K, Freund WR, Cline HE, et al. (1996) A clinical, noninvasive, MR imaging-monitored ultrasound surgery method. Radiographics 16(1):185–195
Ishihara Y, Calderon A, Watanabe H, et al. (1995) A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med 34(6):814–823
Sapareto SA, Dewey WC (1984) Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 10(6):787–800
Young JL, Kolla SB, Pick DL, et al. (2010) In vitro, ex vivo and in vivo isotherms for renal cryotherapy. J Urol 183(2):752–758
Baissalov R, Sandison GA, Donnelly BJ, et al. (2000) A semi-empirical treatment planning model for optimization of multiprobe cryosurgery. Phys Med Biol 45(5):1085–1098
Gorny K, King D, Felmlee J, et al. (2011) In-vitro investigations of the urethral warmer on isotherms during interstitial cryoablations for prostate cancer. AAPM, p 3483
Cooperberg MR, Broering JM, Carroll PR (2010) Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 28(7):1117–1123
Cooperberg MR, D’Amico AV, Karakiewicz PI, et al. (2013) Management of biochemical recurrence after primary treatment of prostate cancer: a systematic review of the literature. Eur Urol 64(6):905–915
Silberstein JL, Vickers AJ, Power NE, et al. (2011) Reverse stage shift at a tertiary care center: escalating risk in men undergoing radical prostatectomy. Cancer 117(21):4855–4860
Wilt TJ, Brawer MK, Jones KM, et al. (2012) T. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 367(3):203–213
Budaus L, Spethmann J, Isbarn H, et al. (2011) Inverse stage migration in patients undergoing radical prostatectomy: results of 8916 European patients treated within the last decade. BJU Int 108(8):1256–1261
van den Bos W, Pinto PA, de la Rosette JJ (2014) Imaging modalities in focal therapy: patient selection, treatment guidance, and follow-up. Curr Opin Urol 24(3):218–224
Stephenson AJ, Slawin KM (2004) The value of radiotherapy in treating recurrent prostate cancer after radical prostatectomy. Nat Clin Pract Urol 1(2):90–96
Partin AW, Pearson JD, Landis PK, et al. (1994) Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology 43(5):649–659
Uchida T, Shoji S, Nakano M, et al. (2011) High-intensity focused ultrasound as salvage therapy for patients with recurrent prostate cancer after external beam radiation, brachytherapy or proton therapy. BJU Int 107(3):378–382
Hakimi AA, Feder M, Ghavamian R (2007) Minimally invasive approaches to prostate cancer: a review of the current literature. Urol J 4(3):130–137
Menon M, Tewari A, Peabody JO, et al. (2004) Vattikuti Institute prostatectomy, a technique of robotic radical prostatectomy for management of localized carcinoma of the prostate: experience of over 1100 cases. Urol Clin N Am 31(4):701–717
Polascik TJ (2014) How to select the right patients for focal therapy of prostate cancer? Curr Opin Urol 24(3):203–208
Katz AE (2009) Prostate cryotherapy: current status. Curr Opin Urol 19(2):177–181
Lee T, Mendhiratta N, Sperling D, Lepor H (2014) Focal laser ablation for localized prostate cancer: principles, clinical trials, and our initial experience. Rev Urol 16(2):55–66
Rogenhofer S, Ganzer R, Lunz J-C, et al. (2008) Eight years’ experience with high-intensity focused ultrasonography for treatment of localized prostate cancer. Urology 72(6):1329–1333 ((discussion 1333–1324))
Tacke J, Adam G, Haage P, et al. (2001) MR-guided percutaneous cryotherapy of the liver: in vivo evaluation with histologic correlation in an animal model. J Magn Reson Imaging 13(1):50–56
Tuncali K, Morrison PR, Tatli S, et al. (2006) MRI-guided percutaneous cryoablation of renal tumors: use of external manual displacement of adjacent bowel loops. Eur J Radiol 59(2):198–202
Woodrum DA, Kawashima A, Karnes RJ, et al. (2013) Magnetic resonance imaging-guided cryoablation of recurrent prostate cancer after radical prostatectomy: initial single institution experience. Urology 82(4):870–875
Gangi A, Tsoumakidou G, Abdelli O, et al. (2012) Percutaneous MR-guided cryoablation of prostate cancer: initial experience. Eur Radiol 22(8):1829–1835
Josan S, Bouley DM, van den Bosch M, et al. (2009) MRI-guided cryoablation: in vivo assessment of focal canine prostate cryolesions. J Magn Reson Imaging 30(1):169–176
van den Bosch MA, Josan S, Bouley DM, Chen J, et al. (2009) MR imaging-guided percutaneous cryoablation of the prostate in an animal model: in vivo imaging of cryoablation-induced tissue necrosis with immediate histopathologic correlation. J Vasc Interv Radiol 20(2):252–258
McNichols RJ, Gowda A, Kangasniemi M, et al. (2004) MR thermometry-based feedback control of laser interstitial thermal therapy at 980 nm. Lasers Surg Med 34(1):48–55
McNichols RJ, Gowda A, Gelnett MD, et al. (2005) Percutaneous MRI-guided laser thermal therapy in canine prostate. In: SPIE, San Jose, pp 214–225
Stafford RJ, Shetty A, Elliott AM, et al. (2010) Magnetic resonance guided, focal laser induced interstitial thermal therapy in a canine prostate model. J Urol 184(4):1514–1520
Woodrum DA, Gorny KR, Mynderse LA, et al. (2010) Feasibility of 3.0T magnetic resonance imaging-guided laser ablation of a cadaveric prostate. Urology 75(6):1514.e1511-1516
Raz O, Haider MA, Davidson SR, et al. (2010) Real-time magnetic resonance imaging-guided focal laser therapy in patients with low-risk prostate cancer. Eur Urol 58(1):173–177
Thuroff S, Chaussy C, Vallancien G, et al. (2003) High-intensity focused ultrasound and localized prostate cancer: efficacy results from the European multicentric study. J Endourol 17(8):673–677
Gelet A, Chapelon JY, Bouvier R, et al. (2000) Transrectal high-intensity focused ultrasound: minimally invasive therapy of localized prostate cancer. J Endourol 14(6):519–528
Gelet A, Chapelon JY, Bouvier R, et al. (2001) Transrectal high intensity focused ultrasound for the treatment of localized prostate cancer: factors influencing the outcome. Eur Urol 40(2):124–129
Brandeis J, Pashos CL, Henning JM, et al. (2000) A nationwide charge comparison of the principal treatments for early stage prostate carcinoma. Cancer 89(8):1792–1799
Moul JW (2000) Prostate specific antigen only progression of prostate cancer. J Urol 163(6):1632–1642
Sella T, Schwartz LH, Swindle PW, et al. (2004) Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology 231(2):379–385
Agarwal PK, Sadetsky N, Konety BR, Cancer of the Prostate Strategic Urological Research E, et al. (2008) Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer 112(2):307–314
Kuban DA, Thames HD, Levy LB, et al. (2003) Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys 57(4):915–928
Woodrum DA, Mynderse LA, Gorny KR, et al. (2011) 3.0T MR-guided laser ablation of a prostate cancer recurrence in the postsurgical prostate bed. J Vasc Interv Radiol 22(7):929–934
Porter CAt, Woodrum DA, Callstrom MR, et al. (2010) MRI after technically successful renal cryoablation: early contrast enhancement as a common finding. AJR Am J Roentgenol 194(3):790–793
Rieke V, Kinsey AM, Ross AB, et al. (2007) Referenceless MR thermometry for monitoring thermal ablation in the prostate. IEEE Trans Med Imaging 26(6):813–821
Rieke V, Vigen KK, Sommer G, et al. (2004) Referenceless PRF shift thermometry. Magn Reson Med 51(6):1223–1231
Gage AA, Baust J (1998) Mechanisms of tissue injury in cryosurgery. Cryobiology 37(3):171–186
Favazza CP, Gorny KR, King DM, et al. (2014) An investigation of the effects from a urethral warming system on temperature distributions during cryoablation treatment of the prostate: a phantom study. Cryobiology 69(1):128–133
Acknowledgments
Dr. Woodrum has an NIH grant, “Regulation of molecular thermal ablative resistance in hepatocellular carcinoma.Funded by National Cancer Institute. (R01 CA 177686)” but no grant funds or time was used on this project. Dr. Mynderse, the Mayo Clinic and Philips Healthcare/Invivo have a cooperative research and development agreement, but no grant funds or time was used on this project. Dr. Kawashima and Dr. Gorny do not have funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No conflict of interest for any of the authors.
Rights and permissions
About this article
Cite this article
Woodrum, D.A., Kawashima, A., Gorny, K.R. et al. Targeted prostate biopsy and MR-guided therapy for prostate cancer. Abdom Radiol 41, 877–888 (2016). https://doi.org/10.1007/s00261-016-0681-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0681-3