Abstract
Purpose
Cardiac transthyretin-related amyloidosis (ATTR) is a progressive and fatal cardiomyopathy. The diagnosis of this disease is frequently delayed or missed due to the limited specificity of echocardiography. An increasing amount of data in the literature demonstrate the ability of bone scintigraphy with bone-seeking radiopharmaceuticals to detect myocardial amyloid deposits, in particular in patients with ATTR. Therefore we performed a systematic review and bivariate meta-analysis of the diagnostic accuracy of bone scintigraphy in patients with suspected cardiac ATTR.
Methods
A comprehensive computer literature search of studies published up to 30 November 2017 on the role of bone scintigraphy in patients with ATTR was performed using the following search algorithm: (a) “amyloid” OR “amyloidosis” AND (b) “TTR” OR “ATTR” OR “transthyretin” AND (c) “scintigraphy” OR “scan” OR “SPECT” OR “SPET” OR “bone” OR “skeletal” OR “skeleton” OR “PYP” OR “DPD” OR “HMDP” OR “MDP” OR “HDP”. Pooled sensitivity, specificity, positive and negative likelihood ratios (LR+ and LR−) and diagnostic odds ratio (DOR) of bone scintigraphy were calculated.
Results
The meta-analysis of six selected studies on bone scintigraphy in cardiac ATTR including 529 patients provided the following results: sensitivity 92.2% (95% CI 89–95%), specificity 95.4% (95% CI 77–99%), LR+ 7.02 (95% CI 3.42–14.4), LR− 0.09 (95% CI 0.06–0.14), and DOR 81.6 (95% CI 44–153). Mild heterogeneity was found among the selected studies.
Conclusion
Our evidence-based data demonstrate that bone scintigraphy using technetium-labelled radiotracers provides very high diagnostic accuracy in the non-invasive assessment of cardiac ATTR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac amyloidosis is a protein deposition disease that is increasingly recognized due to enhanced clinical awareness and better diagnostic imaging [1,2,3,4,5]. Although there are many different amyloid diseases, two types account for over 95% of all cardiac amyloidosis: immunoglobulin light chain amyloidosis (AL) and transthyretin-related amyloidosis (ATTR) [1,2,3,4,5]. Both cardiac AL and ATTR involve the diffuse deposition of amyloid fibrils in the heart that causes thickening of both ventricles. Cardiac involvement can occur as part of a systemic disease or as a localized phenomenon [6, 7]. Amyloid deposition can be found throughout the myocardial tissue but is also seen in the atria, pericardium, endocardium, and vasculature [1, 5].
Besides clinical history, physical examination and measurement of plasma brain natriuretic peptide, the diagnosis of cardiac amyloidosis always begins with two-dimensional echocardiography in conjunction with electrocardiography (ECG) which allow the detection of the classic hallmark of the disease: low absolute or relative QRS voltage on ECG along with a nondilated left ventricle (LV) with moderate concentric LV hypertrophy on echocardiography [8]. Laboratory tests, other cardiac imaging modalities or tissue biopsy are used to confirm the diagnosis [1,2,3,4,5]. Cardiac magnetic resonance imaging (CMR) is often used if cardiac amyloidosis is suspected on echocardiography to confirm or refute the diagnosis. However, CMR is less specific for amyloid detection, so other diagnostic methods are needed to confirm the diagnosis [8]. Endomyocardial biopsy is nearly 100% sensitive for cardiac amyloidosis diagnosis but it is obviously an invasive diagnostic procedure with possible complications and is restricted to referral centres with expertise in performing it [1,2,3,4,5].
ATTR results from misfolding of the liver-derived precursor protein transthyretin (TTR), either as an acquired wildtype variant (ATTRwt) or as a hereditary mutant variant (ATTRm). ATTRwt typically affects older men and presents as a late-onset hypertrophic restrictive cardiomyopathy. The ATTRm variant, caused by one of many different point mutations in the TTR gene, can manifest as a polyneuropathy, cardiomyopathy, or a mixed phenotype. The differentiation of the ATTRwt variant from the ATTRm variant is performed by TTR gene mutation testing [1,2,3,4,5]. Overall, ATTR has a better prognosis than AL; however, it is still a progressive disorder that is associated with a significantly reduced survival and quality of life [1,2,3,4,5].
Several diagnostic techniques can be used to differentiate cardiac AL from ATTR. Due to their different treatment strategies, early diagnosis and prompt initiation of therapy are crucial. Laboratory tests such as the serum free light chain ratio and immunofixation of serum and urine are very useful as they have a high negative predictive value for cardiac AL [1,2,3,4,5]. Bone scintigraphy with different technetium-labelled bone-seeking radiopharmaceuticals has been used as a noninvasive diagnostic method for detecting myocardial ATTR amyloid deposits in patients with cardiac amyloidosis. Higher radiopharmaceutical uptake in the heart of patients with cardiac ATTR than in those with cardiac AL is usually expected. The explanation for this differential uptake is unknown, but it has been suggested that the preferential binding of bone radiotracers to ATTR may be a result of higher calcium content [1,2,3,4,5].
We performed a bivariate meta-analysis of the accuracy of bone scintigraphy with different technetium-labelled bone-seeking radiopharmaceuticals in the diagnosis of cardiac ATTR to demonstrate its role as a noninvasive diagnostic method in this setting.
Materials and methods
This meta-analysis was performed according to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement which describes an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses [9]. Furthermore, specific suggestions for meta-analyses of diagnostic accuracy studies were followed [10].
Search strategy
A comprehensive computer literature search of PubMed/MEDLINE and the Cochrane library databases was conducted to find relevant retrospective or prospective published studies on the diagnostic accuracy of bone scintigraphy in patients with cardiac ATTR. We used a search algorithm based on a combination of these terms: (a) “amyloid” OR “amyloidosis” AND (b) “TTR” OR “ATTR” OR “transthyretin” AND (c) “scintigraphy” OR “scan” OR “SPECT” OR “SPET” OR “bone” OR “skeletal” OR “skeleton” OR “PYP” OR “DPD” OR “HMDP” OR “MDP” OR “HDP”. No beginning date limit was used. The search was updated to 30 November 2017. No language restriction was used. To expand our search, references of the retrieved articles were also screened for additional studies.
Study selection
Studies or subsets of studies investigating the diagnostic performance of bone scintigraphy with technetium-labelled radiotracers in detecting myocardial amyloid deposits in patients with ATTR reporting data on sensitivity, specificity or diagnostic accuracy were eligible for inclusion in the qualitative analysis (systematic review). The exclusion criteria were: (a) articles not within the field of interest of this review, (b) studies not performed in humans, (c) review articles, editorials or letters, comments, and conference proceedings, (d) case reports or small case series (fewer than ten patients), and (e) articles with insufficient data to reassess sensitivity or specificity (such as absence of data on true-positive, true-negative, false-positive and false-negative findings). In the quantitative analysis (meta-analysis) studies with possible patient overlap were also excluded. If patient overlap was found, the most complete article was included in the meta-analysis.
Three researchers (G.T., F.B., L.G.) independently reviewed the titles and abstracts of the retrieved articles, applying the inclusion and exclusion criteria listed above. Articles were rejected if they were clearly ineligible. The same three researchers then independently reviewed the full-text version of the remaining articles to determine their eligibility for inclusion. Disagreements were resolved in a consensus meeting.
Data extraction
For each study potentially eligible for the meta-analysis, information was collected concerning the basic aspects of the study (authors, year of publication, country of origin, study design), patient characteristics (mean age, sex ratio, number of patients with cardiac amyloidosis, type of cardiac amyloidosis), and technical aspects (radiotracer used, injected activity, time between radiotracer injection and image acquisition, type of scintigraphic acquisition, image analysis, applied reference standard). For each study the numbers of true-positive, false-positive, true-negative and false-negative bone scintigraphy findings in the diagnosis of cardiac ATTR were recorded.
Quality assessment
The 2011 Oxford Centre for Evidence-Based Medicine checklist for diagnostic studies was used to assess the quality of the included studies [11]. This checklist has five major parts, as follows: representative spectrum of the patients, consecutive patient recruitment, ascertainment of the gold standard regardless of the index test results, independent blind comparison between the gold standard and index test results, enough explanation of the test to permit replication [11].
Statistical analysis
Sensitivity and specificity, positive and negative likelihood ratio (LR+ and LR−) and diagnostic odds ratio (DOR) of bone scintigraphy (using visual analysis of planar images) in patients with cardiac ATTR was obtained from individual studies in a per-patient-based analysis. A bivariate random effects model was used for statistical pooling of data; this technique is considered an appropriate method for pooling sensitivity and specificity from multiple diagnostic test accuracy studies because it takes into account any correlation that may exist between sensitivity and specificity [10]. Pooled data are presented with 95% confidence intervals (95% CI). Furthermore, a summary receiver operating characteristic (sROC) curve was obtained using a bivariate random effects model [10]. Heterogeneity was estimated using the I-squared index (I2) which represents the percentage variation across studies that is due to heterogeneity rather than chance [12]. A subanalysis of sensitivity and specificity of bone scintigraphy taking into account the different radiopharmaceuticals used was also performed. Statistical analyses were performed using OpenMetaAnalyst software that uses R as the underlying statistical engine. OpenMetaAnalyst (http://www.cebm.brown.edu/openmeta/) is funded by the Agency for Healthcare Research and Quality (Rockville, MD, USA).
Results
Literature search
The comprehensive computer literature search of PubMed/MEDLINE and the Cochrane database revealed 214 articles. Review of titles and abstracts led to the exclusion of 174 articles: 127 not in the field of interest of this review, 31 reviews, editorials or letters, 16 case reports or small case series. Thus, 40 articles were selected and retrieved in full-text version [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. Subsequently, 28 full-text articles were excluded from the analysis due to insufficient data to reassess sensitivity and specificity [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. No additional studies were found on screening the references of these articles. Finally, 12 articles including data on the diagnostic performance of bone scintigraphy in cardiac ATTR were eligible for the qualitative analysis (systematic review) [41,42,43,44,45,46,47,48,49,50,51,52]. After reviewing the full-text articles, six were excluded from the quantitative analysis (meta-analysis) due to patient data overlap [41,42,43,44,45,46] and six including 529 patients were selected for the meta-analysis [47,48,49,50,51,52] (Fig. 1). The characteristics of the selected studies are presented in Tables 1, 2, 3 and 4.
Qualitative analysis (systematic review)
The database search led to the selection of 12 full-text articles including quantitative data on the diagnostic performance (sensitivity and specificity) of bone scintigraphy in cardiac ATTR published over the past 16 years (Table 1) [41,42,43,44,45,46,47,48,49,50,51,52]. Most of the studies were retrospective (75%) and single-centre (67%). Several countries of Europe, North America and Oceania were represented. The mean age of the patients included in these studies ranged from 54 to 76 years, and approximately 76% were men. Most studies included a mixed population of patients with cardiac amyloidosis including ATTR (ATTRm and ATTRwt) and other amyloidosis such as AL (Table 1).
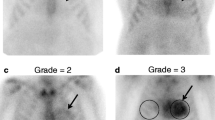
The bone scintigraphy in the included studies showed heterogeneous technical imaging aspects (Table 2). The radiotracer used was technetium-99m diphosphono-propano-dicarboxylic acid (99mTc-DPD) in seven studies, technetium-99m pyrophosphate (99mTc-PYP) in four studies and technetium-99m hydroxymethylene diphosphonate (99mTc-HMDP) in three studies. In one article three different radiotracers were used [49]. The mean injected radiotracer activity and time between radiotracer injection and image acquisition were quite different among the included studies. The acquisition protocol included planar images (thorax or whole-body) in all studies and additional tomographic acquisitions (SPECT or SPECT/CT) in 67% of studies (Table 2). The scintigraphic images were analysed qualitatively (visual analysis) in all studies, and also semiquantitatively in 75% of studies (Table 2). The visual analysis was performed using the following grading system: grade 0 absent cardiac uptake, grade 1 mild cardiac uptake less than that of bone, grade 2 moderate cardiac uptake equal to that of bone, and grade 3 high cardiac uptake greater than that of bone (Fig. 2). For the semiquantitative analysis different methods were used including heart/contralateral thorax count ratio (H/CL), heart tracer retention (HR), whole-body tracer retention (WBR), skull retention (SR), heart to whole-body uptake ratio (H/WB), and heart to skull uptake ratio (H/S; Table 2). The reference standards used included biopsy and genotyping/immunohistochemistry in most of the studies (Table 3).
Example of planar bone scintigraphy images showing the visual grading of cardiac uptake. From Glaudemans et al. [35] with modifications
Overall, bone scintigraphy with different technetium-labelled radiotracers has been used as a very useful noninvasive tool for the assessment of cardiac ATTR with high sensitivity and specificity values using visual analysis of planar images (Table 3) [41,42,43,44,45,46,47,48,49,50,51,52]. Semiquantitative analysis may further increase the diagnostic accuracy of the scintigraphic method [41, 42, 44,45,46,47,48, 50, 52]. No significant difference in diagnostic performance of this scintigraphic method in relation to country of origin of the patients with cardiac ATTR was found. On the other hand, a slightly higher sensitivity of bone scintigraphy in patients with cardiac ATTRwt than in those with ATTRm was found in the multicentre study by Gillmore et al. (89.5% vs. 80.2%, respectively, using a grading score of 2 as the positivity threshold), thus demonstrating the possible influence of the genetic profile of cardiac ATTR on the scintigraphic findings [49].
Quantitative analysis (meta-analysis)
Six studies including 529 patients were selected for the meta-analysis [47,48,49,50,51,52]. The pooled performance of bone scintigraphy in the diagnosis of cardiac ATTR using visual analysis (a visual grading score of ≥2 was considered positive for ATTR) are presented in Figs. 3 and 4.
The sensitivity of bone scintigraphy ranged from 83% to 100%, with a pooled estimate of 92.2% (95% CI 89–95%), whereas the specificity ranged from 67% to 100%, with a pooled estimate of 95.4% (95% CI 77–99%). The pooled LR+, LR− and DOR were 7 (95% CI 3.4–14.4), 0.09 (95% CI 0.06–0.14), and 81.64 (95% CI 43.4–153.4), respectively. The heterogeneity among the included studies was moderate (I2 < 50%). The sROC curve (Fig. 3) shows excellent performance of bone scintigraphy with technetium-labelled radiotracers in detecting myocardial amyloid deposits in patients with ATTR. With regard to the subanalysis taking into account the different radiopharmaceuticals used, the pooled sensitivity and specificity of 99mTc-DPD scintigraphy were 94.6% (95% CI 90–97%) and 88.4% (95% CI 81–93%), respectively. The pooled sensitivity and specificity of 99mTc-PYP scintigraphy were 87.4% (95% CI 79–93%) and 75.4% (95% CI 57–88%), respectively. The pooled sensitivity and specificity of 99mTc-HMDP scintigraphy were 85.7% (95% CI 66–95%) and 97.5% (95% CI 84–99%), respectively. No statistically significant differences in diagnostic performance were found in relation to the different radiopharmaceuticals used.
Discussion
Several studies have used bone scintigraphy with technetium-labelled bone-seeking radiopharmaceuticals in the differential diagnosis of cardiac ATTR from other cardiac amyloidosis, and have shown different values of sensitivity and specificity. However, most of these studies had limited power, due to the relatively small numbers of patients enrolled and assessed. In order to derive more robust estimates of the diagnostic performance of bone scintigraphy with technetium-labelled radiotracers in this setting we pooled the data from the published studies. The pooled analysis indicated that bone scintigraphy with technetium-labelled bone-seeking radiopharmaceuticals shows excellent accuracy in diagnosing cardiac ATTR by visual image analysis with a visual grading score of ≥2 considered positive for ATTR. Using a visual grading score of 1 would increase sensitivity at the price of a significant reduction in specificity as a consequence of low cardiac radiotracer uptake in patients with cardiac amyloidosis other than ATTR [41,42,43,44,45,46,47,48,49,50,51,52].
A possible influence of the TTR genetic profile and ATTR amyloid fibril composition on scintigraphic findings has been hypothesized [23, 29]. Two different types of amyloid fibrils have been identified (type A and type B). Type B fibrils have been found predominantly in patients with early-onset ATTRm amyloidosis with V30M mutation and in patients carrying the Y114C mutation, whereas type A fibrils are noted in patients with all other mutations currently examined as well as in ATTRwt [29]. As demonstrated in some studies, bone scintigraphy can often be negative in patients with type B amyloid fibrils and usually positive in patients with type A amyloid fibrils [23, 29].
Positive bone scintigraphy with technetium-labelled bone-seeking radiopharmaceuticals (grade 2 or 3 cardiac uptake on visual analysis) in the absence of detectable monoclonal protein in the serum and urine on serum free light chain ratio testing and immunofixation allows the diagnosis of cardiac ATTR without the need for histological confirmation [49]. The combination of abnormal results on serum free light chain assay or serum/urine immunofixation and grade 0 cardiac uptake on bone scintigraphy in a patient with suspected cardiac amyloidosis makes the diagnosis of AL likely, but this needs to be confirmed by biopsy including immunohistochemistry [1]. A visual grading score of 1 on bone scintigraphy in a patient with suspected cardiac amyloidosis may be due to either ATTR or AL, and in such a patient measurement of free light chains and abdominal fat biopsy are needed to clarify the diagnosis [1]. In addition, from a clinical viewpoint it remains necessary to exclude other possible causes of cardiac bone-seeking tracer uptake, such as myocardial infarction, hypercalcaemia or a postradiation effect [53, 54].
We were able to include only six studies in our meta-analysis, limiting the statistical power of the analysis. Nevertheless, a significant number of patients was obtained by pooling data from these six studies (n = 529). We determined the diagnostic performance of bone scintigraphy in patients with cardiac ATTR using visual analysis of planar images. However, tomographic acquisition (SPECT) may be useful to evaluate the regional distribution of radiopharmaceutical uptake in the heart. Sperry et al. found that there is decreased 99mTc-PYP uptake in the apical segment compared with the mid and basal segments of the LV in patients with cardiac ATTR, mimicking apical-sparing longitudinal strain seen on echocardiography [14]. This apical-sparing scintigraphic pattern in the LV of patients with cardiac ATTR has also been observed in studies using 99mTc-HMDP and 99mTc-DPD [17, 21]. The evaluation of regional distribution of bone-seeking radiopharmaceuticals in the myocardium might be used in clinical trials to measure the response to treatment in patients with cardiac ATTR or to demonstrate the segmental progression of the disease [14, 17, 21].
Heterogeneity among the studies may be a potential source of bias in a meta-analysis. This heterogeneity is likely to arise through methodological diversity among the different studies (Table 2). The baseline differences among the patients in the included studies (Table 1) and the study quality (Table 4) may also contribute to the heterogeneity of the results. However, we failed to detect significant heterogeneity among the studies in the pooled analysis (I2 < 50%).
The diagnostic accuracy of a test is not a measure of clinical effectiveness and improved accuracy does not necessarily result in improved patient outcomes. Overall, our systematic review and meta-analysis demonstrated that bone scintigraphy with technetium-labelled radiotracers has excellent accuracy for the noninvasive diagnosis of cardiac ATTR. Studies on the cost-effectiveness of bone scintigraphy in patients with cardiac ATTR are needed to confirm the usefulness of this functional imaging method in this setting. The inclusion of bone scintigraphy in the guidelines for the diagnostic work-up of patients with suspected cardiac ATTR will be the next step.
References
Donnelly JP, Hanna M. Cardiac amyloidosis: an update on diagnosis and treatment. Cleve Clin J Med. 2017;84(12 Suppl 3):12–26.
Mankad AK, Shah KB. Transthyretin cardiac amyloidosis. Curr Cardiol Rep. 2017;19(10):97. https://doi.org/10.1007/s11886-017-0911-5.
Siddiqi OK, Ruberg FL. Cardiac amyloidosis: an update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018;28:10–21.
Tuzovic M, Yang EH, Baas AS, Depasquale EC, Deng MC, Cruz D, et al. Cardiac amyloidosis: diagnosis and treatment strategies. Curr Oncol Rep. 2017;19(7):46. https://doi.org/10.1007/s11912-017-0607-4.
Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357–77.
Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007;50(22):2101–10.
Desai HV, Aronow WS, Peterson SJ, Frishman WH. Cardiac amyloidosis: approaches to diagnosis and management. Cardiol Rev. 2010;18(1):1–11.
Habib G, Bucciarelli-Ducci C, Caforio AL, Cardim N, Charron P, Cosyns B, et al. Multimodality Imaging in Restrictive Cardiomyopathies: an EACVI expert consensus document In collaboration with the "Working Group on myocardial and pericardial diseases" of the European Society of Cardiology Endorsed by The Indian Academy of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18(10):1090–121.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Sadeghi R, Treglia G. Systematic reviews and meta-analyses of diagnostic studies: a practical guideline. Clin Transl Imaging. 2017;5:83–7.
Oxford Centre for Evidence-Based Medicine. Critical Appraisal tools. Oxford: Nuffield Department of Primary Care Health Sciences. http://www.cebm.net/blog/2014/06/10/critical-appraisal/. Accessed 16 April 2018.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Castaño A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879–87.
Sperry BW, Vranian MN, Tower-Rader A, Hachamovitch R, Hanna M, Brunken R, et al. Regional variation in technetium pyrophosphate uptake in transthyretin cardiac amyloidosis and impact on mortality. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):234–42.
Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, et al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol. 2017;70(4):466–77.
Cariou E, Bennani Smires Y, Victor G, Robin G, Ribes D, Pascal P, et al. Diagnostic score for the detection of cardiac amyloidosis in patients with left ventricular hypertrophy and impact on prognosis. Amyloid. 2017;24(2):101–9.
Van Der Gucht A, Cottereau AS, Abulizi M, Guellich A, Blanc-Durand P, Israel JM, et al. Apical sparing pattern of left ventricular myocardial (99m)Tc-HMDP uptake in patients with transthyretin cardiac amyloidosis. J Nucl Cardiol. 2017. https://doi.org/10.1007/s12350-017-0894-z.
González-López E, Gagliardi C, Dominguez F, Quarta CC, de Haro-Del Moral FJ, Milandri A, et al. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J. 2017;38(24):1895–904.
Hutt DF, Fontana M, Burniston M, Quigley AM, Petrie A, Ross JC, et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur Heart J Cardiovasc Imaging. 2017;18(12):1344–50.
Vranian MN, Sperry BW, Hanna M, Hachamovitch R, Ikram A, Brunken RC, et al. Technetium pyrophosphate uptake in transthyretin cardiac amyloidosis: associations with echocardiographic disease severity and outcomes. J Nucl Cardiol. 2017. https://doi.org/10.1007/s12350-016-0768-9.
Abulizi M, Cottereau AS, Guellich A, Vandeventer S, Galat A, Van Der Gucht A, et al. Early-phase myocardial uptake intensity of (99m)Tc-HMDP vs (99m)Tc-DPD in patients with hereditary transthyretin-related cardiac amyloidosis. J Nucl Cardiol. 2018;25(1):217–22.
Bennani Smires Y, Victor G, Ribes D, Berry M, Cognet T, Méjean S, et al. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging. 2016;32(9):1403–13.
Pilebro B, Arvidsson S, Lindqvist P, Sundström T, Westermark P, Antoni G, et al. Positron emission tomography (PET) utilizing Pittsburgh compound B (PIB) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis (ATTR). J Nucl Cardiol. 2018;25(1):240–8.
Bokhari S, Morgenstern R, Weinberg R, Kinkhabwala M, Panagiotou D, Castano A, et al. Standardization of (99m)Technetium pyrophosphate imaging methodology to diagnose TTR cardiac amyloidosis. J Nucl Cardiol. 2018;25(1):181–90.
Treibel TA, Fontana M, Gilbertson JA, Castelletti S, White SK, Scully PR, et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016;9(8):e005066. https://doi.org/10.1161/CIRCIMAGING.116.005066.
Di Bella G, Minutoli F, Piaggi P, Casale M, Mazzeo A, Zito C, et al. Quantitative comparison between amyloid deposition detected by (99m)Tc-diphosphonate imaging and myocardial deformation evaluated by strain echocardiography in transthyretin-related cardiac amyloidosis. Circ J. 2016;80(9):1998–2003.
de Gregorio C, Dattilo G, Casale M, Terrizzi A, Donato R, Di Bella G. Left atrial morphology, size and function in patients with transthyretin cardiac amyloidosis and primary hypertrophic cardiomyopathy – comparative strain imaging study. Circ J. 2016;80(8):1830–7.
Galat A, Guellich A, Bodez D, Slama M, Dijos M, Zeitoun DM, et al. Aortic stenosis and transthyretin cardiac amyloidosis: the chicken or the egg? Eur Heart J. 2016;37(47):3525–31.
Pilebro B, Suhr OB, Näslund U, Westermark P, Lindqvist P, Sundström T. (99m)Tc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Ups J Med Sci. 2016;121(1):17–24.
Castaño A, DeLuca A, Weinberg R, Pozniakoff T, Blaner WS, Pirmohamed A, et al. Serial scanning with technetium pyrophosphate ((99m)Tc-PYP) in advanced ATTR cardiac amyloidosis. J Nucl Cardiol. 2016;23(6):1355–63.
Di Bella G, Minutoli F, Piaggi P, Casale M, Mazzeo A, Zito C, et al. Usefulness of combining electrocardiographic and echocardiographic findings and brain natriuretic peptide in early detection of cardiac amyloidosis in subjects with transthyretin gene mutation. Am J Cardiol. 2015;116(7):1122–7.
González-López E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–94.
Treibel TA, Bandula S, Fontana M, White SK, Gilbertson JA, Herrey AS, et al. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J Cardiovasc Comput Tomogr. 2015;9(6):585–92.
Kristen AV, Scherer K, Buss S, Aus dem Siepen F, Haufe S, Bauer R, et al. Noninvasive risk stratification of patients with transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7(5):502–10.
Glaudemans AW, van Rheenen RW, van den Berg MP, Noordzij W, Koole M, Blokzijl H, et al. Bone scintigraphy with (99m)technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid. 2014;21(1):35–44.
Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK, et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):157–65.
Minutoli F, Di Bella G, Mazzeo A, Donato R, Russo M, Scribano E, et al. Comparison between (99m)Tc-diphosphonate imaging and MRI with late gadolinium enhancement in evaluating cardiac involvement in patients with transthyretin familial amyloid polyneuropathy. AJR Am J Roentgenol. 2013;200(3):W256–65.
Russo M, Mazzeo A, Stancanelli C, Di Leo R, Gentile L, Di Bella G, et al. Transthyretin-related familial amyloidotic polyneuropathy: description of a cohort of patients with Leu64 mutation and late onset. J Peripher Nerv Syst. 2012;17(4):385–90.
Rapezzi C, Quarta CC, Guidalotti PL, Pettinato C, Fanti S, Leone O, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2011;4(6):659–70.
Di Bella G, Minutoli F, Mazzeo A, Vita G, Oreto G, Carerj S, et al. MRI of cardiac involvement in transthyretin familial amyloid polyneuropathy. AJR Am J Roentgenol. 2010;195(6):W394–9.
Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1(8):880–9.
Galat A, Rosso J, Guellich A, Van Der Gucht A, Rappeneau S, Bodez D, et al. Usefulness of (99m)Tc-HMDP scintigraphy for the etiologic diagnosis and prognosis of cardiac amyloidosis. Amyloid. 2015;22(4):210–20.
Hutt DF, Quigley AM, Page J, Hall ML, Burniston M, Gopaul D, et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging. 2014;15(11):1289–98.
Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6(2):195–201.
Rapezzi C, Quarta CC, Guidalotti PL, Longhi S, Pettinato C, Leone O, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38(3):470–8.
Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46(6):1076–84.
Cappelli F, Gallini C, Di Mario C, Costanzo EN, Vaggelli L, Tutino F, et al. Accuracy of 99mTc-hydroxymethylene diphosphonate scintigraphy for diagnosis of transthyretin cardiac amyloidosis. J Nucl Cardiol. 2017. https://doi.org/10.1007/s12350-017-0922-z.
Moore PT, Burrage MK, Mackenzie E, Law WP, Korczyk D, Mollee P. The utility of (99m)Tc-DPD scintigraphy in the diagnosis of cardiac amyloidosis: an Australian experience. Heart Lung Circ. 2017;26(11):1183–90.
Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–12.
Papantoniou V, Valsamaki P, Kastritis S, Tsiouris S, Delichas Z, Papantoniou Y, et al. Imaging of cardiac amyloidosis by (99m)Tc-PYP scintigraphy. Hell J Nucl Med. 2015;18(Suppl 1):42–50.
de Haro-del Moral FJ, Sánchez-Lajusticia A, Gómez-Bueno M, García-Pavía P, Salas-Antón C, Segovia-Cubero J. Role of cardiac scintigraphy with 99mTc-DPD in the differentiation of cardiac amyloidosis subtype. Rev Esp Cardiol. 2012;65(5):440–6.
Puille M, Altland K, Linke RP, Steen-Müller MK, Kiett R, Steiner D, et al. 99mTc-DPD scintigraphy in transthyretin-related familial amyloidotic polyneuropathy. Eur J Nucl Med Mol Imaging. 2002;29(3):376–9.
Panagiotidis E, Price G, Harland S, Bomanji J, Kayani I. Myocardial uptake of 99mTc-HDP and reduced perfusion on CT in subacute myocardial infarction. Clin Nucl Med. 2014;39(1):e117–20.
Caobelli F, Paghera B, Pizzocaro C, Guerra UP. Extraosseous myocardial uptake incidentally detected during bone scan: report of three cases and a systematic literature review of extraosseous uptake. Nucl Med Rev Cent East Eur. 2013;16(2):82–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
This article does not describe any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Treglia, G., Glaudemans, A.W.J.M., Bertagna, F. et al. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: a bivariate meta-analysis. Eur J Nucl Med Mol Imaging 45, 1945–1955 (2018). https://doi.org/10.1007/s00259-018-4013-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4013-4