Abstract
Objectives
In this study, we aimed to compare conventional and T1-weighted volumetric magnetic resonance arthrography (MRA) in the diagnosis and grading of glenoid cartilage defects that accompany labral pathologies.
Materials and methods
A total of 79 patients who were prediagnosed with labrum pathologies based on shoulder magnetic resonance imaging (MRI) had MRA and CTA between December 2021 and May 2022. CTA was regarded as reference standard. CTA images were examined by a radiologist experienced in musculoskeletal radiology, and MRA images were examined by two radiologists independently to determine presence, grade, and localization of any glenoid cartilage defect, if present. Sensitivity, specificity, and accuracy were calculated separately for conventional and T1-weighted volumetric MRA. In addition, at the last stage, two observers examined all MRAs together, and the presence of a cartilage defect was decided by consensus, and the overall sensitivity, specificity, and accuracy were calculated.
Results
Cartilage defect was detected on CTAs of 48 (60.75%) cases of among 79 patients with labrum pathology. The sensitivity, specificity, and accuracy of conventional MRA for two examiners were 17–19%, 100–100%, and 49–51%, respectively, while those values were 67–65%, 92–97%, and 84–77%, respectively, for T1-weighted volumetric MRA. Inter-examiner agreement was excellent for diagnosis of cartilage defects on all MRAs. The overall sensitivity, specificity, and accuracy for detection of glenoid cartilage lesions by MRA were 69%, 97%, and 80%, respectively.
Conclusion
T1-weighted volumetric MRA seems to demonstrate cartilage defects accompanied with labrum pathologies accurately with high sensitivity, specificity, and excellent inter-examiner agreement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The shoulder joint, which secures its stability through dynamic forces rather than its structural characteristics, is an almost unrestricted joint. However, even minor anatomical alterations such as loss of glenoid cartilage and bone may cause joint instability, resulting in early osteoarthritis [1, 2]. In some patients, these lesions may cause activity-related shoulder pain years after a minor shoulder trauma [3, 4]. Pain can cause a significant reduction in active range of motion and, as a result, impairs quality of life. Osteochondral lesions of the knee joint have been proven as a source of pain and precursor of degenerative changes and have been identified in routine physical examination of the knee; however, glenohumeral joint (GHJ) cartilage has been studied less extensively.
MRA and CTA may be employed for imaging of the articular cartilage. MRA has been considered as the gold standard for joint and particularly cartilage imaging. However, CTA may demonstrate articular cartilage damage better than MRA, thanks to its high spatial resolution, ability to scan at a submillimeter scale, and multi-planar imaging [5]. Long scan time, sensitivity to motion, or foreign body artifacts are the disadvantages of MRA. However, avoiding radiation exposure is a significant advantage [6]. Volumetric interpolated breath-hold examination (VIBE) sequence is a T1-weighted gradient echo sequence with relatively short exposure time, high contrast, and spatial resolution and has been employed to visualize cartilage defects in various joints [7].
Recent studies reported that fat-suppression VIBE sequence enables three-dimensional (3D) multi-planar imaging and thin sections, and it has taken its place in MRA [8,9,10]. 2D MR arthrograms have good in-plane resolution and conspicuity to evaluate labroligamentous structures. A three-dimensional (3D) fat-suppressed T1-weighted VIBE MR arthrography sequence also allows multiplanar reconstruction using thinner image slices (with a submillimetric thickness) and provides perfect contrast for the surrounding soft tissues in shoulder MR arthrography [8, 11, 12]. Various authors have demonstrated the effectiveness of conventional MRA and T2 volumetric MRA in the detection of glenoid cartilage defects. However, to our knowledge, up to date, no studies in the literature showed the effectiveness of T1 volumetric MRA scan in the detection of cartilage defects accompanying glenoid labrum pathologies.
In this study, we aimed to compare conventional and T1 volumetric MRA with CTA in cases with clinical indications in order to investigate their contribution to diagnosis and grading of the glenoid cartilage defects accompanying labral pathologies.
Material and methods
Patients
In this prospective study, the MRA and CTA images of 83 patients referred to our hospital for shoulder examination between December 2021 and May 2022 were analyzed. The arthroscopic correlation could not be investigated. The inclusion criteria were absence of any history for surgery for labrum pathologies and presence findings in favor of glenoid labrum pathologies in the light of physical examination, history, and routine shoulder MRI. The exclusion criteria were history of intra-articular steroid injection, presence of skin lesions with or without rash on the shoulder, scar tissue or open wound, pregnancy, suspicion of septic arthritis, patients with severe dyspnea or claustrophobia, immunosuppression, presence of a pacemaker, being on anticoagulants or history of bleeding diathesis, history of open or arthroscopic shoulder surgery, the patients with insufficient joint distension on CTA or MRA or with massive contrast agent extravasation that made the diagnosis difficult, failure of completing all imaging parameters on CTA or MRA or insufficient resolution due to artifacts, and the patients that did not volunteered to participate in the study. Four shoulder MR arthrograms were excluded. Figure 1 shows exclusion criteria and patient selection into a flowchart. MRA and CTA scans of 79 (58 male and 21 female) patients aged 16–68 years were included in the study for further analysis. The study protocol was approved by the local Ethics Committee (Dec 06, 2021–2021/238), and all patients provided their consents for the study procedure (injection and imaging).
Injection technique, CT arthrograms, and MR arthrograms
Shoulder arthrographies were performed through an ultrasound-guided posterior approach. Diluted paramagnetic contrast material solution was prepared by adding 0.8–1 ml of 1.0 mmol/ml gadobutrol (Gadovist, Bayer, Germany) into 100 cc SF under sterile conditions using an insulin syringe. In order to obtain both CTA and MRA images in the same session, 10 cc of nonionic iodinated contrast agent iohexol (Kopaq 300 mg / ml, Koçsel, Turkey) was drawn into a 20-cc syringe. Another 10 cc of the existing diluted paramagnetic contrast material solution was withdrawn, and 20 cc of BTA and MRA solution was prepared. The solution was injected into the shoulder joint using a 20-G needle. Between 10 and 15 cc of the prepared solution was injected depending on the patient’s age and shoulder capacity. Resistance to further injection and distension of the capsule was determined as criteria for an adequate the amount for injected contrast material. After injection, ultrasonography (US) was performed to examine the distension of the joint capsule, and it was confirmed that the contrast material was injected into the joint space without any leak out of the joint capsule. The patients injected with intra-articular diluted contrast material were taken to the CT unit, just before MRA imaging. The patients were placed on the CT table in the supine position for a multiplanar CTA (Somatom Definiton, Siemens, Erlangen, Germany). First, standard scenography images were obtained, then the position for the scan was determined, covering the shoulder joint. CTA was performed using the following technical parameters: 120 kVp, Care Dose4D, focal spot size, 0.8 mm × 1.2 mm; FOV, 16 cm; effective thickness, 0.4 mm; and matrix, 512 × 512 and bone reconstruction kernel. MR arthrograms were acquired on a 3 T MR imaging system (Magnetom Skyra; Siemens Healthcare, Erlangen, Germany) with a superficial shoulder coil, 15–30 min after injection into the shoulder joint. Shoulder MR arthrography scans were axial and coronal oblique fat-suppressed 2D T1-weighted TSE (TR/TE, 562/10; 3-mm slice thickness), sagittal oblique T1-weighted TSE (TR/TE, 725/10; 4-mm slice thickness), coronal oblique T2-weighted TSE (TR/TE, 4790/72; 1-mm slice thickness), and fat-suppressed T1-weighted 3D thin section VIBE (TR/TE, 7.76/3.62; 11° flip angle; 0.4-mm section thickness) sequences.
Image analysis
The CTA and MRA data were transferred to Sectra Picture Archiving and Communication Systems (PACS) software for later evaluation (Sectra AB, Linköping, Sweden). All MR arthrograms were analyzed independently by two musculoskeletal radiologists (AA, BB) with 5 and 15 years of experience, respectively. CTA was regarded as the reference standard since correlation with arthroscopy could not be performed, and CTA images were independently evaluated by a radiologist (CC) with 17 years of experience in arthrographic procedures. After the sensitivity, specificity, and accuracy of each MRA technique were evaluated separately by two observers, the two observers came together and examined all conventional and volumetric MRAs together and decided by consensus on the presence of a glenoid cartilage defect. Labrum pathologies were classified in the MRA images of 79 patients included in the study. Tears in the anteroposterior direction, including the superior glenoid labrum and biceps origin, were evaluated as SLAP lesions, while the complete tear of the anterior inferior labroligamentous complex from the glenoid bone and the tear of the scapular periosteum were considered as Bankart lesions. Lesions affecting the posterior inferior labrum, posterior inferior scapular periosteum, and the posterior band of the inferior glenohumeral ligament were classified as reverse Bankart lesion, posterior capsulolabral and periosteal detachment, and rupture of the adjacent cartilage plate as Kim’s lesion [13]. For each patient, the glenoid cartilage was divided into nine zones using a grid system (Fig. 2). A three-grade system was used to stage cartilage defects for convenience in routine clinical practice. These were determined as normal cartilage and superficial and deep lesions. If the cartilage loss was less than 50%, it was defined as a superficial lesion, and if it was more than 50%, it was defined as a deep lesion (Fig. 3). In each case with labrum pathology, the grade of the lesion was determined on arthrographic images by examining cartilage surfaces, the involvement of subchondral bony structures, presence of any leak of the contrast material, and by comparing the images with symmetrical localization at each level. At the same time, the anatomical zones of the cartilage defects were determined. The “bare area” which is defined as the thin central area of the hyaline cartilage covering the glenoid fossa was excluded from the evaluation; however, cartilage defects adjacent to this area were taken into consideration [14].
Statistical analysis
Assumption of normality was tested with Kolmogorov–Smirnov test, Levene test was used for homogeneity assumption of group variances, and independent samples t test was used for intergroup comparisons. Chi-square test (post hoc, Bonferroni test) was used to compare categorical ratios. Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy rates were calculated for both conventional MRA and volumetric MRA in the detection of glenoid cartilage defects. Pearson Chi-square and Fisher-Freeman-Halton tests were used to determine the relationships between categorical variables. Kappa coefficient was calculated for the agreement of the examiners. The statistical significance level was sat at p < 0.05. All statistical analyses were performed using a statistical software (SPSS software, version 25, Chicago, IL, USA).
Results
Of the 79 patients included in the study, 58 (73.4%) were male and 21 (26.6%) were female. Left shoulders of 44 patients and right shoulders of 35 patients were evaluated. The ages of the subjects included in the study ranged between 16 and 68 years with a mean age of 35.38 ± 12.5 years. The ages of the male patients ranged between 16 and 66 years, with a mean age of 31.91 ± 10.9 years, and those values were 18–68 years and 44 ± 10.8 years in the female patients. Among 79 cases with labrum pathology, 42 (53.2%) had a SLAP (superior labrum from anterior to posterior tear) lesion, 24 (30.4%) had Bankart lesion, 7 (8.9%) had Bankart variant, 4 (5.1%) had Bankart + SLAP lesion, 1 (1.3%) had reverse Bankart + SLAP lesion, and 1 had (1.3%) Kim’s lesion. Cartilage defect was detected in 48 (60.8%) of the shoulder CTAs performed on 79 patients. Of the 48 cartilage defects, 24 (50%) were superficial lesions, and 24 (50%) were deep lesions. Analysis of the type of the labrum pathology in relation with gender and age did not reveal any statistically significant differences for the cartilage defect detection rate (p > 0.05). Conventional and T1 volumetric MRA were examined independently by two different examiners. Examiner 1 determined cartilage defects on 8 (10.1%) conventional MRAs and on 28 (35.4%) T1 volumetric MRAs. Examiner 2 determined cartilage defects on 9 (11.4%) conventional MRAs and 31 (39.2%) T1 volumetric MRAs. No superficial lesion was detected by either examiner on conventional MRA (Figs. 4 and 5). For the detection of all glenoid cartilage lesions including superficial lesions, sensitivities, specificities, and accuracies were 67%/92%/84% for examiner 1 (65%/97%/77% for examiner 2) for the T1 volumetric MRA and 17%/100%/49% (19%/100%/51% for examiner 2) for the conventional MRA images (Table 1). For solely deep cartilage lesions, sensitivities, specificities, and accuracies were 83%/87%/86% for examiner 1 (83%/93%/90% for examiner 2) for the T1 volumetric MRA images and 29%/98%/77% (33%/98%/78% for examiner 2) for the conventional MRA. There was an excellent agreement between the two examiners for determining the cartilage defects on conventional MRA (κ = 0.934, p < 0.001) and on T1 volumetric MRA (κ = 0.815, p < 0.001). In addition, there was an excellent agreement between the two examiners for determining grade of cartilage defects on T1 volumetric MRA (κ = 0.100, p < 0.001). For all MRAs, the overall sensitivity, specificity, and accuracy rates in detecting glenohumeral cartilage lesions, evaluated by consensus of two observers, were calculated as 69%, 97%, and 80%, respectively (Table 2).
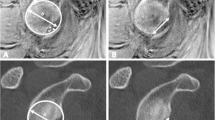
Right shoulder arthrograms of a 66-year-old man with revers Bankart lesion. Conventional MR arthrography (A), VIBE MR arthrography (B), and CT arthrography images show nearly normal chondrium (circle in A), more than 50% chondral defect (circle in B), and full-thicknes cartilage damage with bone involvement (circle in C) adjacent the posterior labral defect, respectively
Cartilage defects detected on CTA showed a statistically significant difference in relation with their localization (p < 0.001). Compared to others, more cartilage defects were detected in the zones 4 and 7 (p < 0.05). In addition, both examiners detected more cartilage defects in the zones 4 and 7 compared to other zones on volumetric MRA (p < 0.05). At zone 4, the rates of cartilage defects detected by CTA and T1 volumetric MRA were significantly different in relation with the type of labrum pathology (p = 0.023), and at this zone, more cartilage defects were observed in Bankart and SLAP lesions compared to other types of labrum pathologies (p < 0.05). On zone 7, the rates of cartilage defects detected on CTA were not significantly different in relation with the type of the labrum pathology (p = 0.836).
The incidence of cartilage defects on conventional MRA and T1 volumetric MRA was not significantly different between two examiners in relation with the labrum pathology. For Bankart and SLAP lesions which are the two most common labrum pathologies and other labrum pathologies, the cartilage defect detected by both examiners did not differ significantly when right and left sides were compared.
Discussion
The results of our study revealed that T1-weighted volumetric MRA showed better diagnostic performance compared to conventional MRA in detecting cartilage defects accompanying glenoid labrum pathologies with excellent inter-examiner agreement. To the best of our knowledge, there are no T1 volumetric MRA studies in the literature that investigated cartilage defects accompanying glenoid labrum pathologies; however, few T2 volumetric MRA studies had investigated cartilage defects in joints other than the shoulder.
When we compared our results with Dietrich et al.’s T2 volumetric MRA study [15] that evaluated shoulder cartilage and labrum lesions, we concluded that volumetric MRA has contributed to the diagnosis in both studies; however, our results are not in parallel when T1 volumetric MRA and T2 volumetric MRA results of two studies are compared. The main differences of our study from Dietrich et al.’s study are that we employed T1-weighted 3D VIBE scans; the magnetic power of our device is higher and we had a thinner slice thickness. Dietrich et al. compared 75 T2 volumetric MRA scans with conventional and 3D true fast imaging with steady state precession (FISP) scans and confirmed their findings with arthroscopy. The specificity and sensitivity values of two examiners for conventional and volumetric true FISP MRA scans were reported as 60–70% and 74–86% and 60–80% and 76–88% for all cartilage defects including superficial lesions, and as 33–33% and 97–98% and 100–100% and 89–92% for deep lesions, respectively. True FISP scan, which is a 3D T2-weighted GRE scan, was used in MRA in which contrast is obtained by preserving the cartilage signal intensity when the fluid has a high signal. In the VIBE scan employed in our study, T1-weighted images are obtained by eliminating transverse magnetization with a disruptive RF pulse, the fluids do not shine, and the contrast difference obtained in MRA is due to the T1 time-shortening effect of the gadolinium-containing contrast agent. Accordingly, spatial resolution and contrast power are higher, and the partial volume effect is less in our technique. In addition, the advantage of our technique is the absence of fluid shine around the joint, which can be misleading in T2-weighted scans. Despite this, although the sample sizes of two studies were similar (75 vs. 79), the reason for our relatively low sensitivity values in cartilage defects may be higher mean age of Dietrich et al.’s study population (35 (16–68) years in our study and 53 (21–83) years in Dietrich et al.’s study) and accordingly, higher probability of age-related degenerative cartilage defects independent of labrum pathologies. In addition, the kappa coefficient is 0.55 for T2 volumetric MRA in the detection of glenoid cartilage defects in Dietrich et al.’s study, which is smaller than the value in our study. This result shows that our study’s dependence on examiners is less.
One of the few T2 volumetric MRA studies in the literature was performed by Knuesel et al. [16]. They compared acetabular cartilages of 50 surgically correlated patients’ conventional MRIs with T2 volumetric MRA with 3D double-echo steady-state (DESS) scans. They stated that there was no difference between the two imaging modalities in terms of diagnostic performance; however. T2 volumetric MRA demonstrated cartilage defects more clearly.
Recently, Mars et al. [17] compared T1-weighted and T2-weighted volumetric scans for the knee joint cartilage in 40 healthy volunteers with a 1.5 T MRI. They stated that these scans provided imaging with a relatively short acquisition time, minimal artifacts, and a more accurate cartilage thickness measurement and signal-to-noise ratio. They demonstrated that multiple echo data ımage combination (MEDIC) and VIBE scans provided the best contrast, the True FISP and sampling perfection with application-optimized contrasts using different flip angle evolution (SPACE) scans displayed the highest signal-to-noise ratio (SNR), the DESS scan which is more sensitive to artifacts compared to other 3D sequences provided the lowest SNR, and the VIBE scan provided the best cartilage thickness measurement potential compared to the others. The 3D fat-suppression VIBE sequance we used in T1 volumetric MRA was also very successful in imaging of bony structures. Tian et al. [18] compared conventional CT and T1 volumetric MRA for glenoid bone lesions on 56 patients and reported that the sensitivity and specificity of volumetric MRA were almost similar to conventional CT in detecting glenoid bone pathologies. In our study, the sensitivity and specificity of volumetric MRA were 83–83%, and 87–93% in deep lesions, and false positivity was detected in 6 cases by researcher 1 and in 8 cases by researcher 2. This may be because the VIBE sequence morphologically exaggerates these defects or shows subchondral bone involvement better than CTA. When we later looked at some of the cases that were false positive on volumetric MRA, it was noted that these lesions with subchondral bone involvement could not be clearly seen on CTA. For a better understanding, arthroscopy and cadaver studies are needed in this field.
In a retrospective MRA study with arthroscopy correlation conducted by Guntern et al. [19], the sensitivity and specificity of glenoid cartilage lesions in patients with subacromial impingement syndrome were found to be 75% and 63%, respectively. For conventional MRA, moderate inter-reviewer agreement was 66%, and accuracy was 65–67%. The main differences of this study from our study are that a 1 T MRI device was used, and the section thickness was determined as 4 mm. In the study of Guntern et al., the number of false positives in detecting glenoid cartilage defects for conventional MRA was 15 for observer 1 and 16 for observer 2, and the specificity was reported as 66% and 63%, respectively. On the other hand, in our study, the specificity was found to be 100%, as there were no false positive cases for conventional MRA for both observers. While the number of superficial and deep cartilage lesions in our study was equal, 62.5% of the defects in Guntern et al.’s study were deep lesions and 37.5% were superficial lesions. In our study, the sensitivity and diagnostic accuracy of conventional MRA in detecting glenoid cartilage defects were found to be lower than in the study by Guntern et al. The reason for this may be that the superficial cartilage lesion rate in our study was higher than in Guntern et al.’s study and was below the conventional MRA imaging resolution. Additionally, in our study, while the sensitivity of superficial lesions was low, the sensitivity of conventional MRA increased as the lesions deepened, and the sensitivity of deep lesions was similar to the study by Guntern et al.
In our study, the sensitivity and specificity of the T1 volumetric MRA sequence on the demonstration of the glenoid cartilage defects were found as 58–65% and 90–97%, respectively. Compared to conventional MRA, sensitivity was higher for both examiners, while specificity was slightly lower. The reason for higher sensitivity may be the thinner slice thickness in the volumetric MRA (3 mm vs. 0.4 mm) compared to the conventional MRA, therefore higher spatial resolution, lower partial volume effect, and less motion artifacts due to higher SNR and relatively shorter duration of the VIBE scans. As a result of all these factors, the detectability of small cartilage defects is higher while the specificity is lower due to the higher number of false positives. We believe that the specificity of conventional MRA is relatively high due to the small number of cartilage defects detected, which limited the statistical analysis. However, when the diagnostic accuracy rates are compared, it can be considered that it is more useful in detecting cartilage defects since it is higher in T1 volumetric MRA. Moreover, the excellent inter-examiner agreement in T1 volumetric MRA in this study shows that the dependence on the examiner is low.
The glenoid labrum is not an isolated structure; it is continuous with the cartilage, with a fibrocartilaginous transition zone [20]. Therefore, depending on the development mechanism of trauma, the force that damages the labrum may also damage the cartilage. As a result, cartilage damage is likely to occur in labrum pathologies that most commonly occur due to traumatic shoulder dislocations. Primary or recurrent shoulder dislocations most commonly cause Bankart lesion, SLAP lesion, and Bankart variant lesions. Abnormal translation of the humeral head during dislocation causes excessive load and strain on the labrum and capsuloligamentous structures [21]. This excessive stretching can cause the labrum to tear and injure adjacent cartilages. For example, GLAD (glenolabral articular distruption) lesion, defined by Nevaiser, in which the anterior-inferior labrum is affected and adjacent cartilage damage is observed, can be considered [22]. In Bankart lesions affecting the anterior-inferior labrum, such as this lesion, damage may also occur to the cartilages adjacent to the labrum. In addition to the primary effect of trauma, it has been reported that biomechanical effects change as a result of glenohumeral joint instability caused by labrum pathologies as a secondary effect, and damage to the glenohumeral joint cartilage develops over time. The most common labrum injury in cases of anterior instability is the Bankart lesion, which causes avulsion of the anterior inferior labrum. In parallel, the two most common labrum pathologies in our study are Bankart and SLAP lesions. In a systematic review, Ruckstuhl et al. [23] reported the incidence of glenoid cartilage defects in unstable shoulders as 57%. In their cohort study, O’Brien et al. reported that 46% of the cases with anterior instability and labrum tear had cartilage damage on MRA [24]. In our study, we found cartilage defects in 60% of the cases with shoulder instability, in parallel with the study of Ruckstuhl et al. Patzer et al. reported that instability resulting from SLAP lesions is a factor in the development of glenoid cartilage lesions [2]. Additionally, anterior glenoid cartilage defects have been reported in the literature at rates of up to 64% in the setting of anterior instability [2, 24,25,26]. In our study, cartilage defects were found more frequently in zones 4 and 7 in CTA and volumetric MRA than in other localizations, and in cases with Bankart and SLAP lesions, cartilage defects were detected significantly more frequently in zone 4, which corresponds to the middle-lower part of the glenoid cavity. For this reason, we recommend that these areas be examined for cartilage defects accompanying frequently encountered labrum pathologies, especially Bankart and SLAP lesions. Knowing this situation allows these places to be examined more carefully in arthrographic reports, and therefore, more accurate treatment planning can be made.
Today, arthroscopy is accepted as the gold standard in the diagnosis of glenohumeral articular cartilage lesions, but arthroscopy is a relatively expensive and invasive diagnostic tool and cannot be applied to every patient before surgery for possible articular cartilage defects [27]. There are many studies in the literature showing that CT arthrography is more reliable than MR arthrography in evaluating hyaline cartilage in various joints [28,29,30]. In a histological study by Nakasa et al., x-ray absorption of the subchondral bone plate was found to correlate with the degree of cartilage degeneration, revealing that CT is an important tool in determining surgical treatment [31]. With the advances in CT detector technology, CT arthrographic examinations have been used with high sensitivity and specificity to evaluate preoperative and postoperative labrum tears in the shoulder joint and to reveal articular cartilage damage [32,33,34,35,36,37,38,39,40,41,42]. In fact, it has been accepted as the reference standard in articular cartilage damage and in distinguishing between normal variant and defect [38, 39, 41, 42]. Advances in CT technology have increased interest in CT arthrography, and recent studies suggest that flat-panel computed tomography (FPCT) arthrography allows precise evaluation of cartilage defects [40]. Pagliano et al. accepted multidetector CT (MDCT) arthrography as the reference standard in detecting cartilage defects in the ankle joint, as in our study, and demonstrated that FPCT was superior to MRA [40]. However, FPCT arthrography is a relatively new imaging modality with very limited in vivo studies, and further in vivo research is needed. The biggest disadvantage of CTA is that it involves radiation, and in shoulder CTA, the thyroid gland, lung parenchyma, or breast tissue may be exposed to x-ray [43]. Lead-free shields and tube current modulation are used to reduce radiation dose during CT imaging. In the study by Kim et al., in which the image quality of the extremities was compared with low-dose radiation (tube current 50% of the standard value) and standard dose radiation, the image quality of the low-dose radiation for the shoulder was within the acceptable range, although there was a decrease in image quality [44]. Because the scan area of shoulder CT may include radiosensitive organs, dose reduction protocols may be used in selective cases.
The first and most important limitation of our study is that our findings were not confirmed by arthroscopy, which is the gold standard examination in the diagnosis of glenoid cartilage defects. The second one is the relatively small number of patients included. Another limitation is the possibility that we could not demonstrate small cartilage defects in the area where the cartilage in the glenoid center is thin, which is called the “bare area,” which corresponds to the zone 5, due to the division of the glenoid cartilage into zones with a grid system. For this reason, larger cadaver and arthroscopic studies are needed.
In conclusion, preoperatively unrecognized and untreated glenoid cartilage defects accompanying labrum pathologies affect patient comfort adversely and carry the risk for future osteoarthritis. Our results indicated that T1-weighted 3D volumetric MRA detects glenoid cartilage defects with higher sensitivity, specificity, and excellent inter-examiner agreement and higher diagnostic accuracy compared to conventional MRA. Conventional MRA, which is preferred in shoulder disorders, can demonstrate rotator cuff tears and labro-ligamentous pathologies that cause shoulder instability; however, in cases with recurrent shoulder dislocation and suspected labrum pathology, T1 volumetric MRA should be preferred to determine glenoid cartilage defects with a high accuracy.
Abbreviations
- κ:
-
Unweighted kappa
- CTA:
-
Computed tomography arthrography
- DESS:
-
Double echo steady state
- GHJ:
-
Glenohumeral joint
- MRA:
-
Magnetic resonance arthrography
- US:
-
Ultrasonography
- VIBE:
-
Volumetric interpolated breath-hold examination
- 3D:
-
Three-dimensional
- TE:
-
Echo time
- TR:
-
Repetition time
- TSE:
-
Turbo spin echo
- PD:
-
Proton density
References
Patzer T, Habermeyer P, Hurschler C, Bobrowitsch E, Wellmann M, Kircher J. The influence of superior labrum anterior to posterior (SLAP) repair on restoring baseline glenohumeral translation and increased biceps loading after simulated SLAP tear and the effectiveness of SLAP repair after long head of biceps tenotomy. J Shoulder Elbow Surg. 2012;21:1580–7.
Patzer T, Lichtenberg S, Kircher J, Magosch P, Habermeyer P. Influence of SLAP lesions on chondral lesions of the glenohumeral joint. Knee Surg Sports Traumatol Arthrosc. 2010;18:982–7.
Romeo AA, Cole BJ, Mazzocca AD, Fox JA, Freeman KB, Joy E. Autologous chondrocyte repair of an articular defect in the humeral head. Arthroscopy. 2002;18:925–9.
Johnson DL, Warner JJ. Osteochondritis dissecans of the humeral head: treatment with a matched osteochondral allograft. J Shoulder Elbow Surg. 1997;6:160–3.
Perdikakis E, Karachalios T, Katonis P, Karantanas A. Comparison of MR-arthrography and MDCT-arthrography for detection of labral and articular cartilage hip pathology. Skeletal Radiol. 2011;40:1441–7.
Klaan B, Wuennemann F, Kintzelé L, Gersing AS. Weber MA [MR and CT arthrography in cartilage imaging : indications and implementation]. Radiologe. 2019;59:710–21.
Zheng ZZ, Shan H, Li X. Fat-suppressed 3D T1-weighted gradient-echo ımaging of the cartilage with a volumetric ınterpolated breath-hold examination. AJR Am J Roentgenol. 2010;194:W414–9.
Ogul H, Karaca L, Can CE, Pirimoglu B, Tuncer K, Topal M, et al. Anatomy, variants, and pathologies of the superior glenohumeral ligament: magnetic resonance imaging with three-dimensional volumetric interpolated breath-hold examination sequence and conventional magnetic resonance arthrography. Korean J Radiol. 2014;15:508–22.
Vandevenne JE, Vanhoenacker F, Mahachie John JM, Gelin G, Parizel PM. Fast MR arthrography using VIBE sequences to evaluate the rotator cuff. Skeletal Radiol. 2009;38:669–74.
Koh E, Walton ER, Watson P. VIBE MRI: an alternative to CT in the imaging of sports-related osseous pathology? Br J Radiol. 2018;91:20170815.
Ogul H, Kantarci M, Topal M, Karaca L, Tuncer K, Pirimoglu B, et al. Extra-articular contrast material leaks into locations unrelated to the injection path in shoulder MR arthrography. Eur Radiol. 2014;24:2606–13.
Ogul H, Taydas O, Sakci Z, Altinsoy HB, Kantarci M. Posterior shoulder labrocapsular structures in all aspects; 3D volumetric MR arthrography study. Br J Radiol. 2021;94:20201230.
Clavert P. Glenoid labrum pathology. Orthop Traumatol Surg Res. 2015;101:S19-24.
Kim HK. Bare spot: a normal variant on shoulder MR arthrography. Pediatr Radiol. 2009;39:1124.
Dietrich TJ, Zanetti M, Saupe N, Pfirrmann CWA, Fucentese SF, Hodler J. Articular cartilage and labral lesions of the glenohumeral joint: diagnostic performance of 3D water-excitation true FISP MR arthrography. Skeletal Radiol. 2010;39:473–80.
Knuesel PR, Pfirrmann CWA, Noetzli HP, Dora C, Zanetti M, Hodler J, et al. MR arthrography of the hip: diagnostic performance of a dedicated water-excitation 3D double-echo steady-state sequence to detect cartilage lesions. AJR Am J Roentgenol. 2004;183:1729–35.
Mars M, Tbini Z, Chelli-Bouaziz M, Ladeb F. Comparison of 3D MR imaging sequences in knee articular cartilage at 1.5 T. Biomedical Research. 2018;29.
Tian CY, Shang Y, Zheng ZZ. Glenoid bone lesions: comparison between 3D VIBE images in MR arthrography and nonarthrographic MSCT. J Magn Reson Imaging. 2012;36:231–6.
Guntern DV, Pfirrmann CW, Schmid MR, Zanetti M, Binkert CA, Schneeberger AG, et al. Articular cartilage lesions of the glenohumeral joint: diagnostic effectiveness of MR arthrography and prevalence in patients with subacromial impingement syndrome. Radiology. 2003;226(1):165–70.
Prodromos CC, Ferry JA, Schiller AL, Zarins B. Histological studies of the glenoid labrum from fetal life to old age. J Bone Joint Surg Am. 1990;72:1344–8.
Tupe RN, Tiwari V. Anteroinferior glenoid labrum lesion (Bankart lesion).: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
Neviaser TJ. The anterior labroligamentous periosteal sleeve avulsion lesion: a cause of anterior instability of the shoulder. Arthroscopy. 1993;9:17–21.
Ruckstuhl H, de Bruin ED, Stussi E, Vanwanseele B. Post-traumatic glenohumeral cartilage lesions: a systematic review. BMC Musculoskelet Disord. 2008;9:107.
O’Brien J, Grebenyuk J, Leith J, Forster BB. Frequency of glenoid chondral lesions on MR arthrography in patients with anterior shoulder instability. Eur J Radiol. 2012;81:3461–5.
Krych AJ, Sousa PL, King AH, Morgan JA, May JH, Dahm DL. The effect of cartilage ınjury after arthroscopic stabilization for shoulder ınstability. Orthopedics. 2015;38:e965–9.
Duchman KR, Hettrich CM, Glass NA, Westermann RW, Wolf BR, Baumgarten K, et al. The Incidence of glenohumeral bone and cartilage lesions at the time of anterior shoulder stabilization surgery: a comparison of patients undergoing primary and revision surgery. Am J Sports Med. 2018;46:2449–56.
Hayes ML, Collins MS, Morgan JA, Wenger DE, Dahm DL. Efficacy of diagnostic magnetic resonance imaging for articular cartilage lesions of the glenohumeral joint in patients with instability. Skeletal Radiol. 2010;39:1199–204.
Omoumi P, Rubini A, Dubuc JE, Vande Berg BC, Lecouvet FE. Diagnostic performance of CT-arthrography and 15T MR-arthrography for the assessment of glenohumeral joint cartilage: a comparative study with arthroscopic correlation. Eur Radiol. 2015;25:961–9.
Pirimoglu B, Ogul H, Polat G, Kantarci M, Levent A. The comparison of direct magnetic resonance arthrography with volumetric interpolated breath-hold examination sequence and multidetector computed tomography arthrography techniques in detection of talar osteochondral lesions. Acta Orthop Traumatol Turc. 2019;53:209–14.
Schmid MR, Pfirrmann CWA, Hodler J, Vienne P, Zanetti M. Cartilage lesions in the ankle joint: comparison of MR arthrography and CT arthrography. Skeletal Radiol. 2003;32:259–65.
Nakasa T, Ikuta Y, Yoshikawa M, Sawa M, Tsuyuguchi Y, Adachi N. Added value of preoperative computed tomography for determining cartilage degeneration in patients with osteochondral lesions of the talar dome. Am J Sports Med. 2018;46:208–16.
Choi JY, Kim SH, Yoo HJ, Shin SH, Oh JH, Baek GH, et al. Superior labral anterior-to-posterior lesions: comparison of external rotation and active supination CT arthrography with neutral CT arthrography. Radiology. 2012;263:199–205.
Kim YJ, Choi JA, Oh JH, Hwang SI, Hong SH, Kang HS. Superior labral anteroposterior tears: accuracy and interobserver reliability of multidetector CT arthrography for diagnosis. Radiology. 2011;260:207–15.
De Filippo M, Araoz PA, Pogliacomi F, Castagna A, Petriccioli D, Sverzellati N, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252:781–8.
De Filippo M, Bertellini A, Sverzellati N, Pogliacomi F, Costantino C, Vitale M, et al. Multidetector computed tomography arthrography of the shoulder: diagnostic accuracy and indications. Acta Radiol. 2008;49:540–9.
Zappia M, Negri G, Grassi S, Pecoraro C, Rotondo A. The CT-arthrography in the antero-inferior glenoid labral lesion: pictorial presentation and diagnostic value. Int J Shoulder Surg. 2008;2:7–12.
Lecouvet FE, Dorzée B, Dubuc JE, Vande Berg BC, Jamart J, Malghem J. Cartilage lesions of the glenohumeral joint: diagnostic effectiveness of multidetector spiral CT arthrography and comparison with arthroscopy. Eur Radiol. 2007;17:1763–71.
Lecouvet FE, Simoni P, Koutaïssoff S, Vande Berg BC, Malghem J, Dubuc JE. Multidetector spiral CT arthrography of the shoulder. Clinical applications and limits, with MR arthrography and arthroscopic correlations. Eur J Radiol. 2008;68:120–36.
Rhee RB, Chan KK, Lieu JG, Kim BS, Steinbach LS. MR and CT arthrography of the shoulder. Semin Musculoskelet Radiol. 2012;16:3–14.
Pagliano S, Chemouni D, Guggenberger R, Pauly V, Guenoun D, Champsaur P, et al. Flat-panel CT arthrography for cartilage defect detection in the ankle joint: first results in vivo. Skeletal Radiol. 2020;49:1259–65.
Ozel MA, Ogul H, Koksal A, Kose M, Tuncer K, Eren S, et al. Detection of the glenoid bare spot by non-arthrographic MR imaging, conventional MR arthrography, and 3D high-resolution T1-weighted VIBE MR arthrography: comparison with CT arthrography. Eur Radiol. 2023;33:3276–85.
Jarraya M, Roemer FW, Gale HI, Landreau P, D’Hooghe P, Guermazi A. MR-arthrography and CT-arthrography in sports-related glenolabral injuries: a matched descriptive illustration. Insights Imaging. 2016;7:167–77.
Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91:1882–9.
Kim JN, Park HJ, Kim MS, Kook SH, Ham SY, Kim E, et al. Radiation dose reduction in extremity multi-detector CT: a comparison of image quality with a standard dose protocol. Eur J Radiol. 2021;135:109405.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the Duzce University Faculty of Medicine, the Health Research Ethics Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Guarantor
The scientific guarantor of this publication is Prof. Dr. Hayri Ogul.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Methodology
• Prospective.
• Cross-sectional study.
• Performed at one institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gokce, A., Guclu, D., Unlu, E.N. et al. Comparison of conventional MR arthrography and 3D volumetric MR arthrography in detection of cartilage defects accompanying glenoid labrum pathologies. Skeletal Radiol 53, 1081–1090 (2024). https://doi.org/10.1007/s00256-023-04536-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04536-9