Abstract
Purpose
The use of [177Lu]Lu-PSMA-617 radioligand therapy has become increasingly recognized as a viable therapeutic approach for patients in the advanced stages of metastatic castration-resistant prostate cancer (mCRPC). However, there is limited data regarding its effectiveness and safety in earlier lines. This study aims to present our institution’s experience with [177Lu]Lu-PSMA-617 as a first-line systemic therapy for mCRPC.
Methods
We collected and analyzed data from consecutive mCRPC patients who underwent first-line treatment with [177Lu]Lu-PSMA-617 at our center from 2015 to 2023. The various outcome measures included best prostate-specific antigen-response rate (PSA-RR) (proportion of patients achieving a ≥ 50% decline in PSA); objective radiographic response rate (ORR) (proportion of patients achieving complete or partial radiographic responses); radiographic progression-free survival (rPFS) (measured from treatment initiation until radiographic progression or death from any cause); overall survival (OS) (measured from treatment initiation until death from any cause); and adverse events.
Results
Forty treatment-naïve mCRPC patients with PSMA-positive disease on [68Ga]Ga-PSMA-11 PET/CT were included (median age: 68.5 years, range: 45–78; median PSA: 41 ng/mL, range: 1-3028). These patients received a median cumulative activity of 22.2 GBq (range: 5.55–44.4) [177Lu]Lu-PSMA-617 over 1–6 cycles at 8–12 week intervals. A ≥ 50% decline in PSA was observed in 25/40 (62.5%) patients (best PSA-RR). Radiographic responses were evaluated for thirty-eight patients, with thirteen showing partial responses (ORR 34.2%). Over a median follow-up of 36 months, the median rPFS was 12 months (95% confidence interval, CI: 9–15), and the median OS was 17 months (95% CI: 12–22). Treatment-emergent grade ≥ 3 anemia, leucopenia, and thrombocytopenia were noted in 4/40 (10%), 1/40 (2.5%), and 3/40 (7.5%) patients, respectively.
Conclusion
The findings suggest that [177Lu]Lu-PSMA-617 is a safe and effective option as a first-line treatment in mCRPC. Further trials are needed to definitively establish its role as an upfront treatment modality in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) currently holds the top position among cancers affecting males, with an estimated 288,300 new cases and 34,700 deaths projected in the United States for the year 2023 [1]. Approximately 10–20% of PCa patients progress to castration-resistant PCa (CRPC), and metastatic CRPC (mCRPC) is known for its aggressive nature and poor outcomes [2, 3]. Since the approval of docetaxel chemotherapy, treatment options for mCRPC have seen substantial improvements over the last decade [4,5,6,7,8]. The use of the androgen-receptor pathway inhibitors (ARPIs), abiraterone and enzalutamide, have contributed to significant survival benefits in both taxane-treated and taxane-naïve mCRPC patients [5,6,7,8]. About 20–30% of metastatic PCa patients harbor mutations in homologous recombination repair (HRR)-related genes, and novel agents like olaparib and talazoparib targeting Poly-(ADP‐ribose)‐polymerase (PARP) have shown promise in inducing ‘synthetic lethality’ in tumors with HRR mutations [9, 10]. While PARP-inhibitor monotherapy has been restricted to the second-line or later settings in HRR-mutated mCRPC, the combined use of an ARPI and a PARP-inhibitor in the first-line mCRPC setting has demonstrated improved survival outcomes compared to ARPI alone in recent studies [11,12,13,14]. However, both these classes of drugs come with significant clinical and financial toxicities, posing challenges in real-world scenarios. Further, with the increasing prevalence of cardiometabolic diseases, the initiation of ARPIs is often a difficult choice for the patients as well as the treating physicians [15]. Thus, there exists a need for alternative efficacious and safer treatment options in these cases.
Prostate-specific membrane antigen (PSMA)-targeted radioligand therapy (RLT) has emerged as a promising treatment option for end-stage mCRPC patients [16]. Landmark trials like TheraP and VISION have shown enhanced response and survival outcomes with the beta-emitting small molecule PSMA-inhibitor, [177Lu]Lu-PSMA-617, in mCRPC patients who have progressed on at least one taxane and/or at least one ARPI [17, 18]. Another phase 2 trial has also reported non-inferior outcomes with [177Lu]Lu-PSMA-617 compared to docetaxel in chemotherapy-naïve mCRPC [19, 20]. In a meta-analysis of 24 studies, [177Lu]Lu-PSMA-RLT demonstrated an excellent safety profile, with grade 3/4 side effects like nausea, fatigue, xerostomia, and anemia occurring in only 1%, 1%, 2%, and 8% of patients, respectively [21]. Given these promising results, it is essential to explore the efficacy and safety of this targeted therapy even earlier in the disease course. This study aims to evaluate our initial experience with the efficacy and safety of [177Lu]Lu-PSMA-617 as a first-line systemic treatment in mCRPC patients.
Materials and methods
Patient population
A prospective registry was maintained for metastatic prostate cancer (PCa) patients treated at a tertiary-care institution. The study focused on consecutive first-line metastatic castration-resistant prostate cancer (mCRPC) patients who received [177Lu]Lu-PSMA-617 between 2015 and 2023. This treatment was offered on a compassionate basis after multidisciplinary tumor board discussion, primarily to patients ineligible for conventional chemotherapy/ ARPIs due to cardiometabolic disorders or those unwilling to undergo such treatments due to potential clinical/ financial toxicities. Patients who received one line of chemotherapy/ ARPI in the metastatic hormone-sensitive setting were also included provided they were treated with [177Lu]Lu-PSMA-617 in the first-line mCRPC setting. Informed written consent was obtained from all patients, emphasizing the experimental nature of upfront [177Lu]Lu-PSMA-617 therapy. The study received approval from the Institute Ethics Committee and adhered to the Declaration of Helsinki guidelines.
Baseline assessments included [68Ga]Ga-PSMA-11 PET/CT, complete blood count (CBC), liver function test (LFT), renal function test (RFT), and serum prostate-specific antigen (PSA) testing. Eligibility for [177Lu]Lu-PSMA-617 therapy required PSMA-positive lesions (defined as lesional uptake more than that of normal liver), hemoglobin ≥ 8 g/dL, total leucocyte count ≥ 3000/mcL, neutrophils ≥ 1500/mcL, platelets ≥ 75,000/mcL, estimated glomerular filtration rate (eGFR) ≥ 30 mL/min, serum albumin ≥ 2.5 g/dL, and Eastern Cooperative Oncology Group (ECOG) performance scores 0–4.
Treatment characteristics
Treatment involved obtaining Lutetium chloride ([177Lu]LuCl3) from the Board of Radiation and Isotope Technology, Bhabha Atomic Research Centre (BARC), Mumbai, India, and PSMA peptide from ABX (GmBH, Radeberg, Germany). In-house radiolabeling of PSMA with [177Lu]Lu was performed in the hospital radiopharmacy, ensuring a labeling efficiency of > 96%. The patients were scheduled for ~ 7.4 GBq of [177Lu]Lu-PSMA-617 per cycle every 6–8 weeks, up to 6 cycles. However, logistic issues like radionuclide production, transportation, and final available activity of the radionuclide were limitations in a real-world, out of trial setting. Finally, [177Lu]Lu-PSMA-617 was intravenously administered at a median activity of 5.55 GBq (range: 5.55–7.4 GBq) per cycle, up to 6 cycles, at 6–12 week intervals. Pre-treatment with ondansetron and dexamethasone was conducted, and patients were monitored for adverse events, undergoing a post-therapy scan 24–48 h after infusion. Patients received standard supportive care, e.g. blood transfusions, granulocyte colony-stimulating factor injections, bisphosphonates, or denosumab, as clinically indicated. Patients also continued to receive androgen deprivation therapy (ADT) to maintain castrate levels of testosterone, unless prior orchiectomy was done.
Treatment outcomes
The patients were followed up every 3 weeks for physical complaints and with laboratory values of CBC, LFT, RFT, and PSA. The various outcome measures of this study included best PSA-response rate (PSA-RR), objective radiographic response rate (ORR), radiographic progression-free survival (rPFS), overall survival (OS), and adverse events (AEs). Best PSA-RR was defined according to the Prostate Cancer Clinical Trials Working Group-3 (PCWG3) as the proportion of patients achieving a ≥ 50% decline in PSA from baseline [22]. Radiographic response was assessed as per the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) for soft tissue lesions and PCWG3 criteria for bone lesions every 12 weeks [22, 23]. ORR was defined as the proportion of patients achieving a complete or partial radiographic response (CR or PR). rPFS was estimated from the time of treatment initiation till documented radiographic progression (as per RECIST 1.1 and PCWG3) or death due to any cause. OS was calculated from the time of treatment initiation till death from any cause. AEs were evaluated using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY; IBM Corp. Categorical variables were expressed as numbers and percentages and compared between groups using Chi-square test or Fisher-exact test. Continuous variables were expressed as medians and ranges. Paired continuous variables were compared using Wilcoxon-signed rank test and independent variables using Mann-Whitney U test. Survival analysis was done using Kaplan-Meier curves and univariate/ multivariate Cox-proportional hazards model. Only those factors with a p-value less than 0.1 on univariate analysis were included in the multivariate model. Median follow-up duration was estimated using the reverse Kaplan-Meier method. A p-value < 0.05 was considered to be statistically significant.
Results
Patient characteristics
Out of the 238 metastatic prostate cancer (PCa) patients who underwent [177Lu]Lu-PSMA-617 treatment at our institution from 2015 to 2023, this study included 40 patients with first-line metastatic castration-resistant prostate cancer (mCRPC). Table 1 and Supplementary Table 1 provide insight into the demographic and clinical characteristics of these patients. The median age was 68.5 years (range: 45–78), with 80% (32/40) patients having high-grade prostate cancer (Gleason score ≥ 8). The median PSA at baseline was 41 ng/mL (range: 1-3028). Nearly all patients exhibited skeletal involvement, with over 10 skeletal metastatic sites in 72.5% (29/40) cases; additionally, five patients (12.5%) had visceral disease. Twenty-four of the 40 (60%) patients had underlying cardiometabolic disorder(s). Notably, 21/40 (52.5%) patients received [177Lu]Lu-PSMA-617 as the first-line systemic treatment after medical/surgical castration. Ten patients received prior abiraterone, eight patients received prior docetaxel, and one patient received prior abiraterone plus docetaxel in the metastatic hormone-sensitive setting.
Treatment characteristics
The patients received a median cumulative activity of 22.2 GBq (range: 5.55–44.4) of [177Lu]Lu-PSMA-617 over 1–6 cycles. The median duration between the treatment cycles was 8 weeks (range: 8–12). A total of 135 cycles of [177Lu]Lu-PSMA-617 were administered: five patients received a single cycle; six patients received two cycles; nine patients received three cycles; twelve patients received four cycles; five patients received five cycles; and three patients received six cycles. The reasons for not completing six cycles were: radiographic/ clinical progression over the course of treatment (n = 16), excellent response (n = 6), deaths (n = 5), grade ≥ 2 AEs (n = 4), loss to follow-up (n = 4), and continuing treatment cycles (n = 2).
Efficacy outcomes
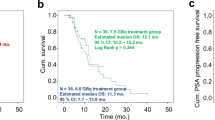
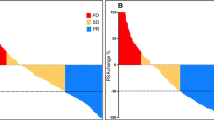
The best PSA-RR was 62.5% (25/40 patients) (Fig. 1). The nadir PSA in these patients was achieved following a median of 2 cycles of [177Lu]Lu-PSMA-617 (range: 1–6). Thirty-eight patients underwent radiographic response assessment, of which thirteen showed partial responses (ORR 34.2%) (Fig. 2 illustrates a case). Further follow-up treatments included: docetaxel (6 patients), abiraterone (4 patients), enzalutamide (4 patients), cabazitaxel (1 patient), and [225Ac]Ac-PSMA-617 (5 patients) (Supplementary Table 2). The median follow-up duration was 36 months (95% confidence interval, CI: 26–46), with 36 recorded events for rPFS and 25 for OS. The median rPFS was 12 months (95% CI: 9–15) (Fig. 3(a)), while the median OS was 17 months (95% CI: 12–22) (Fig. 3(b)). Cox-regression analyses identified a PSA decline of ≥ 50% as the sole independent predictor of favorable rPFS (univariate hazard ratio, HR: 0.35, 95% CI: 0.18–0.70, p = 0.003) (Table 2). For OS, extensive skeletal metastases at baseline (> 20 sites) (univariate HR: 2.70, 95% CI: 1.12–6.52, p = 0.027) and a PSA decline of ≥ 50% (univariate HR: 0.37, 95% CI: 0.16–0.82, p = 0.015) were significant predictors (Table 3).
A 47-year old man having treatment-naïve mCRPC presented with a baseline serum PSA of 29.3 ng/mL and extensive skeletal lesions (a). The patient was a known case of poorly controlled hypertension, had only undergone prior orchiectomy, and was unwilling for taxane-based chemotherapy. Following multidisciplinary tumor board discussion, he was started on [177Lu]Lu-PSMA-617 RLT. Partial biochemical and radiographic responses were observed following three cycles of [177Lu]Lu-PSMA-617 (b). The patient subsequently underwent three more cycles of [177Lu]Lu-PSMA-617 RLT resulting in a nadir PSA of 4.1 ng/mL. Eventually, he experienced biochemical and radiographic progression at 18 months from the start of the treatment (c) and died two months later
Adverse events
Most treatment-emergent AEs were of grade 1/2. Common symptomatic AEs included grade 1/2 xerostomia (12/40 patients, 30%) and fatigue (6/40 patients, 15%). Grade 1/2 anemia was the most common laboratory-related AE (21/40 patients, 52.5%). Five patients (12.5%) had grade 1/2 increase in serum creatinine. The changes in median values of serum creatinine and eGFR over the treatment course were non-significant and are illustrated in Supplementary Fig. 1. Treatment-emergent grade ≥ 3 anemia, leucopenia, and thrombocytopenia were noted in 4/40 (10%), 1/40 (2.5%), and 3/40 (7.5%) patients, respectively (Table 4). These toxicities were generally transient, resolving within 6–8 weeks post-therapy.
Discussion
[177Lu]Lu-PSMA-617 has emerged as a viable third-line treatment for mCRPC in the post-taxane and post-ARPI setting, as evidenced by results from the TheraP and VISION trials [17, 18]. Favorable outcomes have also been reported in a non-inferiority trial comparing [177Lu]Lu-PSMA-617 with docetaxel in chemotherapy-naïve mCRPC patients [19, 20]. Further, preliminary findings from the PSMAfore trial indicate the potential superiority of [177Lu]Lu-PSMA-617 over ARPI switch in chemotherapy-naïve mCRPC patients who have previously received one line of ARPI [24]. Encouraging results with [177Lu]Lu-PSMA-617 have also been reported in the metastatic hormone-sensitive setting [25, 26]. Despite these advancements, real-world data on the use of [177Lu]Lu-PSMA-617 in the first-line mCRPC setting have been scarce. This institutional series, comprising 40 first-line mCRPC patients in a real-world context, demonstrates meaningful clinical benefits post-[177Lu]Lu-PSMA-617 treatment, including a PSA-RR of 62.5%, ORR of 34.2%, median PFS of 12 months, and median OS of 17 months. Importantly, the incidence of major treatment-emergent AEs was minimal. These results suggest that [177Lu]Lu-PSMA-617 could be a valuable addition to the repertoire of effective and safe treatment options for first-line mCRPC, particularly benefiting patients unwilling or unfit for standard chemohormonal treatments. It is necessary to emphasize here that over half of our first-line mCRPC patients had only received medical/ surgical castration in the hormone-sensitive setting and were otherwise naïve to any form of active systemic treatment.
The historical landscape of mCRPC treatments began with docetaxel as the first approved chemotherapeutic agent. The TAX327 study which compared docetaxel plus prednisone to mitoxantrone plus prednisone showed longer median overall survival in the docetaxel arm (18.9 versus 16.5 months) [4]. Subsequent trials with ARPIs, abiraterone (COU-AA-302) and enzalutamide (PREVAIL), reported improved PFS (median 16.5 months and 20 months, respectively) and OS (median 34.7 months and 35.3 months, respectively) in first-line chemotherapy-naïve mCRPC patients [6, 8]. Nevertheless, it is essential to note that these outcomes were reported under rigid trial conditions and may not always reflect in real-world practice. Real-world studies with docetaxel, abiraterone, and enzalutamide in first-line mCRPC suggest median PFS of 8.2–13 months and median OS of 18.9–33 months [27,28,29,30,31,32,33,34]. The current study’s results with [177Lu]Lu-PSMA-617 in first-line mCRPC align well with these real-world outcomes. Notably, majority of our patients had poor performance status (ECOG ≥ 2 in 72.5% patients) which was in sharp contrast to the abovementioned pivotal trials comprising only ECOG 0–1 patients.
In recent times, there has been a focus on intensified first-line treatments for mCRPC. PARP inhibitors, previously used in HRR-mutated progressive mCRPC only, have shown promise when combined with ARPIs in first-line settings. Trials like PROPEL and TALAPRO-2, evaluating combinations such as ‘abiraterone plus olaparib’ and ‘enzalutamide plus talazoparib,’ respectively, have reported positive outcomes with improved PFS [13, 14]. However, the increased toxicity associated with these combinations, along with unproven OS benefits, raises challenges for routine application in real-world settings, especially among patients with poor performance status or cardiometabolic disorders. In this context, [177Lu]Lu-PSMA-617, with its high safety profile and comparable clinical benefits, emerges as a reasonable first-line treatment option for mCRPC, particularly for those with significant cardiometabolic disorders.
While mCRPC remains an incurable disease, treatment goals aim to prolong time to progression and overall survival. With increasing use of ARPIs in the metastatic hormone-sensitive setting, first-line mCRPC treatment options include taxane-based chemotherapy, [177Lu]Lu-PSMA-617, and PARP inhibitors. This study provides crucial real-world evidence for the use of [177Lu]Lu-PSMA-617 in the first-line mCRPC setting. For patients with high PSMA-expressing lesions and no significant PSMA-negative lesions, [177Lu]Lu-PSMA-617, with its efficacy and safety, could be a preferred first-line treatment. This aligns with the findings of a previous meta-analysis, which demonstrated better outcomes for taxane-naïve mCRPC patients treated with [177Lu]Lu-PSMA-617 compared to taxane-treated patients [35]. Moreover, the efficacy of [177Lu]Lu-PSMA-617 appears maintained even in HRR-mutated patients [36, 37]. While further randomized trials are needed to establish the optimal treatment sequence in first- and second-line mCRPC post ARPI progression in the metastatic hormone-sensitive space, the current study contributes valuable insights.
One perplexing scenario regarding the use of [177Lu]Lu-PSMA-617 is the presence of liver metastasis. In a meta-analysis comprising over 1500 patients, the presence of visceral metastasis, particularly liver metastasis, was associated with poor PSA response and survival outcomes in mCRPC patients treated with [177Lu]Lu-PSMA-RLT [38]. However, subgroup analyses in the VISION trial suggest liver metastasis to be associated with poorer OS, but not predictive of rPFS [18]. Our results with [177Lu]Lu-PSMA-617 in first-line mCRPC are thus, consistent with these outcomes (Table 3). It can be therefore, concluded that liver metastasis in mCRPC portends an overall poor prognosis and is not necessarily a predictor of response to [177Lu]Lu-PSMA-617 therapy. However, further studies are required in this direction.
Despite the positive results with [177Lu]Lu-PSMA-617 in mCRPC, its cost-effectiveness remains an area of interest. Based on the data from the VISION trial, a comprehensive cost-effectiveness analysis reported that combined treatment using [177Lu]Lu-PSMA-617 and standard-of-care led to an increased effectiveness of 0.42 quality-adjusted life years (QALYs) at increased costs of $83,712 and incremental cost-effectiveness ratio (ICER) of $200,708/QALY [18, 39]. Here, it is important to note that these costs are based on the commercially available [177Lu]Lu-PSMA-617 in the United States and local costs may differ. Further, with increasing demands for [177Lu]Lu-PSMA-617 and easing of accessibility, the cost-effectiveness may improve in the future. Notably, a similar cost-effectiveness analysis in 2014 for abiraterone in the pre-docetaxel setting had reported an ICER of $389,000/QALY [40].
This study has certain limitations, key among which are the relatively small number of patients included, and the absence of a control arm, which collectively impact the robustness of our observations. Quality-of-life outcomes were not assessed, and the analysis of germline or somatic mutations in HRR-related genes was beyond the study’s scope.
Conclusion
In this study, we evaluated our initial experience with [177Lu]Lu-PSMA-617 as a first-line systemic treatment in 40 patients with mCRPC. [177Lu]Lu-PSMA-617 was observed to have clinical benefit with a PSA-RR of 62.5%, median rPFS of 12 months, and median OS of 17 months. Further, grade 3/4 toxicities were minimal. Our observations, therefore, suggest that [177Lu]Lu-PSMA-617 can be a valuable addition to the treatment armamentarium for first-line mCRPC, and particularly beneficial for those ineligible/unwilling for standard chemohormonal treatment options. Randomized trials evaluating [177Lu]Lu-PSMA-617 in first-line mCRPC are now required to validate our results and definitively establish its role in this upfront setting.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Mansinho A, Macedo D, Fernandes I, et al. Castration-resistant prostate Cancer: mechanisms, targets and treatment. Adv Exp Med Biol. 2018;1096:117–33.
Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate Cancer. Part II-2020 update: treatment of relapsing and metastatic prostate Cancer. Eur Urol. 2021;79:263–82.
Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60.
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with Chemotherapy-naïve metastatic castration-resistant prostate Cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151–4.
Mateo J, Boysen G, Barbieri CE, et al. DNA repair in prostate Cancer: Biology and Clinical implications. Eur Urol. 2017;71:417–25.
Rose M, Burgess JT, O’Byrne K, et al. PARP inhibitors: clinical relevance, mechanisms of Action and Tumor Resistance. Front Cell Dev Biol. 2020;8:564601.
Tisseverasinghe S, Bahoric B, Anidjar M, et al. Advances in PARP inhibitors for prostate Cancer. Cancers (Basel). 2023;15:1849.
Chi KN, Rathkopf D, Smith MR, et al. Niraparib and Abiraterone acetate for metastatic castration-resistant prostate Cancer. J Clin Oncol. 2023;41:3339–51.
Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 2022;1:EVIDoa2200043.
Agarwal N, Azad AA, Carles J, et al. Talazoparib plus Enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402:291–303.
Tanaka A, Node K. The Emerging and Promising Role of Care for Cardiometabolic syndrome in prostate Cancer. JACC CardioOncol. 2019;1:307–9.
Yadav MP, Ballal S, Sahoo RK, et al. Radioligand Therapy with 177Lu-PSMA for metastatic castration-resistant prostate Cancer: a systematic review and Meta-analysis. AJR Am J Roentgenol. 2019;213:275–85.
Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804.
Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate Cancer. N Engl J Med. 2021;385:1091–103.
Satapathy S, Mittal BR, Sood A, et al. 177Lu-PSMA-617 versus docetaxel in chemotherapy-naïve metastatic castration-resistant prostate cancer: a randomized, controlled, phase 2 non-inferiority trial. Eur J Nucl Med Mol Imaging. 2022;49:1754–64.
Satapathy S, Mittal BR, Sood A, et al. [177Lu]Lu-PSMA-617 Versus Docetaxel in Chemotherapy-Naïve metastatic castration-resistant prostate Cancer: final survival analysis of a phase 2 Randomized, Controlled Trial. J Nucl Med. 2023;64:1726–9.
Sadaghiani MS, Sheikhbahaei S, Werner RA, et al. 177 Lu-PSMA radioligand therapy effectiveness in metastatic castration-resistant prostate cancer: an updated systematic review and meta-analysis. Prostate. 2022;82:826–35.
Scher HI, Morris MJ, Stadler WM, et al. Trial Design and objectives for castration-resistant prostate Cancer: updated recommendations from the prostate Cancer clinical trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Sartor O, Castellano Gauna DE, Herrmann K, et al. LBA13 phase III trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore). Ann Oncol. 2023;34(Suppl 2):S1324–5.
Satapathy S, Das N, Sood A, et al. Short-course 177Lu-PSMA-617 Radioligand Therapy in High-volume metastatic hormone-sensitive prostate Cancer: time to take the Leap? Eur Urol. 2021;80:390–2.
Privé BM, Peters SMB, Muselaers CHJ, et al. Lutetium-177-PSMA-617 in low-volume hormone-sensitive metastatic prostate Cancer: a prospective pilot study. Clin Cancer Res. 2021;27:3595–601.
Shore ND, Laliberté F, Ionescu-Ittu R, et al. Real-world treatment patterns and overall survival of patients with metastatic castration-resistant prostate Cancer in the US prior to PARP inhibitors. Adv Ther. 2021;38:4520–40.
Galli L, Chiuri VE, Di Lorenzo G, et al. First-line treatment of metastatic castration-resistant prostate cancer: the real-world Italian cohort of the prostate Cancer Registry. Tumori. 2023;109:224–32.
Shayegan B, Wallis CJD, Malone S, et al. Real-world use of systemic therapies in men with metastatic castration resistant prostate cancer (mCRPC) in Canada. Urol Oncol. 2022;40:e1921–9.
Anton A, Pillai S, Semira MC, et al. Real-world first-line systemic therapy patterns in metastatic castration-resistant prostate cancer. BJUI Compass. 2021;3:205–13.
Chowdhury S, Bjartell A, Lumen N, et al. Real-world outcomes in First-Line treatment of metastatic castration-resistant prostate Cancer: the prostate Cancer Registry. Target Oncol. 2020;15:301–15.
Bjartell A, Lumen N, Maroto P, et al. Real-world safety and efficacy outcomes with abiraterone acetate plus prednisone or prednisolone as the First- or second-line treatment for metastatic castration-resistant prostate Cancer: data from the prostate Cancer Registry. Target Oncol. 2021;16:357–67.
Freedland SJ, Davis M, Epstein AJ et al. Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population. Prostate Cancer Prostatic Dis. 2023 Oct 2. https://doi.org/10.1038/s41391-023-00725-8. Epub ahead of print.
George DJ, Sartor O, Miller K, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate Cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18:284–94.
Satapathy S, Sahoo RK, Bal C. [177Lu]Lu-PSMA-Radioligand Therapy Efficacy outcomes in Taxane-Naïve Versus taxane-treated patients with metastatic castration-resistant prostate Cancer: a systematic review and metaanalysis. J Nucl Med. 2023;64:1266–71.
Privé BM, Slootbeek PHJ, Laarhuis BI, et al. Impact of DNA damage repair defects on response to PSMA radioligand therapy in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2022;25:71–8.
Satapathy S, Das CK, Aggarwal P, et al. Genomic characterization of metastatic castration-resistant prostate cancer patients undergoing PSMA radioligand therapy: a single-center experience. Prostate. 2023;83:169–78.
Satapathy S, Mittal BR, Sood A. Visceral metastases as predictors of response and survival outcomes in patients of castration-resistant prostate Cancer treated with 177Lu-Labeled prostate-specific membrane Antigen Radioligand Therapy: a systematic review and Meta-analysis. Clin Nucl Med. 2020;45:935–42.
Mehrens D, Kramer KKM, Unterrainer LM, et al. Cost-effectiveness analysis of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-resistant prostate Cancer. J Natl Compr Canc Netw. 2023;21:43–e502.
Gong CL, Hay JW. Cost-effectiveness analysis of abiraterone and sipuleucel-T in asymptomatic metastatic castration-resistant prostate cancer. J Natl Compr Canc Netw. 2014;12:1417–25.
Acknowledgements
None.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Drs. Satapathy and Bal conceived the idea; Drs. Satapathy, Yadav, Ballal, and Sahoo collected the data; Drs. Satapathy, and Ballal analyzed the data; Dr. Satapathy wrote the initial draft of the manuscript; Drs. Yadav and Ballal modified the manuscript; Drs. Sahoo and Bal critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institute Ethics Committee, All India Institute of Medical Sciences, New Delhi (Ref. No. IESC/T-229/05.05.2015, RT-46/2015).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Satapathy, S., Yadav, M., Ballal, S. et al. [177Lu]Lu-PSMA-617 as first-line systemic therapy in patients with metastatic castration-resistant prostate cancer: a real-world study. Eur J Nucl Med Mol Imaging 51, 2495–2503 (2024). https://doi.org/10.1007/s00259-024-06677-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-024-06677-y