Abstract
Multiparametric MRI has revolutionized the imaging in the diagnostic pathway of prostate cancer. Multiparametric MRI followed by MR-targeted biopsy of suspicious lesions may preclude biopsy in at least a quarter of at-risk men. Over the past 10 years, various strategies have been developed for performing MR-targeted biopsy of prostate. A direct in-bore MR-guided biopsy allows real-time MR imaging and prostate sampling while patient is lying inside MR gantry, while MR-ultrasound fusion-targeted biopsy registers previously acquired MRI data with the real-time ultrasound images at the day of biopsy to simulate an MR-targeted approach. MR-ultrasound fusion-targeted biopsy and in-bore MR-guided biopsy have been shown to be safe and outperform traditional systematic 12-core transrectal ultrasound-guided (TRUS) biopsy, with significantly higher detection of clinically significant prostate cancer and lower detection of clinically insignificant cancer. Cost-effectiveness assessments have shown both MR-based approaches to have higher quality-adjusted life-year benefits. This chapter reviews the technical aspects, the current literature, and future directions for MR-ultrasound fusion and in-bore MR-guided biopsy of prostate. While there is no consensus on the preferred MR-targeted approach for sampling prostate, each MR-targeted biopsy technique provides its advantages and disadvantages. The selection of the preferred method needs to be tailored per patient basis and considering the availability of the technique and expertise of the center.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Systematic transrectal ultrasound-guided biopsy (TRUS bx) of prostate has been regarded as the standard of care for sampling prostate in men with clinical suspicion of prostate cancer (PCa), mainly based on the increased prostate-specific antigen (PSA) or abnormal digital rectal exam (DRE).
While the traditional goal of TRUS bx was cancer detection, the contemporary goal of targeted prostate biopsy is to maximize the detection rate of clinically significant prostate cancer (csPCa) (Gleason score (GS) ≥ 7 or Gleason group (GG) ≥ 2) and minimize the detection of clinically insignificant cancer (low-volume cancer with Gleason group 1). Long-term cohort studies have shown that patients with low-grade, clinically insignificant prostate cancers do not benefit from invasive therapy, because cancer-related mortality in this subgroup of patients was similar to patients on active surveillance, while they show higher rates of morbidity [1, 2]. Traditional TRUS bx , adopted widely since 1986, has several drawbacks. First, it is a random sampling of the peripheral, posterior half of the prostate gland and may miss clinically significant foci of PCa located outside the biopsy zones. Transition zone and anterior region lesions are often missed on systematic TRUS bx. Out of 121 anterior PCa lesion in the study of Volkin et al., 48.7% would have been missed by TRUS bx alone [3]. Second, it is associated with an unfavorably high detection of low-grade PCa. Third, TRUS bx underestimates PCa grade and leads to error rates in up to 49% in patients on active surveillance [4]. Fourth, the risk of bleeding and infection/sepsis is up to 5% in men undergoing TRUS bx. Other strategies such as transperineal-ultrasound biopsy (TPUS bx) also have these limitations. Overall, despite the simplicity and widespread availability of TRUS bx, the nontargeted nature of the technique undersamples csPCa and oversamples clinically insignificant PCa.
The accuracy of prostate imaging for detection of PCa has significantly increased with the advances in multiparametric MRI (mpMRI) hardware, imaging sequences, and postprocessing software [5]. A standardized method has been developed to increase inter-reader reliability for interpretation of prostate mpMRI . Based in part on prior published single-center scoring systems (e.g., UCLA score, NIH score), the Prostate Imaging-Reporting and Data System (PIRADS) was introduced in 2012 with ongoing updates (PIRADS v2 and v2.1) [6, 7]. The PRECISION trial, a multicenter, randomized trial of 500 men, suggested that initial assessment with mpMRI and MR-US fusion biopsy (MRUS-Fbx) of MR-positive lesions resulted in 12% increase in detection of csPCa and 13% decrease in detection of clinically insignificant cancers, compared to systematic TRUS bx alone. A retrospective review of 4259 individuals [8] who underwent mpMRI between January 2012 and December 2017 showed that biopsy was precluded in 53% of individuals at risk by using PIRADS assessment scoring. csPCa diagnosis-free survival was 99.6% after 3 years in this group of individuals. The PROMIS study, which was a paired-cohort confirmatory study [9], showed that mpMRI has higher sensitivity and negative predictive value for the detection of clinically significant PCa, compared to TRUS bx. A high negative predictive value is important because a negative mpMRI result would assure that csPCa would be highly unlikely, potentially precluding biopsy in many individuals at risk. The PROMIS study indicated that targeted biopsy in individuals with suspicious mpMRI could potentially avoid biopsy in up to 25% of men at risk. The study reported that targeted biopsy can improve the detection of clinically significant PCa with significant reduction in the number of diagnosed clinically insignificant cancer.
Various strategies for performing MR-targeted biopsy of prostate have been proposed without an absolute consensus on the preferred method. Overall, MR-targeted biopsy might be performed via three approaches including MRUS-Fbx, cognitive guidance (MRUS-Cbx), and direct in-bore MR-guided biopsy (IBMR-Bx). MRUS-Fbx strategy fuses previously acquired MRI data with the real-time TRUS or TPUS images on the day of biopsy. In the MRUS-Cbx, the operator mentally maps the previously acquired MR target on the real-time TRUS or TPUS biopsy images, thus “cognitively fusing” and approximating the MRI target on the real-time US image and guiding the needle to this location. Finally, the IBMR-Bx can be performed using transrectal (TR), transperineal (TP), or transgluteal (TG) approaches with a direct in-bore approach while patient is lying in the MR gantry.
Both MRUS-Fbx and MRUS-Cbx use pre-acquired MRI-based suspicious targets (PIRADS 3–5), while IBMR-Bx uses these pre-acquired targets supplemented by real-time anatomic (T2-weighted) and functional (diffusion-weighted) MR images during biopsy of suspicious targets. A number of studies have shown the significantly higher detection of clinically significant disease with lower required biopsy cores in MR-targeted methods compared to TRUS bx. A systematic review of 43 studies on MR-targeted biopsy of prostate found that omitting TRUS bx would result in missing 10% of csPCa and 50% of clinically insignificant cancers [10].
10.2 MRI-Ultrasound Fusion Biopsy
10.2.1 Biopsy Technique
Prior to a TRUS-bx procedure, the patient is instructed to eat a low-residue diet and undergo a bowel preparation on the day prior to biopsy to ensure no fecal matter remains in the rectum. Transperineal approach doesn’t require bowel preparation. MRUS-Fbx using either the TRUS or TPUS route essentially converts a systematic partial organ sampling procedure into a targeted biopsy of suspicious MRI foci with or without systematic TRUS bx. After obtaining either 3-Tesla or 1.5-Tesla mpMRI of the prostate, the outline of the prostate gland and the suspicious targets is contoured on T2-weighted images using one of several automated, semiautomated, or manual software programs (e.g., DynaCAD (InVivo Inc., Gainesville Fl), Profuse ((Artemis Inc., Grass Valley, Ca), SyngoVIA (Siemens Healthineers), Osirix (Osirix)) that enables contouring of the whole gland and individual suspicious lesions. The data is then transferred to one of several MRUS-Fbx platforms (UroNav, Artemis, Koelis, etc.). To perform the TRUS bx, the patient is placed in lateral decubitus position on the operating table. An anesthetic gel (e.g., lidocaine) is administered into the rectum to reduce pain during TRUS probe manipulation. A 2D TRUS probe is then inserted into the rectum to perform an ultrasound sweep, which captures small slices of the prostate. These slices are sent to the fusion platform to generate a 3D segmented model of the prostate. For TPUS biopsy, the patient lies supine with anesthetic gel injected bilaterally to anesthetize the pudendal nerves [11]. The perineum is imaged with a needle guide attached to the probe, and the images are sent to the fusion platform. The vendor-specific software then co-registers ultrasound images on the previously acquired segmented MR images by fusing the contours of the prostate [12, 13]. The platform will create a live “tracking” guidance for prostate biopsy. The platforms for software-assisted MR-ultrasound fusion prostate biopsy mainly differ by the type of image registration, the needle tracking method, and the biopsy approach (TP vs. TR) (Fig. 10.1).
Steps required to obtain an MR-ultrasound fusion-guided biopsy. ERC endorectal coil, T2W T2 weighted, DWI diffusion-weighted imaging, DCE dynamic contrast enhanced, TRUS transrectal ultrasound, 3D three dimensional. (Reprinted from Siddiqui et al. [14], with permission from Elsevier)
10.2.1.1 Image Registration
Image registration can be either rigid or nonrigid (elastic). In rigid image registration, mpMRI images are superimposed on TRUS or TPUS images without considering the possible prostate deformation by position difference between mpMRI and ultrasound (e.g., supine position for mpMRI and TPUS biopsy vs. left lateral decubitus position for TRUS), ultrasound probe pressure, or possible patient movement. In the elastic image registration, the deformation of prostate by biopsy probe adjusted, and therefore elastic registration supposed to be a more accurate method with the elimination of the residual cognitive fusion for targeting the lesion. Several FDA-approved devices such as Artemis (Eigen, Grass Valley, CA) and UroNav (Philips-InVivo, Gainesville, FL) use a combined elastic and rigid registration. A systematic review by Venderink et al. [15] pooled the results of studies, which compared the performance of MRUS-Fbx to systematic TRUS bx, including 11 studies with elastic and 10 studies with rigid image registration techniques. Venderink et al. reported OR of 1.45 (95% CI: 1.21–1.73, p < 0.0001) and 1.40 (95% CI: 1.13–1.75; p = 0.002) for elastic and rigid registration subgroups , respectively. They did not find any superiority for either image registration method to detect PCa (P: 0.19) and csPCa (P: 0.83).
10.2.1.2 Transrectal vs. Transperineal Approach
MR-ultrasound fusion-targeted biopsy may be used via the TR or TP approaches. As stated, the incidence of infection, rectal bleeding, and septic shock is reportedly much lower using a TP approach [16] as compared to the dominant TR approach (up to 5% compared to near 0%) [17]. Reasons include tracking of inadequately treated fecal bacteria into the bloodstream or bacterial resistance to ciprofloxacin during biopsy [17]. A comparison between TRUS and TPUS biopsy showed similar minor complication rates for hematuria, lower urinary tract symptoms, and dysuria, while TRUS was associated with significantly higher rate of infection and rectal bleeding [18]. A drawback of TPUS guidance is that it is significantly more painful than TRUS guidance, and, therefore, it requires a pudendal block or spinal anesthesia but can also easily performed under local anesthesia in an outpatient setting [11]. Moreover, TRUS approach is more likely to miss the anterior prostate lesions [19].
10.2.1.3 Fusion Platforms
A number of commercially available platforms have been developed for performing MRUS-Fbx of prostate. These platforms differ based on the needle tracking system (e.g., electromagnetic tracking, position-encoded joints tracking, and image-based software tracking), type of ultrasound probe, the biopsy route, and image registration method [20]. Several examples of common commercially available systems are outlined below.
-
1.
UroNav (Philips/InVivo, Gainesville, Fl) : The first and currently the most common MRUS-Fbx platforms for prostate sampling, UroNav uses a passive electromagnetic field generator, similar to global positioning system (GPS), to track the motion of TRUS probe on previously acquired axial MR imaging (Fig. 10.2). This platform allows elastic and rigid image registration and enables freehand ultrasound for prostate biopsy [21]. Another feature of the platform is its documentation of the biopsy location for future reference, to enable repeat biopsy from previous positive targets. The tracking error of this platform is approximately 2–3 mm on average but can be much larger due to many factors. Siddiqui et al. detected 30% more csPCa detection by UroNav device compared to systematic TRUS bx [22].
-
2.
Artemis (Eigen) : One of the first commercially available MRUS-Fbx platforms, Artemis uses a fixed mechanical arm to TRUS probe with embedded angle-sensing encoders, which track the position of the probe and needle. This system immobilizes the probe from target acquisition to firing the probe. Similar to UroNav, Artemis utilizes both rigid and nonrigid image registration and has also the advantage of recording the biopsy site for potential future sampling (Fig. 10.3). Studies with this device have shown that PCa detection was three times more likely with Artemis MRUS fusion platform compared to systematic TRUS bx, with 38% of csPCA which were detected only on MRUS-FBx and not on TRUS bx [23].
-
3.
Urostation (Koelis) : Widely used in Europe and the United States, this MRUS-Fbx platform uses a TRUS-TRUS registration tracking in which fusion of 3D ultrasound images with previously acquired MRI is performed and then biopsy is taken. Immediately after biopsy, an additional 3D TRUS is obtained to retrospectively determine the accuracy of biopsy needle position (Fig. 10.4). Unlike other fusion biopsy devices, this platform does not provide real-time prospective targeting but enables an automatic TRUS probe rotation and elastic image registration [24]. Mozer et al. showed significantly higher csPCa detection rate (43% vs. 37%), higher csPCa core positivity rate (31% vs. 7.5%), and higher positive cores length (8 mm vs. 4 mm) using Urostation MRUS-Fbx, compared to systematic TRUS bx [25].
-
4.
HI-RVS/Real-time Virtual Sonography (Hitachi) and Virtual Navigator (Esaote) : Similar to UroNav, these platforms use a freehand TRUS probe with electromagnetic tracking sensors, enable only rigid image registration. Target delineation can only be performed after MR images are transferred into fusion platform. HI-RVS platform has the advantage of utilizing both TR and TP approaches [20].
-
5.
BioJet (GeoScan Medical) : This system is also based on a position-encoded mechanical arm. This platform uses rigid registration and allows for both transrectal and transperineal biopsies. Using this MRUS-Fbx platform, Shoji et al. identified 79% of csPCa using a whole-mount histopathology (WMHP) as reference standard [26].
-
6.
BiopSee (PiMedical/ MedCom) : This is a fusion platform for performing TP fusion biopsy. TRUS probe is mounted on a stepper that is fixed to the operating table. A grid mounted on the mechanical stepper is used to place the biopsy needles. In a study of 120 patients who underwent MRUS-Fbx using BiopSee, 79% of csPCa was detected with WMHP correlation [27].
Transrectal MRUS fusion biopsy using UroNav platform. (a) The snapshot shows the operator view while performing the procedure with overlay of ultrasound image (upper) and previously acquired MRI (lower). (b) This platform allows elastic image registration and freehand manipulation of ultrasound probe. The yellow line shows the position of the biopsy needle. (Image courtesy of Allan J. Pantuck, MD, UCLA Institute of Urologic Oncology, Department of Urology, UCLA)
A longitudinal view of the output from Artemis fusion platform. The target lesions are volume-rendered, while the lines show the biopsy needle positions. The center of the biopsy needle where the cores were obtained is indicated by blue and purple dots, while the green dots represent the location of the acquired systematic biopsy sites. (Image courtesy of Leonard S. Marks, MD, Clark Urology Center, UCLA)
Operator view while performing MRUS fusion biopsy using Urostation (Koelis) platform. The grid planning and the operator view in transrectal and transperineal biopsy approaches are shown. (Adapted and reprinted with permission from Koelis Academy. https://Koelis.Academy [28])
10.2.2 Current Opinions on MRUS Fusion Biopsy
Several studies have found that MRUS-Fbx increases the detection rate of csPCa and decreases the detection of clinically insignificant PCa, compared to systematic TRUS bx . The ability of this technique to register MRI to TRUS images leads to a targeted sampling of the most suspicious PCa foci and thus improves yields for csPCa and risk stratification of PCa. In a head-to-head comparison of TRUS bx and Urostation MRUS-Fbx in 582 men, Siddiqui et al. found that adding MRUS-Fbx to TRUS bx yielded a 67% increase in the detection of high-grade (GS ≥ 4 + 3) PCa and upgraded GS in 32% of men compared to systematic TRUS bx alone [22]. Wu et al. were the first to perform a meta-analysis on all MRUS-Fbx studies dating prior to August 2015 [29]. In their study of 3105 individuals from 16 paired cohort studies, MRUS fusion biopsy detected more csPCa (RR: 1.19, P < 0.05) and less clinically insignificant cancers (RR: 0.68, P < 0.01), compared to systematic TRUS bx. In addition, significantly higher core positivity percentage was achieved by MRUS-Fbx compared to TRUS bx (26.6% vs. 10.2%, RR: 2.75, P < 0.01).
Eliminating the TRUS bx remains somewhat controversial despite the high negative predictive value of multiparametric prostate MRI . Some investigators have suggested combining systematic and targeted biopsies would result in enhanced detection of prostate cancer. PAIREDCAP, a paired cohort study [30], which recruited 300 biopsy-naïve men to undergo three sets of biopsy at the same setting including systematic, MRUS-Fbx (Artemis) and MRUS-Cbx, reported detection of csPCa ranging from 15% in MR-negative to 70% in MR-positive individuals.
MRUS-Fbx has been shown to improve selection of patients for active surveillance (AS) . In a study of 113 AS patients, an mpMRI and MRUS-Fbx resulted in reclassification of the disease grade in 36% of patients [31]. As many as 21% of all PCa lesions are found in the anterior region of the prostate gland which are usually more challenging and often missed in physical examination and systematic TRUS bx. Puech et al. found twice as many csPCa detection in anterior of prostate gland by MRUS-Fbx, compared to systematic TRUS bx [32]. Volkin et al. performed 12-core systematic TRUS biopsy and MRUS-FBx in all 241 suspected anterior lesions on prostate mpMRI and found that overall 50.2% (121/241) of targets were positive for PCa, of which 62 (25.7%) were positive on systematic TRUS biopsy , while 97 (40.2%) were positive on MRUS-FBx. (P: 0.001) [3]. Overall, 59 PCa lesions (48.7%) would have been missed on systematic TRUS bx alone, of which 34 were GS ≥ 3 + 4.
The quality of the MRUS-Fbx of prostate and the final outcome depends on several important steps including optimal MRI acquisition, MRI interpretation by an experienced radiologist, standard sweep ultrasound of the prostate to construct a 3D prostate volume, fusion platform accuracy, and the expertise of the physician performing the fusion biopsy [33]. Structured training of the new users in tertiary centers and courses at international meeting are encouraged to ensure the quality of the procedure.
10.3 Cognitive MR-Guided Biopsy of Prostate
Cognitive or so-called visual MRUS biopsy is the simplest method for targeted biopsy of prostate, as it does not require the sophisticated equipment required for MRUS-FBx and IBMR-Bx. In MRUS-Cbx, the operator reviews the pre-acquired MRI data prior and mentally registers the PIRADS score 3–5 targets to their approximate location on US (apex, midgland or base, anterior or posterior, transitional or peripheral gland). The registered MRUS-Cbx target is usually a hypoechoic nodule or an area with some degree of contrast to the surrounding tissue; however, the visualized target on MRI might not be simply recognized on MRUS-Cbx in some individuals [34]. Current evidence suggest that this visual-targeted technique detects a higher number of PCa lesions, compared to systematic TRUS bx [35]. This technique, however, is highly dependent in the operator expertise, without any tracking or guidance system, making it highly susceptible to human error. Overall, studies have not shown a definite superiority for MRUS-Fbx over MRUS-Cbx for detection of csPCa, although some studies have shown a trend toward improved PCa detection with MRUS-Fbx comparted to MRUS-Cbx. A study of 50 individuals who underwent MRUS-Cbx and MRUS-Fbx showed that the detection rate of csPCa was slightly but not significantly higher for MRUS-Fbx (52% vs. 43% at target level, p: 0.24) [36]. In a cohort of 231 patients who underwent either MRUS-Fbx or MRUS-Cbx, Oberlin et al. showed that MRUS-Fbx detected significantly more lesions (48% vs. 35%, p:0.04), with a nonsignificant increased rate of csPCa (61.5% vs. 48%, p:0.07) [37]. In contrast, in an analysis of a cohort of 391 individuals, both elastic and rigid fusion-targeted techniques were superior to MRUS-Cbx for detection of high-grade PCa [38]. Overall, MRUS cognitive biopsy is the cheapest MR-guided biopsy technique and is the most compatible method for performing prostate biopsy in an office setting. As with other MR-targeted techniques, the quality of communication between the radiologist who interpreted the MRI and the one who performs the biopsy would increase the accuracy of this technique [34].
10.4 In-Bore MR-Guided Biopsy of Prostate
Direct in-bore MR-guided biopsy (IBMR-Bx) of prostate allows within-gantry sampling of prostate. This technique has several advantages . First, MR images are acquired during biopsy with T2- and diffusion-weighted imaging to ensure that the previously noted findings are reproducible. Occasionally sampling may be obviated if MR findings of prostatitis resolve. Second, it is the only technique that ensures MR confirmation of the needle within an MR target. All other techniques are based on approximations. Third, IBMR-Bx usually requires less obtained cores for diagnosis of PCa since oversampling is not required for diagnosis. Finally, IBMR-Bx is versatile and may be performed via TR, TP, or TG routes. Relative drawbacks of the technique include its limited availability relative to MRUS fusion or MRUS cognitive biopsies. Second, the procedure may be more time-consuming at some centers with a median procedure time of 25–68 min. Third , it may be more costly than MRUS-Fbx or MRUS-Cbx.
This technique requires obtaining and interpreting mpMRI images before biopsy planning to identify suspicious targets as with other MR-guided biopsy techniques. IBMR-Bx may be performed with an open- or closed-bore scanner with a commercially available MR-compatible biopsy device (DynaTRIM, Philips-InVivo, Gainesville, Fl). Biopsy localization DynaCAD software is used to identify the target and guide adjustment of the biopsy device in three planes to accurately place the needle within the lesion. On the day of the procedure, patients are placed in prone position in the scanner table, and T2-weighted and DWI sequences are performed to localize the lesion (10–12 min) and transfer this data to the DynaCAD workstation to enable lesion localization for biopsy coordinates (5 min). Individual targets are selected on the workstation with three unique coordinates for each target in anteroposterior, left-to-right, and craniocaudal directions. These coordinates are then adjusted in the biopsy device to keep the proper alignment between the needle guide and the target. After obtaining the appropriate coordinates, the biopsy device with the needle guide is adjusted accordingly, and then a repeat T2-weighted sequence is acquired to confirm appropriate needle guide placement, followed by further manual adjustments of the needle guide as necessary, to allow lesion sampling and sampling of additional targets (15–30 min). During the procedure, patients may move in and out of the gantry several times to confirm needle position within targets and for confirmation of the additional targets. The cycle is repeated to ensure the needle guide orientation toward the desired target (Fig. 10.5). In addition, after obtaining biopsy cores, another confirmatory MR image is acquired. An MR-compatible robotic devices have also been be developed and undergone US FDA clearance to assist this procedure [39].
DynaTRIM device (InVivo-Philips, Gainesville, Fl) for performing in-bore MR-guided biopsy of prostate. The workstation allows localization of the target during biopsy procedure (a), with target coordinations, adjusted on the dial (arrowhead on b) and finally biopsy needle position confirmation within the desired target (Arrow on c). (Adapted and reprinted from Tan et al. [41], with permission from Radiological Society of North America (RSNA))
The procedure might be carried out through TR, TP, and TG approaches using intravenous conscious sedation (with midazolam and fentanyl) with the TR route being the most commonly used. The TP route allows a freehand use of the needle and carries a much lower risk of sepsis and rectal bleeding, compared to TR route, as discussed earlier. A TP approach, moreover, is helpful in individuals with history of previous rectal surgery, anal stricture, or perianal disease. A TG route is used in patients with a surgically resected rectum or with anal stricture and may better access to prostate apex.
Overall, in-bore MR-guided biopsy is a safe procedure with rare occurrence of serious complications . Transient hematuria and short-term rectal bleeding may occur in 1–4% of patients. Urinary retention and urosepsis may also occur in up to 2% of patients [40].
10.4.1 Current Opinions on In-Bore MR-Guided Biopsy
In single and multicenter series, IBMR-Bx has detected significantly more csPCa along with a significantly less low-grade PCa compared to systematic TRUS bx with a higher core positivity rate compared to all other targeted techniques. Application of mpMRI and IBMR-Bx of MR-positive lesions can reduce the need for biopsy up to 51% [42]. A systematic review on ten studies dating since 2013 found median PCa and csPCa detection rate of 42% and 81–93%, respectively, for IBMR-Bx [40]. In an update on the review of 23 IBMR-Bx cohorts dated back to mid-2018 [43], csPCa detection rate was 63% among 2632 individuals, much higher than PCa and csPCa detection rates of 30–50% and 10–40% for systematic TRUS bx [44]. In a head-to-head comparative study, van der Leest et al. [45] examined the efficacy of MRI with subsequent MR-guided biopsy against TRUS bx for the diagnosis of prostate cancer in 626 biopsy-naïve men. They found that the MRI pathway leads to avoidance of biopsy in half of individuals, 11% reduction in the number of clinically insignificant PCa and 89% less biopsy cores for diagnosis of PCa. Hambrock et al. also reported that IBMR-Bx cores predicted final Gleason grade at RP in 88% of individuals, much higher than the 55% RP correlation rate of 10-core TRUS bx [46].
The role of IBMR-Bx for the detection of csPCa has been reported in three distinct populations including:
-
1.
Biopsy-naïve individuals with high suspicion of PCa
-
2.
Individuals with high suspicion of csPCa despite a history of prior negative TRUS-guided biopsy
-
3.
Individuals with known low-grade PCa under active surveillance
Current guidelines recommend performing mpMRI when suspicion for PCa remains high after a negative systematic TRUS biopsy, followed by MR-targeted biopsy of the suspicious lesions. A single study of patients with elevated PSA and repeat negative TRUS biopsy found PCa detection rate of 59% by IBMR-Bx. The results showed a significantly better performance for IBMR-Bx over TRUS bx for PCa detection of except in individuals with very high suspicion (PSA > 20 ng/ml, prostate volume > 65 cc, PSAD<0.15, or > 0.5015–0.5 ng/ml/cc), in whom the yield of both techniques was comparable [47]. Pokorny et al. reported the PCa and csPCa detection rate of 60% and 81% in individuals with prior negative prostate biopsy [43]. In one of the largest multicenter IBMR-Bx series of 461 patients to date, Felker et al. [4] reported a PCa and csPCa detection rate of 51% and 65%, suggesting that both biopsy-naïve patients and patients with history of negative TRUS bx can benefit from IBMR-Bx. Felker et al. also found that Gleason grade was upgraded in 49% of patients under AS. Moreover, they reconfirmed that PIRADS assessment categories of 3, 4, and 5 correlated with csPCa detection rates of 10%, 43%, and 84%, respectively. Another large single-center IBMR-Bx on 475 targets in 379 individuals found an overall PCa of 69.1% and csPCa detection rate of 36.8%, 52.8%, and 50.7% in prior negative TRUS bx patients, biopsy-naïve patients, and active surveillance patients. PIRADSv2.1 score significantly correlated with PCa and csPCa detection (OR: 3.97 and 1.41, respectively) (Figs. 10.6, 10.7, and 10.8).
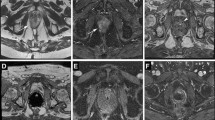
Multiparametric MRI (using a 3-Tesla scanner) of a 65-year-old man with suspicious rise in PSA despite having a negative TRUS bx 6 months ago shows a moderately hypointense lesion on T2-weighted image (a) with obscured margins, located in anterior transitional zone. Marked signal hypointensity (407 mic2/s) is noted on ADC image (b). Early and intense focal enhancement and washout was seen on dynamic contrast-enhanced images, shown as increased exchange constant in pharmacokinetic map for K-trans (c). Overall PIRADS v2.1 score of 5/5 was assigned. In-bore transrectal MRGB (d, e, f) was performed with the specimen showing clinically significant PCa (GS: 4 + 3)
Multiparametric MRI and IBMR-Bx using a 3-Tesla scanner in a 71-year-old biopsy-naïve patient with PSA of 8.2. The arrow points to an oval and markedly hypointense lesion with obscured margins at TSE T2-weighted image (a) with moderate restricted diffusion (b) in left posterolateral peripheral zone in midgland prostate. Overall PIRADS V2.1 score was 4/5. Patient underwent IBMR-Bx (c, d), and four core biopsies were obtained. Pathology assessment of the specimen revealed prostate cancer with Gleason score of 3 + 4
Multiparametric MRI and in-gantry MRGB using a 3-Tesla scanner in a 62-year-old patient with rising PSA and prior negative TRUS bx. Axial TSE T2-weighted image (a) showed a suspicious oval, mildly hypointense lesion (arrow) with blurred margins in left anterior transition zone of prostate with moderate restricted diffusion (b). PIRADS v2.1 score of 3/5 was assigned, and IBMR-Bx of prostate was performed. An MR-compatible, 18-gauge needle was introduced through a needle guide, and 6-core needle biopsies were obtained from the target (c, d). Pathology of the specimen yielded benign lesion
The yield of IBMR-Bx for PCa and csPCa has been similar in both transition (TZ) and peripheral (PZ) zone targets [41, 48]. This is in contrast with TRUS-bx results, in which 70% of positive lesions come from PZ. The anterior and transitional zones are not sampled in a systematic 12-core TRUS bx, and PCa in these areas is more likely to be undetected on TRUS bx.
10.5 IBMR-Bx vs. MRUS-Fbx
Both IBMR-Bx biopsy and MRUS-Fbx have higher spatial resolution and have the notable advantage of documenting the target location before biopsy, compared to systematic TRUS bx. Both techniques are safe and have similar complication rate to TRUS bx. Both techniques require extra training of the operator (e.g., radiologist, urologist) and staff. Detection rates of PCa and csPCa by targeted techniques have been shown to be directly influenced by the operator experience. IBMR-Bx, however, is less dependent to operator experience with a small increase in PCa yield and the obtained cancer core length and also a significant decrease in biopsy time from first to second year [49]. In contrast, MRUS-Fbx has a steep learning curve with improved PCa detection rate over time. In a study of 340 individuals who underwent transperineal MRUS-Fbx, the PCa detection rate increased from 27% to 63% over 22 months [50]. IBMR-Bx allows concurrent and direct visualization of the suspicious target and the needle guide in MRI with fewer biopsy cores required for the definite diagnosis. However, drawbacks of IBMR-Bx compared to MRUS-Fbx include its relatively higher expense and time and relatively less widespread availability.
A systematic review of 11 IBMR-Bx, 17 MRUS-Fbx, and 11 MRUS-Cbx and 4 combined MR-targeted biopsy studies showed a significantly higher detection rate of csPCa (RR: 1.16) and a significantly lower detection rate of clinically insignificant cancer (RR:0.47) with MR-targeted methods over TRUS bx. The review did not detect any significant difference between three MR-targeted techniques for the detection of csPCa [10].
In the FUTURE study, a multicenter randomized controlled trial of 665 men with suspected PCa and prior negative systematic biopsy (<4 year) compared three MR-targeted techniques: MRUS-Fbx, MRUS-Cbx, and IBMR-Bx. The study reported no significant difference was found between three subcohorts in the detection rate of prostate cancer (MRUS-Fbx, 49.4%; MRUS-Cbx, 43.6%; IBMR-Bx, 54.5%; p, 0.4) and clinically significant prostate cancer (MRUS-Fbx, 34.2%; MRUS-Cbx, 33.3%; IBMR-Bx, 32.5%; P > 0.9); however, core positivity rate was significantly higher by the IBMR bx technique (IBMR-Bx, 47.7%; MRUS-Cbx, 33.3%; MRUS-Fbx, 31.3%) [51]. Another prospective trial also did not find any significant difference in clinically significant prostate cancer detection rate between combined TRUS- MRUS-Fbx and IBMR-Bx [52]. In contrast, a study by Costa et al. [53] found significantly higher detection of csPCa by IBMR-Bx compared to MRUS-Fbx (61% vs. 47%, p < 0.0001) and significantly lower detection of insignificant PCa by in-bore technique compared to the fusion technique (11% vs. 18%, P:0.001).
It is noteworthy that systematic reviews of studies on MR-targeted biopsies are usually limited by several factors. First, by heterogeneous population of patients undergoing MR-targeted biopsy, as there are no clear criteria for performing MR-targeted biopsy. The European Association of Urology suggests that in patients with high clinical suspicion of PCa who need a repeat biopsy, mpMRI should be considered with subsequent MR-targeted biopsy if mpMRI is positive (PIRADS category 3 and higher). Second, the definition of clinically significant prostate cancer varies widely between published studies on MR-targeted biopsy. Third, there are variations in the number of obtained cores and the preferred technical aspects.
As there is no general consensus on the preferred MR-targeted method, the optimal biopsy technique should be decided on a per-patient basis and based on the availability of the systems and expertise of the staff.
10.6 Cost-Effectiveness of MR-Targeted Biopsy Strategies
Both MRUS-Fbx and IBMR-Bx have higher initial procedure costs, in comparison with TRUS biopsy; however, both targeted techniques have the beneficial sensitivity of multiparametric MRI to detect clinically significant cancers. Therefore, MR-targeted prostate biopsy methods are cost-effective compared to TRUS bx of prostate, when considering the higher health benefits and the overall lifetime expenses. The use of mpMRI as a screening test before biopsy can avoid the financial burden of unnecessary and repeat biopsies and ultimately unnecessary treatment [20]. In a decision-tree model in biopsy-naïve men in the United States, Pahwa et al. found that the use of mpMRI of prostate followed by MR-guided biopsy of suspicious foci to detect csPCa is cost-effective compared to the standard TRUS bx, when the endpoint was quality-adjusted life-year. IBMR-Bx of MR-positive lesions had the maximum net health benefits compared to systematic TRUS bx and MRUS-Cbx [54]. A study of prostate biopsy cost-effectiveness in a Dutch healthcare setting showed that MRUS-Fbx of MR-positive targets is more cost-effective than systematic TRUS bx. IBMR-Bx may be the most cost-effective method if it has a sensitivity of 89% for detection of csPCa [55].
10.7 Future Directions
When there is suspicion of prostate cancer, systematic TRUS bx remains the standard of care, due to its wide availability, relatively low costs, and ease of use in outpatient setting by urologists. Nevertheless, the future for MR-targeted biopsy is very promising. Several studies have proposed using multiparametric MRI over TRUS bx as the initial screening in patients with abnormal PSA or DRE. Furthermore, there is growing use of multiparametric MRI for the identification of suspicious lesions in patients with negative TRUS bx and in those with known low-grade PCa qualified for active surveillance protocol. Currently, there is little consensus over the preferred method of MR-targeted prostate sampling. Appropriate indications for application of each technique need to be determined. Overall, MRUS-Fbx and IBMR-Bx have been shown to be significantly more effective for detection of clinically significant PCa with each technique having its own advantage and disadvantage; hence the preferred method needs to be chosen per-patient basis, considering the availability, experience of the operators, and patient profile.
References
Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095–101.
Popiolek M, Rider JR, Andren O, Andersson SO, Holmberg L, Adami HO, et al. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol. 2013;63(3):428–35.
Volkin D, Turkbey B, Hoang AN, Rais-Bahrami S, Yerram N, Walton-Diaz A, et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int. 2014;114(6b):E43–E9. Pubmed Central PMCID: 5613950.
Felker ER, Lee-Felker SA, Feller J, Margolis DJ, Lu DS, Princenthal R, et al. In-bore magnetic resonance-guided transrectal biopsy for the detection of clinically significant prostate cancer. Abdom Radiol. 2016;41(5):954–62.
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–757. Pubmed Central PMCID: 3297750.
Barentsz JO, Weinreb JC, Verma S, Thoeny HC, Tempany CM, Shtern F, et al. Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol. 2016;69(1):41–49. Pubmed Central PMCID: 6364687.
Padhani AR, Weinreb J, Rosenkrantz AB, Villeirs G, Turkbey B, Barentsz J. Prostate imaging-reporting and data system steering committee: PI-RADS v2 status update and future directions. Eur Urol. 2019;75(3):385–396. Pubmed Central PMCID: 6292742.
Venderink W, van Luijtelaar A, van der Leest M, Barentsz JO, Jenniskens SFM, Overduin CG, et al. Multiparametric MRI and follow-up to avoid prostate biopsy in 4259 men. BJU Int. 2019;25:775–84.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–22.
Wegelin O, van Melick HHE, Hooft L, Bosch J, Reitsma HB, Barentsz JO, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol. 2017;71(4):517–31.
Smith JB, Popert R, Nuttall MC, Vyas L, Kinsella J, Cahill D. Transperineal sector prostate biopsies: a local anesthetic outpatient technique. Urology. 2014;83(6):1344–9.
Rothwax JT, George AK, Wood BJ, Pinto PA. Multiparametric MRI in biopsy guidance for prostate cancer: fusion-guided. BioMed Res Int. 2014;2014:439171. Pubmed Central PMCID: 4122009.
Kongnyuy M, George AK, Rastinehad AR, Pinto PA. Magnetic resonance imaging-ultrasound fusion-guided prostate biopsy: review of technology, techniques, and outcomes. Curr Urol Rep. 2016;17(4):32. Pubmed Central PMCID: 4928379.
Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64(5):713–719. Pubmed Central PMCID: 6301057.
Venderink W, de Rooij M, Sedelaar JPM, Huisman HJ, Futterer JJ. Elastic versus rigid image registration in magnetic resonance imaging-transrectal ultrasound fusion prostate biopsy: a systematic review and Meta-analysis. Eur Urol Focus. 2018;4(2):219–27.
van den Heuvel S, Loeb S, Zhu X, Verhagen PC, Schroder FH, Bangma CH, et al. Complications of initial prostate biopsy in a European randomized screening trial. Am J Clin Exp Urol. 2013;1(1):66–71. Pubmed Central PMCID: 4219277.
Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, Moon DA, et al. Sepsis and ‘superbugs’: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int. 2014;114(3):384–8.
Huang H, Wang W, Lin T, Zhang Q, Zhao X, Lian H, et al. Comparison of the complications of traditional 12 cores transrectal prostate biopsy with image fusion guided transperineal prostate biopsy. BMC Urol. 2016;16(1):68. Pubmed Central PMCID: 5114768.
Shoji S. Magnetic resonance imaging-transrectal ultrasound fusion image-guided prostate biopsy: current status of the cancer detection and the prospects of tailor-made medicine of the prostate cancer. Investig Clin Urol. 2019;60(1):4–13. Pubmed Central PMCID: 6318202.
Brown AM, Elbuluk O, Mertan F, Sankineni S, Margolis DJ, Wood BJ, et al. Recent advances in image-guided targeted prostate biopsy. Abdom Imaging. 2015;40(6):1788–99.
Singh AK, Kruecker J, Xu S, Glossop N, Guion P, Ullman K, et al. Initial clinical experience with real-time transrectal ultrasonography-magnetic resonance imaging fusion-guided prostate biopsy. BJU Int. 2008;101(7):841–845. Pubmed Central PMCID: 2621260.
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390–397. Pubmed Central PMCID: 4572575.
Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189(1):86–91. Pubmed Central PMCID: 3561472.
Ukimura O, Desai MM, Palmer S, Valencerina S, Gross M, Abreu AL, et al. 3-dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J Urol. 2012;187(3):1080–6.
Mozer P, Roupret M, Le Cossec C, Granger B, Comperat E, de Gorski A, et al. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int. 2015;115(1):50–7.
Shoji S, Hiraiwa S, Ogawa T, Kawakami M, Nakano M, Hashida K, et al. Accuracy of real-time magnetic resonance imaging-transrectal ultrasound fusion image-guided transperineal target biopsy with needle tracking with a mechanical position-encoded stepper in detecting significant prostate cancer in biopsy-naive men. Int J Urol. 2017;24(4):288–94.
Radtke JP, Schwab C, Wolf MB, Freitag MT, Alt CD, Kesch C, et al. Multiparametric magnetic resonance imaging (MRI) and MRI-Transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen. Eur Urol. 2016;70(5):846–53.
Koelis Academy. Available from: https://koelis.academy/. 28 Sep 2019.
Wu J, Ji A, Xie B, Wang X, Zhu Y, Wang J, et al. Is magnetic resonance/ultrasound fusion prostate biopsy better than systematic prostate biopsy? An updated meta- and trial sequential analysis. Oncotarget. 2015;6(41):43571–43580. Pubmed Central PMCID: 4791251.
Elkhoury FF, Felker ER, Kwan L, Sisk AE, Delfin M, Natarajan S, et al. Comparison of targeted vs systematic prostate biopsy in men who are biopsy naive: the prospective assessment of image registration in the diagnosis of prostate Cancer (PAIREDCAP) study. JAMA Surg. 2019;12. Pubmed Central PMCID: 6563598.
Hu JC, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol. 2014;192(2):385–390. Pubmed Central PMCID: 4129939.
Puech P, Rouviere O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology. 2013;268(2):461–9.
Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, et al. Detection of clinically significant prostate Cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol. 2015;68(1):8–19.
Puech P, Ouzzane A, Gaillard V, Betrouni N, Renard B, Villers A, et al. Multiparametric MRI-targeted TRUS prostate biopsies using visual registration. BioMed Res Int. 2014;2014:819360. Pubmed Central PMCID: 4266999.
Giganti F, Moore CM. A critical comparison of techniques for MRI-targeted biopsy of the prostate. Trans Androl Urol. 2017;6(3):432–443. Pubmed Central PMCID: 5503959.
Valerio M, McCartan N, Freeman A, Punwani S, Emberton M, Ahmed HU. Visually directed vs. software-based targeted biopsy compared to transperineal template mapping biopsy in the detection of clinically significant prostate cancer. Urologic Oncol. 2015;33(10):424 e9–16.
Oberlin DT, Casalino DD, Miller FH, Matulewicz RS, Perry KT, Nadler RB, et al. Diagnostic value of guided biopsies: fusion and cognitive-registration magnetic resonance imaging versus conventional ultrasound biopsy of the prostate. Urology. 2016;92:75–79. Pubmed Central PMCID: 4882086.
Delongchamps NB, Peyromaure M, Schull A, Beuvon F, Bouazza N, Flam T, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013;189(2):493–9.
Futterer JJ, Barentsz JO. MRI-guided and robotic-assisted prostate biopsy. Curr Opin Urol. 2012;22(4):316–9.
Overduin CG, Futterer JJ, Barentsz JO. MRI-guided biopsy for prostate cancer detection: a systematic review of current clinical results. Curr Urol Rep. 2013;14(3):209–13.
Tan N, Lin WC, Khoshnoodi P, Asvadi NH, Yoshida J, Margolis DJ, et al. In-bore 3-T MR-guided Transrectal targeted prostate biopsy: prostate imaging reporting and data system version 2-based diagnostic performance for detection of prostate Cancer. Radiology. 2017;283(1):130–139. Pubmed Central PMCID: 5375629.
Pokorny MR, de Rooij M, Duncan E, Schroder FH, Parkinson R, Barentsz JO, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66(1):22–9.
Pokorny M, Kua B, Esler R, Yaxley J, Samaratunga H, Dunglison N, et al. MRI-guided in-bore biopsy for prostate cancer: what does the evidence say? A case series of 554 patients and a review of the current literature. World J Urol. 2019;37(7):1263–79.
Wang Y, Zhu J, Qin Z, Wang Y, Chen C, Wang Y, et al. Optimal biopsy strategy for prostate cancer detection by performing a Bayesian network meta-analysis of randomized controlled trials. J Cancer. 2018;9(13):2237–2248. Pubmed Central PMCID: 6036722.
van der Leest M, Cornel E, Israel B, Hendriks R, Padhani AR, Hoogenboom M, et al. Head-to-head comparison of Transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: a large prospective Multicenter clinical study. Eur Urol. 2019;75(4):570–8.
Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, Scheenen T, Futterer J, Bouwense S, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012;61(1):177–84.
Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183(2):520–7.
Quentin M, Blondin D, Arsov C, Schimmoller L, Hiester A, Godehardt E, et al. Prospective evaluation of magnetic resonance imaging guided in-bore prostate biopsy versus systematic transrectal ultrasound guided prostate biopsy in biopsy naive men with elevated prostate specific antigen. J Urol. 2014;192(5):1374–9.
Friedl A, Schneeweiss J, Sevcenco S, Eredics K, Kunit T, Susani M, et al. In-bore 3.0-T Magnetic Resonance Imaging-guided Transrectal Targeted Prostate Biopsy in a Repeat Biopsy Population: Diagnostic Performance, Complications, and Learning Curve. Urology. 2018;114:139–46.
Gaziev G, Wadhwa K, Barrett T, Koo BC, Gallagher FA, Serrao E, et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int. 2016;117(1):80–6.
Wegelin O, Exterkate L, van der Leest M, Kummer JA, Vreuls W, de Bruin PC, et al. The FUTURE trial: a Multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate Cancer in patients with prior negative biopsies. Eur Urol. 2019;75(4):582–90.
Arsov C, Rabenalt R, Blondin D, Quentin M, Hiester A, Godehardt E, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol. 2015;68(4):713–20.
Costa DN, Goldberg K, Leon AD, Lotan Y, Xi Y, Aziz M, et al. Magnetic resonance imaging-guided in-bore and magnetic resonance imaging-transrectal ultrasound fusion targeted prostate biopsies: an adjusted comparison of clinically significant prostate Cancer detection rate. Eur Urol Oncol. 2019;2(4):397–404.
Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging-guided strategies for detection of prostate Cancer in biopsy-naive men. Radiology. 2017;285(1):157–166. Pubmed Central PMCID: 5621719.
Venderink W, Govers TM, de Rooij M, Futterer JJ, Sedelaar JPM. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: systematic Transrectal ultrasound, direct in-bore MRI, and image fusion. AJR Am J Roentgenol. 2017;208(5):1058–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hosseiny, M., Raman, S.S. (2020). MRI-Guided In-Bore and MRI-Targeted US (Fusion) Biopsy. In: Tirkes, T. (eds) Prostate MRI Essentials. Springer, Cham. https://doi.org/10.1007/978-3-030-45935-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-45935-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45934-5
Online ISBN: 978-3-030-45935-2
eBook Packages: MedicineMedicine (R0)