Abstract
Purpose
We sought to assess the performance of 68 Ga-FAPI-04 PET/MR for the diagnosis of primary tumours as well as metastatic lesions in patients with pancreatic cancer and to compare the results with those of 18F-FDG PET/CT.
Methods
Prospectively, we evaluated 33 patients suspected to have pancreatic adenocarcinoma, of whom thirty-two were confirmed by histopathology, and one had autoimmune pancreatitis confirmed by needle biopsy and glucocorticoid treatment. Within 1 week, each patient underwent both 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT. Comparisons of the detection abilities for primary tumours, lymph nodes, and metastases were conducted for the two imaging approaches. The original maximum standard uptake values (SUVmax) and normalised SUVmax (SUVmax/SUVbkgd) of paired lesions on 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT were measured and compared.
Results
Thirty pancreatic cancer patients and three pancreatitis patients were enrolled. 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT exhibited equivalent (100%) detection rates for primary tumours. The original/normalised SUVmax of primary tumours on 68 Ga-FAPI-04 PET was markedly higher than that on 18F-FDG (p < 0.05). Sixteen pancreatic cancer patients had pancreatic parenchymal uptake, whereas 18F-FDG PET images showed parenchymal uptake in only four patients (53.33% vs. 13.33%, p < 0.001). 68 Ga-FAPI-04 PET detected more positive lymph nodes than 18F-FDG PET (42 vs. 30, p < 0.001), while 18F-FDG PET was able to detect more liver metastases than 68 Ga-FAPI-04 (181 vs. 104, p < 0.001). In addition, multisequence MR imaging helped explain ten pancreatic cancers that could not be definitively revealed due to 68 Ga-FAPI-04 inflammatory uptake and identified more liver metastases than 18F-FDG (256 vs. 181, p < 0.001).
Conclusion
68 Ga-FAPI-04 PET might be better than 18F-FDG PET in the detection of suspicious lymph node metastases. MR multiple sequence imaging of 68 Ga-FAPI-04 PET/MR was helpful for explaining pancreatic lesions in patients with obstructive inflammation and detecting tiny liver metastases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is a malignancy with high mortality. Its global incidence has tripled since the 1950s, ranking as the fourth leading cause of tumour-associated mortalities [1]. Surgical therapy remains the only pancreatic cancer cure. With insidious clinical symptoms, most pancreatic tumours are found at the late stage, resulting in only 10–20% of patients being eligible for surgical resection when detected [2]. Thus, an early pancreatic cancer diagnosis will inform the choice of optimal therapy. Currently, relative to conventional imaging examinations (computed tomography (CT) and magnetic resonance imaging (MRI)), positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) has higher sensitivity and specificity for pancreatic cancer staging [3]. Therapeutic responses and disease recurrence for pancreatic cancer have also been evaluated by 18F-FDG PET/CT [4]. Furthermore, 18F-FDG PET/CT imaging parameters may predict its treatment efficacy and clinical outcome [5]. It should be noted that 18F-FDG sometimes produces false positives for various nonmalignant lesions exhibiting moderate FDG avidity (e.g. reactive lymph nodes or inflammation), and produces false negatives in about 10% of pancreatic cancer patients [6].
Pancreatic cancer is characterised by a prominent desmoplastic reaction. The desmoplastic stroma is produced by mainly pancreatic stellate cells (PSCs) [7]. Cancer-associated fibroblasts (CAFs) are partly derived from PSCs and transform their tumour-promoting biological properties by a cross talk with neoplastic cells [8, 9]. CAFs promote the proliferation and growth of pancreatic cancer cells and thus contribute to the progression, invasion, metastasis, and therapy resistance [10, 11]. Unlike normal fibroblasts, CAFs express specific marker fibroblast activation protein (FAP) on their surface [12, 13]. Based on FAP-specific inhibitors (FAPI), radiopharmaceuticals targeting FAP have been developed. 68 Ga-labelled FAPI (68 Ga-FAPI-04) has been recently introduced as a promising tumour imaging agent targeting CAFs. High uptake of radioactive FAPI has been confirmed in various malignant cancers, including pancreatic cancer [14,15,16]. Röhrich et al. found that relative to contrast-enhanced CT (CECT), 68 Ga-FAPI-04 PET/CT is better at detecting recurrent and metastatic lesions in patients with pancreatic ductal carcinoma (PDAC) [16].
It is well known that the low resolution of low-dose CT does not allow satisfactory anatomic evaluation of lesions in soft tissue. The combination of PET and MRI is a very capable hybrid imaging technique that integrates the superiority of MRI soft-tissue contrast with the molecular specificity and sensitivity of PET [17]. Integrated PET/MR, as a versatile modality, can potentially compensate for the known limitation of PET/CT in detecting small pancreatic cancer, distinguishing mimics, and detecting small hepatic metastases [6, 18].
This is a prospective study to determine if the performance of 68 Ga-FAPI-04 PET/MR is superior to that of 18F-FDG PET/CT in diagnosing primary tumours, involvement of the lymph nodes, and distant metastases in patients with pancreatic cancer and to compare the potential impacts of both on therapeutic management.
Materials and methods
Patients
This prospective study was permitted by the Ethical Committee of the First Affiliated Hospital of Naval Medical University (Changhai Hospital, CHEC2020-071). All patients signed an informed consent form prior to participation. Inclusion criteria were as follows: (i) patients with clinical symptoms or blood tumour marker abnormalities were suspected of pancreatic adenocarcinoma by radiologic examinations (CECT or MRI); (ii) patients willing to accept both 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT scans; (iii) patients were subjected to both 68 Ga-FAPI-04 PET/MR as well as 18F-FDG PET/CT scans within 1 week; (iv) no contraindications to MRI. The exclusion criteria were as follows: (i) pregnancy; (ii) subjected to invasive examinations prior to PET scans, including histopathological biopsy, endoscopic retrograde cholangiopancreatography (ERCP), and stent placement; (iii) received radiotherapy or chemotherapy before PET scans; (iv) no available complete clinical or pathological records; (v) exclusion of typical cystic or blood-rich pancreatic tumours (e.g. solid pseudopapillary neoplasm of the pancreas, pancreatic neuroendocrine neoplasms, etc.); (vi) inability or unwillingness of the research participant, parent, or legal representative to provide written informed consent.
Biological and clinical data, including sex, clinical presentation, age, and laboratory indices, were collected from each patient. The final diagnosis was based on the histopathological assessments of tumour samples harvested by surgical resection or biopsy. For patients for whom tissue diagnosis is not appropriate, radiological follow-up is required. The minimum follow-up time was 3 months.
Radiopharmaceuticals
The synthesis and labelling of 68 Ga-FAPI-04 were performed according to a previously documented method [19]. 68 Ga was obtained from an in-house 68Ge-to-68 Ga generator (ITG, Germany). Chelation was performed after adjusting the pH using sodium acetate. Then, for 10 min, heating of the reaction mixture was performed at 100 °C. The reaction integrity was assessed by radio-liquid chromatography. Solid-phase extraction of 68 Ga compounds was performed before injection. The final product was sterile and pyrogen-free, and the radiochemical purity was > 95%.
18F-FDG injections were obtained from Shanghai Atom Kexing Pharmaceutical Co., Ltd. (their radiochemical purity was > 95%).
68 Ga-FAPI-04 PET/MR imaging
PET/MR assessments were conducted on an integrated PET/MR scanner (Biograph mMR; Siemens Healthcare, Erlangen, Germany) that has a combination of PET and 3.0-T MRI scanners. The intravenous injection activity of 68 Ga-FAPI-04 was 1.85–3.70 MBq/kg. After a fast and simple MRI scout imaging sequence, a PET scan (3 min/bed position) was conducted for the whole body from the skull vertex to mid-thigh in 5–6 bed positions. MRI was concurrently conducted using the protocol: T1-weighted 3D volumetric interpolated breath-hold examination (VIBE) with Dixon fat saturation (T1-VIBE-DIXON) (3D, transversal, TR 4.07 ms, TE 1.28 ms, flip angle 12°, 72 slices, 3-mm slice thickness, the field of view (FOV) 400 × 400, voxel size 1.3 × 1.3 × 3.0 mm3), T2W-BLADE (transversal, TR 3000 ms, TE 89 ms, flip angle 90°, 33 slices, slice thickness 6 mm, FOV 400 × 400, voxel size 1.3 × 1.3 × 6.0 mm3), DWI (2D, transversal, TR 6270 ms, TE 50 ms, 33 slices, 6-mm slice thickness, FOV 400 × 400, voxel size 1.6 × 1.6 × 6.0 mm3, b-values 50, 800 s/mm2). The PET data were reconstructed using high-definition PET (HD-PET) (3 iterations, 21 subsets; matrix 172 × 172, voxel size 2.3 × 2.3 × 5.0 mm3). The Dixon sequence was used to derive MRI-based attenuation correction.
18 F-FDG PET/CT imaging
Prior to the 18F-FDG PET/CT scan, study participants were asked to fast for at least 6 h, ensuring a blood glucose (BG) less than 11.1 mmol/L, and then they were intravenously administered 18F-FDG (3.70–5.55 MBq/kg). All acquisitions were performed on a Biograph 64 PET/CT scanner (Siemens Healthcare, Erlangen, Germany) 45–60 min after 18F-FDG injection. The whole-body CT scanning parameters were set as follows: current (170 mA), voltage (120 kV), and scan layer thickness (3 mm). The PET scan was performed after CT scan acquisition and conducted in 5–6 bed positions. Immediately after CT image acquisition, PET data were acquired for 3 min per bed position. Reconstruction of the acquired data was performed by the postprocessing workstation with an iterative TrueD reconstruction system (Siemens Medical Solutions). Correction attenuation was performed by CT images.
Image interpretation
All reconstructed 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT images were evaluated using Syngo. Via (Siemens Healthcare, Erlangen, Germany) by two groups of experienced nuclear medicine physicians independently. Any discrepancies were discussed to reach a consensus.
Due to the presence of obstructive inflammation, we assessed the readability of PET uptake in primary lesions. Tumours that could not be accurately localised/discerned on PET images were defined as PET negative; otherwise, they were defined as PET positive. Additionally, the number of primary foci and metastases detected by 68 Ga-FAPI-04 or 18F-FDG PET, 68 Ga-FAPI-04 PET/MR, and 18F-FDG PET/CT were recorded, and the evaluations were conducted without any information from the other PET scan. The patient’s previous CECT/MR images were also referenced during the segmentation.
In this study, if 68 Ga-FAPI-04 or 18F-FDG uptake in the lymph node surpassed that in the surrounding tissue, it was considered a positive lymph node. Distant metastases were assessed by abnormal tracer uptake as well as CT or MR imaging findings. Locations and other information on metastases were documented.
To calculate the standard uptake values, circular regions of interest were drawn around the lesions and automatically adapted to a tridimensional volume of interest. For every lesion, the maximum standard uptake value (SUVmax) was automatically calculated by the syngo.via software. To ensure that the SUVmax was relatively comparable, referring to Qin et al. [20], the original SUVmax was normalised using the following formula: \(\mathrm{Normalised SUVmax}=\mathrm{Original SUVmax}/\mathrm{SUVbkgd}.\) SUVbkgd refers to the average SUV of the background tissue. We normalised the average SUV of the descending aorta, liver, and spleen to the original SUVmax.
If there were fewer than five lesions in a single organ/region, all lesions were quantitatively assessed. If there were more than five lesions in a single organ/region, the five lesions with the highest activity were quantitatively evaluated.
Statistical analysis
Data analyses were conducted using SPSS (version 26.0; IBM, Armonk, NY, USA). The quantitative data are presented as the mean ± SD. The chi-squared test was used to compare the number of positive lesions identified by two examinations. We used a paired t test to compare different paired 18F-FDG and 68 Ga-FAPI-04 PET SUVmax. p < 0.05 was the cutoff for significance, and all tests were two-sided.
Results
Patient characteristics
We recruited 33 patients between January 2020 and August 2021. Patient information, including clinical presentation and laboratory indices, was recorded, as summarised in Table 1. The median time interval between the two scans was 2 days (range: 1–6 days); see Supplementary Table 1 for further details.
Clinical diagnosis of suspicious patients
Among the 33 patients, 30 cases were confirmed as pancreatic cancer by histopathological results, with nine patients undergoing surgical resection and 21 patients undergoing endoscopic ultrasound-fine needle aspiration (EUS-FNA) (Supplementary Table 1). Of the nine surgical samples, eight were duct adenocarcinoma, and one was adenosquamous carcinoma.
The other three patients were confirmed to have pancreatitis. Two patients (patients 31 and 32) were confirmed to have chronic pancreatitis by surgery. In the other patient (patient 33), tumour cells were not detected by needle biopsy, and her clinical symptoms improved after glucocorticoid treatment. The diagnosis of autoimmune pancreatitis was established.
Primary tumour detection
68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT exhibited comparable detection abilities for primary pancreatic tumours with a 100% positive detection rate. Table 2 shows the comparison of uptake parameters (including the original and normalised SUVmax) between 68 Ga-FAPI-04 and 18F-FDG. Both the original and normalised SUVmax of the primary tumour on 68 Ga-FAPI-04 PET were higher than those on 18F-FDG (p < 0.05).
Elevated tracer uptake was observed in the adjacent tissue of the pancreas at the same time in 68 Ga-FAPI-04 imaging in 16 patients (53.33%), which masked the tumour uptake in 11 patients (Fig. 1). Density differences were not observed in 12 lesions (75.00%) on low-dose CT images, while all patients (100.0%) showed abnormal signals on multiparameter MR, which enhanced confidence in the interpretation of pancreatic tumours (Table 3). In 68 Ga-FAPI-04 PET, there was no significant difference in SUVmax between obstructive pancreatic inflammation and pancreatic cancer lesions (SUVmax, 13.70 ± 5.15 vs. 12.58 ± 4.44, p = 0.596). Two of the 16 patients had a clinical history of chronic pancreatitis, and thirteen showed dilated pancreatic ducts on MR or CT images. Diffuse or focal parenchymal uptake in 18F-FDG PET imaging was observed in only four patients (13.33% vs. 53.33%, p < 0.001), and the radiographic follow-up data of these four patients were presented in Supplementary Fig. 1. Typical cases are presented in Figs. 2 and 3.
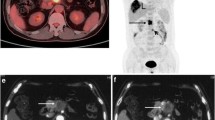
A 53-year-old man (patient 24) with pancreatic ductal adenocarcinoma (PDAC). a–f 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT exhibited high focal uptake (FAPI: SUVmax = 9.59; FDG: SUVmax = 12.10) in the pancreatic head (white arrows) and lymph node metastasis (FAPI: SUVmax = 6.77; FDG: SUVmax = 15.40) in the lesser omentum (yellow arrows). g Haematoxylin–eosin (HE) staining of pancreatic cancer tissues (× 200 magnification). h, i Dilatation of the major pancreatic duct with obstructive pancreatitis-related 68 Ga-FAPI-04 uptake in the body and pancreatic tail (SUVmax = 11.70)
A 72-year-old man (patient 17) with pancreatic ductal adenocarcinoma (PDAC). 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT exhibited high focal uptake (FAPI: SUVmax = 19.30; FDG: SUVmax = 7.64) in the pancreatic head (white arrows). Pancreatitis-related 68 Ga-FAPI-04 uptake could be seen in the pancreatic body and tail (SUVmax = 14.9), and nodular 18F-FDG uptake could also be seen in the body of the pancreas (SUVmax = 4.34). On CT images, the pancreatic head lesion showed soft tissue density (dashed arrow), swelling of the pancreatic body and tail, and the main pancreatic duct appears ill defined, while MR images showed abnormal signal in the lesion (low signal on T1WI, slightly high signal on T2WI, high signal on DWI, and low signal on ADC, yellow arrows), accompanied by slight dilation of the main pancreatic duct, which enhanced the confidence of diagnosing pancreatic head carcinoma on 68 Ga-FAPI-04 images
Lymph node assessment
In this study, each lymph node with obvious 68 Ga-FAPI-04 or 18F-FDG uptake was deemed a positive lymph node. Among the 15 patients with alleged metastasis of lymph nodes, 33.33% (5/15) showed more positive lymph nodes on 68 Ga-FAPI-04 than on 18F-FDG PET. Altogether, 42 and 30 positive lymph nodes were depicted by 68 Ga-FAPI-04 and 18F-FDG PET, respectively (p < 0.001). In all, compared with 18F-FDG PET, 68 Ga-FAPI-04 led to N upstaging in 26.67% (4/15) of patients, and upstaging from N0 to N1 (patient 18) and N0 to N2 (patient 8) occurred in one case. Two patients were upstaged from N1 to N2 (patients 3 and 26). Notably, one 18F-FDG-positive metastatic lymph node was missed by 68 Ga-FAPI-04 PET in a patient (patient 28); however, abnormal signals on multiparametric MR of PET/MR enhanced interpretation confidence. Follow-up CT imaging 3 months after chemotherapy revealed shrinkage of the lymph node. Typical cases are presented in Figs. 4 and 5.
Comparison of 18F-FDG PET/CT and 68 Ga-FAPI-04 PET/MR of para-aortic lymph nodes in a 66-year-old man (patient 28). This lymph node measured 1.2 cm in short diameter and showed marked 18F-FDG accumulation (SUVmax = 5.6). On 68 Ga-FAPI-04 PET/MR imaging, no increased radioactive uptake was seen in the enlarged node, which made it difficult to determine whether it was benign or malignant; however, MR signals showed significant changes (high signal on T2WI and DWI, low signal on ADC), which enhanced the confidence in diagnosing the metastatic lymph node. After chemotherapy, the lymph node was reduced to 0.7 cm on the follow-up CT images
There was no significant difference in the uptake of 18F-FDG and 68 Ga-FAPI-04 between all 23 double-positive lymph nodes, and the normalised indicators did not affect the results (p > 0.05).
Liver metastases
Biopsy of at least one lesion in the liver should be performed in patients with liver metastasis, but it was not mandatory. Of the five patients (patients 8, 9, 26, 29, and 30) with liver metastases (Supplementary Fig. 2), 18F-FDG PET showed more metastatic lesions than 68 Ga-FAPI-04 (181 vs. 104, p < 0.001). 18F-FDG exhibited a higher uptake than 68 Ga-FAPI-04 (SUVmax, 8.64 ± 2.04 vs. 5.97 ± 2.19, p = 0.001) in all 22 double-positive intrahepatic metastasis lesions, and the normalised indicators did not affect the results. In these five patients, the visual analysis revealed that larger intrahepatic metastases often showed ring-shaped 68 Ga-FAPI-04 uptake with tracers around merely the edge of the lesions, and the uptake intensity was significantly lower than that of 18F-FDG. Typical cases are presented in Fig. 6.
In two liver metastatic patients with pancreatic tumours, 68 Ga-FAPI-04 uptake was detected at only the edge of liver metastases, and the 18F-FDG uptake intensity was more obvious. All intrahepatic metastases with increased 18F-FDG metabolism were detected by MRI, and more micrometastases undetectable by CT were identified by MRI (white arrows). These lesions showed typical ring enhancement in contrast-enhanced axial T1-weighted MR images (yellow arrows)
All intrahepatic metastases with increased 18F-FDG or 68 Ga-FAPI-04 uptake were detected by PET/MR MRI, and more micrometastases were detected by MRI (Table 2). However, the results have no influence on tumour M staging, which is important for clinical management and outcomes.
Discussion
The present study was designed as a single-centre and prospective study. We compared the diagnostic and staging efficacy of 68 Ga-FAPI-04 PET/MR with 18F-FDG PET/CT for pancreatic cancer.
Pancreatic cancer is characterised by vascular deficiency and plentiful desmoplastic stroma, accounting for 90% of the tumour volume. The stroma consists of extracellular matrix proteins and CAFs [21]. Our study revealed that the primary tumour could be visualised by 68 Ga-FAPI-04, which was consistent with previous studies [14, 16]. Fibrosis of the pancreas is often a striking feature of chronic pancreatitis [22]. 68 Ga-FAPI-04 was not more tumour-precise than 18F-FDG and has a limitation of false-positive uptake caused by inflammation-induced fibrosis, which has been demonstrated by previous studies [16, 23]. In this study, there seemed to be an overlap of the uptake intensities in the pancreatic mass and obstructive pancreatitis of the pancreatic parenchyma, and it is crucial to differentiate pathological 68 Ga-FAPI-04 uptake from tumour-induced obstructive pancreatitis. The positive 68 Ga-FAPI-04 uptake caused by tumour-induced inflammation sometimes affects the visual interpretation of PET; thus, the qualitative reading of 68 Ga-FAPI-04 PET images sometimes must be combined with other radiological data.
Our results showed that 68 Ga-FAPI-04 might be better than 18F-FDG in the assessment of lymph node metastasis in pancreatic cancer. This result was in accordance with previous research [24]. These findings may significantly impact clinical management. A recent study by Qin et al. proposed the contrary opinion. They found that the number of avid lymph nodes detected by 18F-FDG was higher than that of 68 Ga-FAPI-04 in nasopharyngeal carcinoma (100 vs. 48) [25]. None of the lymph nodes was confirmed by histopathological results, but imaging follow-up was performed. Further studies with histopathological results are required.
We found that larger hepatic metastases usually had “ring-like” 68 Ga-FAPI-04 uptake at the edge of the lesion only. In many animal models, the highest tracer uptake is usually at specific tumour areas with a good blood supply [26]. Early studies showed that in the peritumoural hepatic parenchyma of hepatic metastases, the rate of sinusoidal hyperaemia was 95%, and the rate of fibrous proliferation was 58% [27]. However, the extent of CAF expression was consistent in peritumoural and intratumoural regions of liver metastases [28]. Thus, we speculate that the “ring-like” 68 Ga-FAPI-04 uptake in liver metastases might be related to sinusoidal congestion surrounding metastatic tumours.
18F-FDG PET/CT, which has good accuracy, is a powerful screening tool for metastatic disease assessment. However, the assessment of liver lesions is inhibited by high background liver uptake as well as other intrinsic technical limitations [29]. In contrast, 68 Ga-FAPI-04 demonstrates very low unspecific liver uptake and is expected to be superior for identifying liver metastasis. However, in five patients with liver metastasis in our cases, some 18F-FDG hypermetabolic micrometastases were not detected by 68 Ga-FAPI-04, and the SUVmax was significantly lower in 68 Ga-FAPI-04 than in 18F-FDG PET. This is contrary to previously published studies that showed that 68 Ga-FAPI-04 PET/CT identified more metastatic sites than 18F-FDG with a significantly higher SUV [14, 24]. The reason for this discrepancy might be related to the difference in the origins of CAFs. Differences in progenitor cellular origins result in different phenotypes and functions of CAFs [30, 31]. Patients who were identified with pancreatic cancer as their only tumour or first primary tumour were included in this study, whereas the metastases reported in other studies consisted of mixed primary tumour compositions, with few pancreatic primary tumours.
As reported previously, pancreatic cancer may cause false-positive concentrations of 68 Ga-FAPI-04 in the inflammatory pancreatic parenchyma to some extent; therefore, we introduced PET/MR to explore the added value of MR [16]. Based on our results, obstructive inflammation of the pancreas might affect the interpretation of tumour foci in 68 Ga-FAPI-04 imaging, which is inferior to 18F-FDG for the identification of liver metastases, and false-negative results would be expected for 68 Ga-FAPI-04 imaging in some metastatic lymph nodes. As mentioned above, MR provides more valuable information for its superior soft-tissue contrast and multiple sequence imaging to compensate for the deficiency of 68 Ga-FAPI-04 imaging [6, 17, 32]. Therefore, we think PET/MR may be a better partner for 68 Ga-FAPI-04 imaging of pancreatic tumours.
The present study had several limitations. First, hardware differences between the two devices may lead to data heterogeneity; therefore, we normalised the two sets of data to ensure relative comparability. Second, histopathology results were not obtained for lymph node metastasis. Third, the present study was a single-centre based trial with a small sample size. Further studies with larger patient cohorts are needed to confirm these results.
Conclusion
In this prospective study, 68 Ga-FAPI-04 PET/MR demonstrated an equivalent detection rate to 18F-FDG PET/CT for primary tumours of pancreatic cancer, and MR multiple sequence imaging of integrated PET/MR was helpful for explaining pancreatic lesions in patients with obstructive inflammation. 68 Ga-FAPI-04 PET might be better than 18F-FDG PET in the detection of suspicious lymph node metastases, but the value of 68 Ga-FAPI-04 PET for N staging needs further study. The 68 Ga-FAPI-04 PET shows no obvious superiority over 18F-FDG PET in detecting intrahepatic metastasis, but hybrid MR imaging was helpful in detecting the tiny metastatic foci.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. https://doi.org/10.3322/caac.21590.
Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. https://doi.org/10.1038/nrdp.2016.22.
Kauhanen SP, Komar G, Seppänen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957–63. https://doi.org/10.1097/SLA.0b013e3181b2fafa.
Lee JW, O JH, Choi M, Choi JY. Impact of F-18 fluorodeoxyglucose PET/CT and PET/MRI on initial staging and changes in management of pancreatic ductal adenocarcinoma: a systemic review and meta-analysis. Diagnostics (Basel). 2020;10:952. https://doi.org/10.3390/diagnostics10110952.
Wang L, Dong P, Shen G, Hou S, Zhang Y, Liu X, et al. 18F-Fluorodeoxyglucose positron emission tomography predicts treatment efficacy and clinical outcome for patients with pancreatic carcinoma: a meta-analysis. Pancreas. 2019;48:996–1002. https://doi.org/10.1097/MPA.0000000000001375.
Rijkers AP, Valkema R, Duivenvoorden HJ, van Eijck CH. Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: a meta-analysis. Eur J Surg Oncol. 2014;40:794–804. https://doi.org/10.1016/j.ejso.2014.03.016.
Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–67. https://doi.org/10.1038/nrgastro.2012.115.
Nielsen MFB, Mortensen MB, Detlefsen S. Typing of pancreatic cancer-associated fibroblasts identifies different subpopulations. World J Gastroenterol. 2018;24:4663–78. https://doi.org/10.3748/wjg.v24.i41.4663.
Whittle MC, Hingorani SR. Fibroblasts in pancreatic ductal adenocarcinoma: biological mechanisms and therapeutic targets. Gastroenterology. 2019;156:2085–96. https://doi.org/10.1053/j.gastro.2018.12.044.
Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: a strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873:188356. https://doi.org/10.1016/j.bbcan.2020.188356.
von Ahrens D, Bhagat TD, Nagrath D, Maitra A, Verma A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J Hematol Oncol. 2017;10:76. https://doi.org/10.1186/s13045-017-0448-5.
Mikuła-Pietrasik J, Uruski P, Tykarski A, Książek K. The peritoneal “soil” for a cancerous “seed”: a comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell Mol Life Sci. 2018;75:509–25. https://doi.org/10.1007/s00018-017-2663-1.
Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, et al. FAP Promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 2016;76:4124–35. https://doi.org/10.1158/0008-5472.CAN-15-2973.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5. https://doi.org/10.2967/jnumed.119.227967.
Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W, et al. 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. 2019;60:386–92. https://doi.org/10.2967/jnumed.118.215913.
Röhrich M, Naumann P, Giesel FL, Choyke PL, Staudinger F, Wefers A, et al. Impact of 68Ga-FAPI PET/CT imaging on the therapeutic management of primary and recurrent pancreatic ductal adenocarcinomas. J Nucl Med. 2021;62:779–86. https://doi.org/10.2967/jnumed.120.253062.
Sagiyama K, Watanabe Y, Kamei R, Hong S, Kawanami S, Matsumoto Y, et al. Multiparametric voxel-based analyses of standardized uptake values and apparent diffusion coefficients of soft-tissue tumours with a positron emission tomography/magnetic resonance system: preliminary results. Eur Radiol. 2017;27:5024–33. https://doi.org/10.1007/s00330-017-4912-y.
Yeh R, Dercle L, Garg I, Wang ZJ, Hough DM, Goenka AH. The role of 18F-FDG PET/CT and PET/MRI in pancreatic ductal adenocarcinoma. Abdom Radiol (NY). 2018;43:415–34. https://doi.org/10.1007/s00261-017-1374-2.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–22. https://doi.org/10.2967/jnumed.118.210443.
Qin C, Shao F, Gai Y, Liu Q, Ruan W, Liu F, et al. 68Ga-DOTA-FAPI-04 PET/MR in the evaluation of gastric carcinomas: comparison with 18F-FDG PET/CT. J Nucl Med. 2022;63(1):81–8. https://doi.org/10.2967/jnumed.120.258467.
González-Borja I, Viúdez A, Goñi S, Santamaria E, Carrasco-García E, Pérez-Sanz J, et al. Omics approaches in pancreatic adenocarcinoma. Cancers (Basel). 2019;11:1052. https://doi.org/10.3390/cancers11081052.
Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, et al. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. https://doi.org/10.1038/nrdp.2017.60
Luo Y, Pan Q, Yang H, Peng L, Zhang W, Li F. Fibroblast activation protein-targeted PET/CT with 68Ga-FAPI for imaging IgG4-related disease: comparison to 18F-FDG PET/CT. J Nucl Med. 2021;62:266–71. https://doi.org/10.2967/jnumed.120.244723.
Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–32. https://doi.org/10.1007/s00259-020-04769-z.
Qin C, Liu F, Huang J, Ruan W, Liu Q, Gai Y, et al. A head-to-head comparison of 68Ga-DOTA-FAPI-04 and 18F-FDG PET/MR in patients with nasopharyngeal carcinoma: a prospective study. Eur J Nucl Med Mol Imaging. 2021;48:3228–37. https://doi.org/10.1007/s00259-021-05255-w.
Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C, et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med. 2019;60:1421–9. https://doi.org/10.2967/jnumed.118.224469.
Kanematsu M, Hoshi H, Yamada T, Nandate Y, Kato M, Yokoyama R, et al. Overestimating the size of hepatic malignancy on helical CT during arterial portography: equilibrium phase CT and pathology. J Comput Assist Tomogr. 1997;21:713–9. https://doi.org/10.1097/00004728-199709000-00006.
Peng J, Wang Y, Zhang R, Deng Y, Xiao B, Ou Q, et al. Immune cell infiltration in the microenvironment of liver oligometastasis from colorectal cancer: intratumoural CD8/CD3 ratio is a valuable prognostic index for patients undergoing liver metastasectomy. Cancers (Basel). 2019;11:1922. https://doi.org/10.3390/cancers11121922.
Wang XY, Yang F, Jin C, Fu DL. Utility of PET/CT in diagnosis, staging, assessment of resectability and metabolic response of pancreatic cancer. World J Gastroenterol. 2014;20:15580–9. https://doi.org/10.3748/wjg.v20.i42.15580.
Sharon Y, Raz Y, Cohen N, Ben-Shmuel A, Schwartz H, Geiger T, et al. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015;75:963–73. https://doi.org/10.1158/0008-5472.CAN-14-1990.
Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, et al. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut. 2019;68:159–71. https://doi.org/10.1136/gutjnl-2018-316451.
Chen WS, Li JJ, Hong L, Xing ZB, Wang F, Li CQ. Comparison of MRI, CT and 18F-FDG PET/CT in the diagnosis of local and metastatic of nasopharyngeal carcinomas: an updated meta analysis of clinical studies. Am J Transl Res. 2016;8:4532–47.
Funding
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (Grant Nos. 82001867 and 81871390) and the “234 Discipline Climbing Plan” of the First Affiliated Hospital of Naval Medical University (Grant Nos. 2019YPT002 and 2020YPT002).
Author information
Authors and Affiliations
Contributions
Zeyu Zhang, Guorong Jia, Chao Cheng, and Changjing Zuo designed the study, interpreted the data, and led the writing and review of the manuscript. Kai Cao, Guixia Pan, Lu Zhang, and Tao Wang synthesised 68 Ga-FAPI-04 and performed the examination. Chao Cheng, Changjing Zuo, Zeyu Zhang, Guorong Jia, Qinqin Yang, Hongyu Meng, and Jian Yang collected clinical and imaging data. Chao Cheng and Changjing Zuo participated in the review of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with animals. All procedures of this study followed the principles of the Declaration of Helsinki. This prospective study was approved by the Ethics Committee of the First Affiliated Hospital of Naval Medical University (Changhai Hospital, CHEC2020-071).
Consent to participate
All subjects provided written informed consent.
Consent for publication
Informed consent was obtained from all individual participants included in this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Digestive tract
Supplementary Information
Below is the link to the electronic supplementary material.
259_2022_5729_MOESM1_ESM.tif
Supplementary Fig. 1 Radiographic follow-up date of 4 patients with abnormal 18F-FDG uptake in pancreas parenchyma.(a)A 61-year-old man (Patient 6) with pancreatic head cancer. 18F-FDG PET/CT showed diffuse increased 18F-FDG uptake in the pancreatic parenchyma of the body and tail. Radiographic follow-up 5 months later showed the peripancreatic inflammation and inflammatory exudation, and no abnormal lesions were found in pancreatic parenchyma. (b) A 65-year-old woman (Patient 10) with pancreatic body cancer. 18F-FDG PET/CT showed focal increased uptake of 18F-FDG in the pancreatic tail parenchyma. Radiographic follow-up 8 months later showed no significant lesions in the tail of the pancreas. (c) A 66-year-old man (Patient 28) with pancreatic head cancer. 18F-FDG PET/CT showed diffuse increased 18F-FDG uptake in the pancreatic parenchyma of the body and tail. Radiographic follow-up 4 months later showed dilation of the pancreatic duct in the body andtail, and no obvious lesions in the pancreatic parenchyma. (d) A 72-year-old man (Patient 30) with pancreatic body cancer.18F-FDG PET/CT showed atrophied pancreatic parenchymal in the body and tail with diffuse increased 18F-FDG uptake. Radiographic follow-up 3 months later showed no significant lesions inthe body and tail of the pancreas. (TIF 52555 KB)
259_2022_5729_MOESM2_ESM.tif
Supplementary Fig. 2 MIP images of 68Ga-FAPI-04 PET and 18F-FDG PET in patients with multiple hepatic metastases (patients 8, 9, 29 and 30) (TIF 41013 KB)
Rights and permissions
About this article
Cite this article
Zhang, Z., Jia, G., Pan, G. et al. Comparison of the diagnostic efficacy of 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging 49, 2877–2888 (2022). https://doi.org/10.1007/s00259-022-05729-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05729-5