Abstract
Objectives
To compare MRI features of sporadic and neurofibromatosis syndrome–related localized schwannomas and neurofibromas.
Methods
In this retrospective study, our pathology database was searched for “neurofibroma” or “schwannoma” from 2014 to 2019. Exclusion criteria were lack of available MRI and intradural or plexiform tumors. Qualitative and quantitative anatomic (location, size, relationship to nerve, signal, muscle denervation) and functional (arterial enhancement, apparent diffusion-weighted coefficient) MRI features of sporadic and syndrome-related tumors were compared. Statistical significance was assumed for p < 0.05.
Results
A total of 80 patients with 64 schwannomas (sporadic: 42 (65.6%) v. syndrome-related: 22 (34.4%)) and 19 neurofibromas (sporadic: 7 (36.8%) v. syndrome-related: 12 (41.7%)) were included. Only signal heterogeneity (T2W p=0.001, post-contrast p=0.03) and a diffused-weighted imaging target sign (p=0.04) were more frequent with schwannomas than neurofibromas. Sporadic schwannomas were similar in size to syndrome-related schwannomas (2.9±1.2cm vs. 3.7±3.2 cm, p = 0.6), but with greater heterogeneity (T2W p = 0.02, post-contrast p = 0.01). Sporadic neurofibromas were larger (4.6±1.5cm vs. 3.4±2.4 cm, p = 0.03) than syndrome-related neurofibromas, also with greater heterogeneity (T2W p=0.03, post-contrast p=0.04). Additional tumors along an affected nerve were only observed with syndrome-related tumors). There was no difference in apparent diffusion coefficient values or presence of early perfusion between sporadic and syndrome-related tumors (p > 0.05).
Conclusions
Although syndrome-related and sporadic schwannomas and neurofibromas overlap in their anatomic, diffusion and perfusion features, signal heterogeneity and presence of multiple lesions along a nerve are differentiating characteristics of syndrome-related tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peripheral neural sheath tumors (PNSTs) are predominantly benign tumors accounting for approximately 10–12% of all benign soft tissue tumors and occur most commonly in the form of schwannomas and neurofibromas [1]. They may occur sporadically or as a part of neurocutaneous genetic syndromes such as neurofibromatosis type 1 (NF1) and schwannomatosis (SWN), with schwannomas occuring sporadically in around 95% of cases and neurofibromas occurring in the absence of NF1 in around 60–90% of cases [1,2,3]. While benign PNSTs can be localized/solitary, diffuse, or plexiform in nature, the localized subtype occurrs most commonly for both sporadic and syndrome-related tumors [1, 2, 4].
Distinguishing localized schwannomas from neurofibromas is of clinical value, as surgical resection with nerve preservation maybe be substantially more difficult for a neurofibroma than for a schwannoma. However, differentiation based on imaging is difficult, and prior magnetic resonance imaging (MRI)-based investigations suggest that the accuracy of expert performance when differentiating between localized neurofibromas and schwannomas using anatomic MRI characteristics alone is consistently below 80% [5]. Although syndrome-related and sporadic schwannomas and neurofibromas also exhibit overlapping histological characteristics, the presence of “whorl” formations, multifocal nerve involvement, and, whenever immunohistochemical staining is procurable, a mosaic pattern or patchy loss of SMARCB1/INI1 all favor SR PNSTs [6,7,8]. By imaging, PNSTs, including localized, plexiform, and diffuse plaque-like lesions, can be either sporadic or associated with peripheral nerve tumor syndromes [1, 9]. As such, there are two clinical conondrums that arise when faced with a new suspected localized benign PNST: the characterization of the lesion as a schwannoma or a neurofibroma, and the prediction of whether the lesion is associated with a neurocutaneous syndrome.
While the histology of both syndrome-related (SR) and sporadic PNSTs can be identical, the long-term management of a person with a SR PNST in the setting of NF1 and SWN differs from the management of someone with non-syndromic PNSTs, and the surgical treatment of a symptomatic localized PNST may vary depending on whether the lesion is a schwannoma or neurofibroma [1, 8, 10,11,12,13]. We hypothesized that the analysis of conventional and advanced MRI (diffusion-weighted imaging and dynamic contrast–enhanced sequences) features could provide insight into distinguishing a SR PNST from a sporadic PNST and guide the clinical management of a newly detected PNST. Therefore, the purpose of our study was to systematically evaluate the qualitative and quantitative anatomic and functional MRI features of localized benign PNSTs (schwannomas and neurofibromas) and compare syndrome-related (associated with NF1 and SWN) tumors to sporadic tumors. To our knowledge, no direct comparisons of MRI features between the two entities have been published to date. If significant differences on imaging existed, radiologists could help direct clinicians regarding an important distinction: a peripheral tumor which is associated with an underlying syndrome requires a more comprehensive clinical and imaging evaluation, whereas an isolated peripheral tumor can be managed with fewer resources.
Materials and methods
Subject population
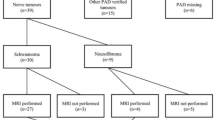
This retrospective study performed at a single tertiary referral center was approved by Institutional Review Board and was compliant with the Health Insurance Portability and Accountability Act (HIPPA). The requirement for informed consent was waived. Subjects were selected consecutively from a retrospective review of our pathology database for “neurofibroma or schwannoma“ between September 2014 and September 2019. Inclusion criteria were any person with a pathologically proven localized neurofibroma or schwannoma within a peripheral nerve, regardless of age. Exclusion criteria were patients with no available MRI studies, exclusively intradural tumors, and localized cutaneous, diffuse cutaneous, and plexiform PNSTs on histology. Localized PNSTs were defined as well-circumscribed lesions with a true capsule, while diffuse PNSTs were defined as unencapsulated, poorly demarcated plaque-like thickenings of the skin and underlying subcutis that frequently contain pseudomeissnerian corpuscles (spherical arrangements of S100-positive cell processes) [9]. Plexiform PNSTs were defined as multinodular enlargements of an involved peripheral nerve with a “bag of worms” or “string of onions” appearance [9]. All histologic diagnoses were rendered by a neuropathologist with more than 13 years of histology experience on samples obtained from surgical or biopsy specimens at our institution. The flow chart of the study design is shown in Fig. 1, and imaging of different localized PNSTs is shown in Figs. 2, 3, 4, 5, and 6.
33-year-old male with schwannomatosis. Axial T2-FS, T1, DWI using b-value 50 mm/s and ADC mapping (A, B, C, D) through the left popliteal fossa show a tibial nerve schwannoma (solid arrow). Axial T2-FS, T1, DWI using b-value 50 mm/s and ADC mapping (E, F, G, H) through the soleal sling show an additional tibial nerve schwannoma (dotted arrow). Note faint target sign (central hypointensity and peripheral hyperintensity) associated with the distal schwannoma visible on axial T2FS (E) and ADC mapping (H). Presence of more than one lesion with along a peripheral nerve on imaging is indicative of an underlying PNST syndrome
33-year-old male with schwannomatosis (same patient as in Fig. 2). Coronal T2-FS (A), sagittal T1 (B), coronal (C) and axial T1 post-contrast FS through the popliteal fossa (D), and soleal sling (E) redemonstrate two schwannomas along the proximal (solid arrow) and slightly distal (dotted arrow) tibial nerve. Note the faint target sign (central hypointensity and peripheral hyperintensity) associated with the distal schwannoma visible on coronal T2FS (A) and axial T1 post-contrast FS (E). Presence of more than one lesion with along a peripheral nerve on imaging is indicative of an underlying PNST syndrome
55-year-old female with sporadic schwannoma. Axial T1 (A), T2-FS (B), DWI using b-value of 50 (C), and ADC map (D) shows a medial right upper arm intermuscular soft tissue mass (arrow) contiguous with the median nerve. DWI (C) and ADC mapping (D) shows absence of restricted diffusion. Note the tail sign suggesting neurogenic origin on the sagittal T1-weighted image (E) and coronal T2-FS image (F). There is late arterial enhancement on dynamic contrast–enhanced MR angiogram (G). Note heterogeneous intralesional enhancement on static coronoal post-contrast T1-FS (H). A target sign is visible on fluid-sensitive sequence (F). The MRI features are compatible with a benign peripheral nerve sheath tumor with greater heterogeneity compatible with schwannoma
31-year-old male with a syndrome-related neurofibroma in the setting of neurofibromtosis type 1. Axial T2-FS (A), T1 (B), DWI using b-value of 50 (C), and ADC mapping (D) through the right tarsal tunnel show a focal soft tissue mass without restricted diffusion contiguous with the tibial nerve compatible with neurofibroma. Sagittal T2-FS (E), intermediate weighted (F), and post-contrast T1-FS (G) shows a focal mass at the level of the tarsal tunnel (solid arrow) and an additional mass in the distal tibial nerve (dotted arrow). Presence of more than one lesion with along a peripheral nerve on imaging is indicative of an underlying PNST syndrome
62-year-old female with a sporadic neurofibroma. Axial T2-FS (A), T1 (B), DWI using b-value of 50 (C), and ADC mapping (D) through the left thigh shows a large soft tissue mass contiguous with the sciatic nerve (solid arrow). Coronal T2-FS (E), sagittal T1 (F), sagittal dynamic contrast–enhanced MR angiogram (G), and sagittal (H) and coronal T1-FS post-contrast images show absence of target sign and heterogeneous signal on fluid-sensitive sequence as well as heterogeneous enhancement on post-contrast images. No other lesion was detected in either thigh (right side is not shown)
Clinical analysis
One experienced observer reviewed each patient’s electronic medical records for demographic information (age and sex), preoperative symptoms, surgical indication, and presence or absence of genetic syndromes. A multidisciplinary team from our institution diagnosed NF1, neurofibromatosis type 2 (NF2), and SWN following the clinical criteria and molecular genetic testing; NF2 and SWN were considered separate diagnoses in accordance with the established guidelines at the time of diagnosis and prior to the updated diagnostic criteria and nomenclature recommendations where the term NF2 was retired [7, 8, 14, 15].
MRI acquisition
MRI studies were performed on 1.5 Tesla (T) (32/83, 38.6%) or 3T (51/83, 61.4%) systems using different multichannel coils, matrix, and field of view (FOV) depending on the anatomic site of the tumors. Of the 83 MRI examinations, 43 (51.8%) were from our institution and 40 (48.2%) were outside studies reviewed at our institution. The FOV varied depending on the tumor location, with 10 cm in the transverse and longitudinal plane for smaller regions like the hand and 40 cm in the transverse and longitudinal plane for larger regions like the thigh. All patients in the study group underwent multiplanar spin-echo T1-weighted images (TR/TE 450–650/20–28), and fluid-sensitive sequences [(fat-suppressed T2-weighted (TR/TE 3000–4800/60–70 or short tau inversion recovery (STIR, TR/TE/TI 2500/60/160)]. Seventy of 83 MRIs (84.3%) included 2D or 3D T1-weighted gradient-echo or spin-echo sequences before and following the administration of an intravenous gadolinium-based contrast agent, and 29 of 83 (34.9%) scans included a time-resolved perfusion sequence (TR/TE, 2.69/1.01ms). Diffusion-weighted imaging with ADC mapping (TR/TE, 760/80 ms; b-values=50,400, and 800 ms/mm2) was performed in 49 of 83 (59%) MR scans.
Image analysis
Two experienced musculoskeletal radiologists (with 15 and 8 years of clinical experience) with no knowledge of the diagnosis (syndromic vs. sporadic) independently reviewed all 83 MRI studies on a picture archiving and communication system (PACS) workstations (Carestream Health Inc., Canada), and discrepancies were resolved by consensus. At the time of analysis, the observers had no knowledge of the patient's electronic medical records, including histopathological results, clinical history, and previous MRI reports.
The observers recorded the following features by MRI: location, largest tumor diameter in any plane of imaging, presence or absence of other tumors in the same nerve, relationship with the nerve (central or excentric) [16, 17], shape (ovoid or fusiform), signal intensity (hypointense, isointense, and hyperintense relative to skeletal muscle), signal heterogeneity (homogeneous [the entire lesion displaying one uniform intensity level], < 25% heterogeneity [<25% of the lesion displaying 2 or more intensity levels and the remainder showing one uniform intensity level], 25–75% heterogeneity [25–75% of the lesion displaying 2 or more intensity levels and the remainder showing one uniform intensity level], >75% heterogeneity [>75% of the lesion displaying 2 or more intensity levels and the small remainder showing one uniform intensity level]), definition of margin (poorly defined [>75% of margin not clear], mixed definition [10–25% of margin not clear], and well-defined [>90% or margin clear]), on T1-weighted and fluid-sensitive sequences. On post-contrast sequences, delayed contrast enhancement heterogeneity (< 25%, 25–50%, 50–75%, and >75%), and the presence or absence of early arterial enhancement on time-resolved perfusion sequences were recorded.
The presence or absence of imaging features previously described as characteristic of PNSTs were recorded, including the target sign on T2W, ADC mapping, DWI (b-value=50, 400, or 800s/mm2), and post-contrast T1W sequence as previously described [18], and the mean and minimum ADC values were recorded. Similarly, the presence or absence of the following imaging features was recorded: fascicular sign, cystic tumor changes, split fat sign, internal low-signal-intensity septations, perilesional edema-like zone, periosteal/cortex/marrow/joint extension, bone remodeling, and muscle denervation [18,19,20,21,22,23].
Statistical analysis
All statistical analyses were performed using Matlab software (R2019a, The Mathworks, Natick, MA, USA). Descriptive statistics were reported. Imaging characteristics of sporadic and SR PNSTs were compared using the chi-squared test, Fisher’s exact test, and the U test of Mann–Whitney for categorized and continuous variables, respectively. Statistical significance was assumed for p < 0.05.
Results
A total of 80 patients (83 MRI and 83 tumors) were included in the study and had 64 schwannomas (64/83, 77.1%) and 19 neurofibromas (19/83, 22.9%). Fifteen neurofibromas and 58 schwannomas were surgically excised, while 4 neurofibromas and 6 schwannomas were biopsied. Twenty-two schwannomas (22/64, 34.3%) and 12 neurofibromas (12/19, 63.1%) were part of a genetic syndrome, whereas the remainder were sporadic. Out of 22 SR schwannomas, 19 were found in patients with SWN and 3 were found in patients with NF2. Out of 12 SR neurofibromas, 9 were found in patients with NF1 and 3 were found in patients with NF2. Table 1 lists subject demographics. Clinical features and surgical indications in patients with schwannomas and neurofibromas (sporadic vs. SR tumors) are summarized in Table 2. Table 3 summarizes anatomic MRI features and Table 4 summarizes functional MRI features of schwannomas and neurofibromas (sporadic vs. SR tumors).
Schwannomas vs. neurofibromas
Demographic information
Both schwannomas and neurofibromas were more common in females, 60.9% (39/64) and 68.4% (13/19), respectively. The most common symptom was pain in isolation or in conjunction with an additional symptom, and these were the most common indications for surgery for both types of tumors. Pain and weakness were more often observed with schwannomas than neurofibromas, but the difference did not reach statistical significance
Anatomic MRI features
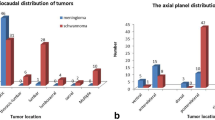
Schwannomas tended to be larger than neurofibromas (schwannomas vs. neurofibromas: 3.0 ± 2.1, range: 0.5–14 cm; vs. 4.2 ± 2.3, range: 0.8–10.1 cm; p=0.02), although similarly located in the body. Schwannomas were more frequently reported as arising central to the parent nerve compared to neurofibromas (central: 46.9% vs. eccentric:15.8%), noting that the relationship to the parent nerve was indeterminate in many neurofibromas (47.4%). Schwannomas exhibited a more heterogeneous appearance on all sequences compared with neurofibromas.
Neurogenic features and anatomic extent
The target sign was more frequently observed in schwannomas (45.3%, 29/64) than neurofibromas (31.6%, 6/19) on T2W images and persisted on DWI and ADC mapping as well as post-contrast sequences (p< 0.05 for DWI using b-value=50s/mm2 and b-value=800s/mm2). There was no difference in other neurogenic features or anatomic extent between the two groups of tumors.
Functional MRI sequences (apparent diffusion coefficient (ADC) and DCE)
Early arterial enhancement was observed in 4.5% of schwannomas and 14.3% of neurofibromas. There was no difference in the average or minimum ADC values between schwannomas and neurofibromas.
Sporadic vs. SR schwannomas
Demographic information
Of 64 schwannomas, 42 (65.6%) were sporadic and 22 (34.4%) were SR tumors. Sporadic schwannomas were more frequently present in women than men, compared with SR schwannomas (p = 0.01) (Table 1). All sporadic schwannomas and nearly all (95.5%) SR schwannomas were symptomatic, most commonly with pain, followed by weakness and a palpable mass.
Anatomic MRI characteristics
Both SR and sporadic schwannomas were most commonly located in the extremities, although SR schwannomas were more frequently located in the lower extremities, while sporadic schwannomas were more evenly distributed throughout the body. There was no difference (p = 0.6) in the size of sporadic (2.9 ± 1.2, range: 1.1–6.5 cm) and SR (3.7 ± 3.2; range: 0.8–14 cm) schwannomas. Approximately one-third of patients with SR tumors had concurrent masses involving the same nerve, whereas none of the patients with sporadic tumors had another lesion affecting the same nerve in the imaged field of view (p=0.0001). On fluid-sensitive images, sporadic tumors exhibited greater signal heterogeneity on T2W and post-contrast imaging compared with SR schwannomas which were more homogeneous.
Neurogenic features and anatomic extent
On T2-weighted images, the target sign (TS) was present in both sporadic (45.2%, 19/42) and SR (45.5%, 10/22) schwannomas. However, TS was more common in SR schwannomas on ADC mapping compared with sporadic schwannomas (sporadic: 30.4%, 7/23 vs. SR: 42.8%, 6/14, p<0.05), but a higher proportion of sporadic schwannomas exhibited TS on high b-value DWI (sporadic: 34.7%, 8/23 vs. SR: 14.3%, 2/14, p<0.05) and post-contrast T1W imaging (sporadic: 19.4%, 7/36 vs. SR: 5.9%, 1/17, p<0.05) compared with SR schwannomas. Otherwise, there was no difference in other neurogenic features.
Functional MRI sequences (apparent diffusion coefficient [ADC] and DCE)
Early arterial enhancement was observed in a small subset (5.5%) of sporadic schwannomas (p = 0.03). There was no difference in average and minimum ADC values (p>0.05) between the two groups of tumors, with minimum ADC values of 1.6 × 10−3 mm2/s (range: 1.3–1.9) and 0.9 × 10−3 mm2/s (range: 0.6–1.5) for sporadic and SR schwannomas, respectively.
Sporadic vs. SR neurofibromas
Demographic information
There were 19 neurofibromas in this study (7 (36.8%) sporadic and 12 (63.2%) SR). There was no significant difference in the distribution of tumors by age and gender between the two groups (p> 0.05). There was no significant difference concerning the main symptoms present in patients with sporadic neurofibroma versus SR neurofibroma (p = 0.1). In all symptomatic patients, pain was present as an isolated symptom in the majority of sporadic and SR neurofibromas. Pain was by far the main surgical indication, especially in patients with SR neurofibromas (p < 0.05). No patients with SR neurofibroma and only 8.3% of patients with sporadic neurofibroma underwent surgery or biopsy based on suspected malignancy detected on preoperative imaging (Table 3.)
Anatomic MRI features
There was no significant difference in tumor location between sporadic and SR neurofibromas (p > 0.05). Sporadic neurofibromas (4.6 ± 1.5, range: 3.9–7.4 cm) tended to be larger than SR neurofibromas (3.4 ± 2.4, range: 0.8–10.1 cm) (p = 0.03). Sporadic neurofibromas tended to arise eccentrically in the nerve (57.1%), whereas SR tumors tended to arise both centrally and eccentrically (25%), with no significant difference between the two groups (p = 0.2). The relationship between tumors and affected nerves could not be determined in 42.8% of sporadic and 50% of SR neurofibromas. Five of the 12 SR tumors (41.7%) and none of the sporadic neurofibromas had other masses on the affected nerve.
Neurogenic features and anatomic extent
There was no significant difference (p > 0.05) between sporadic and SR tumors regarding the presence of the target signal (TS) on T2W, ADC mapping, and DWI. There was no significant difference (p > 0.05) between the two groups of tumors concerning the presence or absence of the neurogenic features, anatomic extent and signs of muscle denervation. Of note, skeletal muscle denervation signs were not identified in any of the reviewed MRI.
Functional MRI sequences (apparent diffusion coefficient (ADC) and DCE)
Early arterial enhancement was observed in 20% (1/5) of sporadic neurofibromas and none (0/2) of the SR tumors. There was no significant difference in the average and minimum ADC values (p > 0.05) between the two groups of tumors, with minimum ADC values of 1.1 × 10−3 mm2/s (range: 0.4–2.3) and 1.2 × 10−3 mm2/s (range: 0.3–1.7) for sporadic and SR neurofibromas, respectively.
Discussion
Benign PNSTs have traditionally been challenging to differentiate by imaging. Our study confirms that schwannomas and neurofibromas overlap in their clinical and MRI presentations, including in their anatomic and functional characteristics. However, tumor heterogeneity may be a differenentiating feature, and perhaps the most useful sign is the presence of multiple lesions along a nerve that predicts an underlying syndrome. This finding is expected, as the occurrence of multiple PNSTs is seldom sporadic [9].
In our case series of histologically proven PNSTs, the majority of lesions were schwannomas, in keeping with data from a study by Zipfel et al. where 90% of sporadic PNSTs were schwannomas and 10% were neurofibromas [19]. This is expected, as schwannomas constitute the most common type of PNSTs in adults [20]. Average sizes, shapes, and appearance at the margins of schwannomas and neurofibromas were similar and less than 5 cm; however, schwannomas were more heterogeneous. One possible explanation is that this observational data is derived from histologically proven PNSTs which may have been more diagnostically challenging on imaging [21]. In addition, almost half of both schwannomas and neurofibromas displayed the target sign appearance on T2W, but schwannomas were more likely to show a target sign appearance on DWI and post-contrast T1W sequences, (a useful tool for identifying a soft tissue tumor as neurogenic in origin, although it was not a main differentiator between schwannomas and neurofibromas). Determination of eccentric or central relationship with the parent nerve was challenging and an unreliable differentiating parameter in our cases series. Often, the relationship could not be discerned, potentially due to the small size of the parent nerve, or, alternatively, because schwannomas arose from a central fascicle simulating a central relationship rather than the expected eccentric relationship previously described with schwannomas [1].
In our population, schwannomas were more commonly sporadic (65.6%) and neurofibromas were more commonly syndrome-related (63.2%). This population breakdown differs from one study of 197 PNSTs by Kim et al. where sporadic neurofibromas were found to be more common (59.5%) and another study by Zipfel et al. where 48.2% of patients with PNSTs were found to have sporadic tumors [2, 19]. The differences in our data and published reports may be due to a number of reasons: we only included PNSTs referred for surgery or biopsy, suggesting that preoperative imaging was adequate at differentiating benign from malignant lesions, and the main indication for biopsy and/or operative management was pain, rendering most of our lesions benign symptomatic PNSTs. A higher number of SR neurofibromas could thus indicate a lower number of sporadic neurofibromas that were symptomatic. Moreover, our institution is a tertiary referral center for cases of systemic neurofibromatoses with experience in non-operative management comprised of observation and symptom management, likely resulting in a smaller subset of patients with sporadic neurofibromas.
In our series, most patients with sporadic and SR tumors were symptomatic, with pain being the most common symptom. Weakness on physical examination was infrequent in patients with schwannomas and neurofibromas, a finding in agreement with a study by Ogose et al. describing a series of 99 benign PNSTs where only 6 lesions were accompanied by preoperative motor weakness as a presenting symptom [22]. Pain was also the primary surgical indication for most neurofibromas and schwannomas, with no difference between sporadic and SR tumors, while surgical indication based solely on suspected malignancy on MRI or PET-CT was rare for all tumors. Similarly, Zipfel et al. assessed 144 sporadic peripheral schwannomas in adults and reported that pain was the main reason for surgery in 66% of cases, while suspected malignancy was the indication for only 1.4% of the interventions [19]. As such, both our data and the existing literature suggest that qualitative and quantitative MRI features can reliably distinguish benign from malignant PNSTs with high accuracy [24, 25].
There was a significant difference in signal heterogeneity between sporadic and SR tumors in our series, with most sporadic tumors showing more heterogeneity than SR tumors. This finding contradicts previous studies that have shown that SWN-related schwannomas tended to be more heterogeneous than sporadic schwannomas due to intralesional myxoid composition that results in T2-hyperintensity [8]. The difference in T2 signal characteristics between sporadic and syndrome-related PNSTs may be due to our inclusion criteria (requiring histological proof) rather than true differences in all PNSTs in this patient population as a whole. As we did not include all patients with systemic neurofibromatoses, the qualitiative MRI characteristics may not be reflective of all PNSTs in patients with PNST syndromes but rather of a smaller subset of patients who require biopsy or surgery for symptomatic disease. Sporadic tumors could also have had more time to mature prior to presentation, rendering them more heterogenous on imaging. It is important to note that signal heterogeneity was qualitatively evaluated using visual assessment, and further research with quantitative assessment using textural mapping and analysis could potentially provide insight into whether statistically significant numerical differences exist between the two subgroups.
With respect to quantitative MRI characteristics, benign PNSTs tend to have a minimum ADC value greater than 1.0 × 10−3 mm2/s, although the existing literature suggests that some schwannomas with higher cellularity may also demonstrate values lower than 1.0 × 10−3 mm2/s [8, 23]. Previous studies have reported variability in minimum ADC values with a wide range of 0.3–2.2 ×10−3 mm2/s in PNSTs in patients with schwannomatosis and a minimum ADC range of 0.8–2.7 × 10−3 mm2/s in schwannomas related to NF2 [8, 26]. Similarly, the majority of the tumors in our series exhibited a minimum ADC value > 1.0 × 10−3 mm2/s, with a rare schwannoma exhibiting restricted diffusion. In addition to that, there was no significant difference in the mean and minimum ADC value between sporadic and SR lesions. The average of ADC values (mean and minimum values) was similar between sporadic and SR schwannomas, while the average ADC values (mean and minimum) of SR neurofibromas was lower than the average values of sporadic neurofibromas, probably due to the presence of tumors with greater cellularity.
The “target sign” (TS) evaluated by conventional anatomic sequences (fluid-sensitive and contrast-enhanced) and DWI/ADC mapping technique is an indicator of a benign SR related or isolated PNSTs [18, 27,28,29,30]. Similar to prior investigations, our results showed that, for pathologically proven benign PNSTs, TS was more often present in schwannomas than neurofibromas on anatomic and functional sequences [18]. Although a statistical difference was not shown in our study, none of the sporadic neurofibromas exhibited a target sign on conventional or functional techniques, and SR neurofibromas tended not to show the TS except on T2W images. Again, histological proof as an inclusion criteria in our case series likely accounts for the relative paucity of the TS in neurofibromas in our population. The presence of TS and lack of restricted diffusion on DWI/ADC have been used as markers for benignity in our clinical practice, potentially limiting unnecessary biopsies of asymptomatic benign peripheral nerve tumors.
Lastly, signs of muscle denervation (muscle edema-like signal change, fatty infiltration, or atrophy) along the surrounding or distally innervated skeletal musculature were present in a small subset of the schwannomas and in none of the neurofibromas. Factors that could partly explain the low frequency of muscle denervation in our study is that muscle changes were beyond the scan field of view in localized MRI. However, Stull et al. similarly reported muscle atrophy with striated increased fat content or decreased size in 25% of schwannomas and 14.3% neurofibromas [31]. Considering only intramuscular PNSTs, Lee et al. also reported that skeletal muscle denervation was observed in 33% of schwannomas and 100% of ancient schwannomas only; no changes were observed with neurofibromas [32]. It is unclear whether the presence or absence of muscle denervation can be a reliable differentiator of schwannomas and neurofibromas based on our data.
This study has limitations. First, our study has a retrospective design with a relatively small number of patients, and the presence of selection bias was unavoidable. Second, some MRI examinations were performed at different institutions using different MR scanners, imaging parameters, and radiofrequency coils. Although there was heterogeneity in the MRI protocols, images were of diagnostics quality and, as such, suitable for analysis. Moreover, the noted MRI protocol variations do not significantly alter or bias most anatomic and neurogenic imaging features outlined and assessed in this study. Of 83 MRI examinations, 43 (51.8%) were from our institution and 40 (48.2%) were outside studies reviewed at our institution. Third, we only included cases that were pathologically confirmed and therefore frequently symptomatic, potentially limiting the generalizability to asymptomatic, incidentally detected lesions. Fourth, post-contrast T1W sequences and functional MRI techniques (DWI/ADC mapping and DCE) were not available in all subjects; thus, the results may be under-representative or incomprehensive. Finally, some qualitative features, such as signal heterogeneity, do not follow standardized assessment methods and can be prone to reader-specific subjectivity. However, such assessments can still shed light on potential differences in imaging features and help highlight areas that could warrant further investigation with quantitative assessments.
In conclusion, for symptomatic benign PNSTs referred for surgery, signal heterogeneity, the presence of a DWI target sign and observation of muscle denervation may be more common with schwannomas than with neurofibromas. Qualitative and quantitative anatomic and functional MRI features of syndrome-related and sporadic PNSTs overlap, although qualitative visual assessment of tumor homogeneity and the presence of more than one tumor along a nerve are potentially predictive of an underlying syndrome, and further textural analysis of signal heterogeneity could possibly help quantify any existing differences. While differentiating neurofibromas from schwannomas and sporadic from SR lesions is currently not possible on MRI alone, our study sheds light on subtle differences that can exist between the two, and further prospective studies with larger and more representative samples are necessary to confirm our findings and potentially reveal more differences.
Data Availability
The data that support the findings of this study are available from the corresponding author, P.D., upon reasonable request.
References
Murphey MD, Smith WS, Smith SE, Kransdorf MJ, Temple HT. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1253–80.
Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246–55.
Neurofibroma - an overview | ScienceDirect Topics. [cited 2023 Sep 4]. Available from: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/neurofibroma. Accessed 4 Sept 2023.
Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295–319.
Zhang M, Tong E, Wong S, Hamrick F, Mohammadzadeh M, Rao V, et al. Machine learning approach to differentiation of peripheral schwannomas and neurofibromas: a multi-center study. Neuro Oncol. 2022;24:601–9.
Caltabiano R, Magro G, Polizzi A, Praticò AD, Ortensi A, D’Orazi V, et al. A mosaic pattern of INI1/SMARCB1 protein expression distinguishes Schwannomatosis and NF2-associated peripheral schwannomas from solitary peripheral schwannomas and NF2-associated vestibular schwannomas. Childs Nerv Syst. 2017;33:933–40.
Plotkin SR, Wick A. Neurofibromatosis and Schwannomatosis. Semin Neurol. 2018;38:73–85.
Ahlawat S, Blakeley JO, Langmead S, Belzberg AJ, Fayad LM. Current status and recommendations for imaging in neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis. Skeletal Radiol. 2020;49:199–219.
Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443–56.
Ahlawat S, Baig A, Blakeley JO, Jacobs MA, Fayad LM. Multiparametric whole-body anatomic, functional, and metabolic imaging characteristics of peripheral lesions in patients with schwannomatosis. J Magn Reson Imaging. 2016;44:794–803.
Gunasekaran A, Newhart H, Kumar M, Kazemi N. Utility of MRI neurography in neurofibromatosis type I: case example and review of MRI neurography literature. Surg Neurol Int. 2019;10:12.
Salamon J, Veldhoen S, Apostolova I, Bannas P, Yamamura J, Herrmann J, et al. 18F-FDG PET/CT for detection of malignant peripheral nerve sheath tumours in neurofibromatosis type 1: tumour-to-liver ratio is superior to an SUVmax cut-off. Eur Radiol. 2014;24:405–12.
Schwabe M, Spiridonov S, Yanik EL, Jennings JW, Hillen T, Ponisio M, et al. How effective are noninvasive tests for diagnosing malignant peripheral nerve sheath tumors in patients with neurofibromatosis type 1? Diagnosing MPNST in NF1 patients. Sarcoma. 2019;2019:4627521.
Plotkin SR, Blakeley JO, Evans DG, Hanemann CO, Hulsebos TJM, Hunter-Schaedle K, et al. Update from the 2011 International Schwannomatosis Workshop: from genetics to diagnostic criteria. Am J Med Genet A. 2013;0:405–16.
Plotkin SR, Messiaen L, Legius E, Pancza P, Avery RA, Blakeley JO, et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: an international consensus recommendation. Genet Med. 2022;24:1967–77.
Lin J, Martel W. Cross-sectional imaging of peripheral nerve sheath tumors: characteristic signs on CT, MR imaging, and sonography. AJR Am J Roentgenol. 2001;176:75–82.
Lee SK, Kim J-Y, Jeong HS. Benign peripheral nerve sheath tumor of digit versus major-nerve: comparison of MRI findings. PLoS One. 2020;15:e0230816.
Ahlawat S, Fayad LM. Imaging cellularity in benign and malignant peripheral nerve sheath tumors: utility of the “target sign” by diffusion weighted imaging. Eur J Radiol. 2018;102:195–201.
Zipfel J, Al-Hariri M, Gugel I, Grimm A, Steger V, Ladurner R, et al. Surgical management of sporadic peripheral nerve schwannomas in adults: indications and outcome in a single center cohort. Cancers (Basel). 2021;13:1017.
Hilton DA, Hanemann CO. Schwannomas and their pathogenesis. Brain Pathol. 2014;24:205–20.
Debs P, Fayad LM, Ahlawat S. MR neurography of peripheral nerve tumors and tumor-mimics. Semin Roentgenol. 2022;57:232–40.
Ogose A, Hotta T, Morita T, Yamamura S, Hosaka N, Kobayashi H, et al. Tumors of peripheral nerves: correlation of symptoms, clinical signs, imaging features, and histologic diagnosis. Skeletal Radiol. 1999;28:183–8.
Fayad LM, Blakeley J, Plotkin S, Widemann B, Jacobs MA. Whole body MRI at 3T with quantitative diffusion weighted imaging and contrast-enhanced sequences for the characterization of peripheral lesions in patients with neurofibromatosis type 2 and Schwannomatosis. ISRN Radiol. 2013;2013:627932.
Yun JS, Lee MH, Lee SM, Lee JS, Kim HJ, Lee SJ, et al. Peripheral nerve sheath tumor: differentiation of malignant from benign tumors with conventional and diffusion-weighted MRI. Eur Radiol. 2021;31:1548–57.
Li C-S, Huang G-S, Wu H-D, Chen W-T, Shih L-S, Lii J-M, et al. Differentiation of soft tissue benign and malignant peripheral nerve sheath tumors with magnetic resonance imaging. Clin Imaging. 2008;32:121–7.
Soldatos T, Fisher S, Karri S, Ramzi A, Sharma R, Chhabra A. Advanced MR imaging of peripheral nerve sheath tumors including diffusion imaging. Semin Musculoskelet Radiol. 2015;19:179–90.
Bhargava R, Parham DM, Lasater OE, Chari RS, Chen G, Fletcher BD. MR imaging differentiation of benign and malignant peripheral nerve sheath tumors: use of the target sign. Pediatr Radiol. 1997;27:124–9.
Jee W-H, Oh S-N, McCauley T, Ryu K-N, Suh J-S, Lee J-H, et al. Extraaxial neurofibromas versus neurilemmomas: discrimination with MRI. AJR Am J Roentgenol. 2004;183:629–33.
Söderlund V, Göranson H, Bauer HCF. MR imaging of benign peripheral nerve sheath tumors. Acta Radiol. 1994;35:282–6.
Kakkar C, Shetty CM, Koteshwara P, Bajpai S. Telltale signs of peripheral neurogenic tumors on magnetic resonance imaging. Indian J Radiol Imaging. 2015;25:453–8.
Stull MA, Moser RP, Kransdorf MJ, Bogumill GP, Nelson MC. Magnetic resonance appearance of peripheral nerve sheath tumors. Skeletal Radiol. 1991;20:9–14.
Lee SK, Kim J-Y, Lee Y-S, Jeong HS. Intramuscular peripheral nerve sheath tumors: schwannoma, ancient schwannoma, and neurofibroma. Skeletal Radiol. 2020;49:967–75.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Debs, P., Luna, R., Fayad, L.M. et al. MRI features of benign peripheral nerve sheath tumors: how do sporadic and syndromic tumors differ?. Skeletal Radiol 53, 709–723 (2024). https://doi.org/10.1007/s00256-023-04479-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04479-1