Abstract
Objective
The objective of this study is to introduce cooled radiofrequency ablation technical feasibility as an alternative percutaneous image-guided treatment of chronic pain and stiffness in the setting of uncomplicated total knee arthroplasty.
Material and method
This retrospective pilot study includes a total of 19 consecutive patients experiencing persistent chronic pain after total knee arthroplasty, without underlying hardware complications who had failed conservative care. Patients initially underwent anesthetic blocks of the genicular nerve branches to determine C-RFA candidacy. After adequate response to the anesthetic blocks (> 50% immediate pain relief), patients were subjected to cooled radiofrequency ablations 2–3 weeks later. Treatment response was evaluated utilizing clinically validated questionnaires (KOOS, the Knee Injury and Osteoarthritis Outcome Score) and visual analog scale (VAS) to assess pain severity, stiffness, functional activities of daily living, and use of pain medication. Follow-up outcome scores were collected up to 1 year after C-RFA procedure.
Result
A total of 21 knees were treated consecutively between 4/2019 and 1/2020 (mean age 70.5 years; 5 M:14F). The mean total KOOS score improved significantly from baseline at 35.0 ± 14.0 to 64.2 ± 14.7 at a mean of 10.2 months after treatment (p < 0.0001), with significant improvement in mean stiffness score from 44.8 ± 16.7 to 68.8 ± 20 (p < 0.0001). The mean VAS score improved significantly from baseline at 8.30 ± 1.1 to 2.45 ± 1.8 (p < 0.0001). No major complications were encountered. No patients went on to receive re-treatment, surgical revision, or other intervention.
Conclusion
Image-guided genicular nerve cooled radiofrequency ablation offers a promising alternative in treating chronic pain/stiffness in the setting of uncomplicated TKA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is one of the most common diseases afflicting individuals over the age of 40 worldwide [1]. Pathogenesis involves articular cartilage degeneration and microstructural remodeling of the joint, translating to restricted range of motion, swelling, and pain. At an 83% prevalence rate, knee OA is one of the most common forms of OA [2], with symptomatic knee OA estimated to affect over 27 million adults in the USA alone [3].
Clinical management of knee OA includes non-surgical (non-pharmacological and pharmacological therapies) and surgical pathways. Non-pharmacological treatments aim at deaccelerating the progress of OA through education, lifestyle modification, and use of orthotic devices [4,5,6,7,8]. Pharmacological therapy provides short-term symptomatic relief with well-known short- and long-term side effects including chondrotoxicity/acceleration of OA and addiction potential [4, 9, 10]. First-line treatment includes topical/oral non-steroidal anti-inflammatory drugs (NSAIDs) and oral acetaminophen with intra-articular anesthetic-corticosteroid injection and oral opioids reserved for refractory cases.
Elective surgical management is the terminal therapeutic option for end-stage knee OA, ranging from joint-preserving intervention to partial and total knee arthroplasty (TKA) [4]. Averaging at 500,000 procedures a year, TKA is one of the most commonly performed procedures in the USA [2]. Even after TKA, one fifth of patients experience persistent chronic symptoms with no underlying hardware complication [11]. Although the pain is multifactorial in etiology, there often is an underlying neuropathic component related to peripheral nerve injury or impaired pain modulation with central sensitization [12,13,14].
Thermal ablation delivers targeted thermal damage to nerves, disrupting transmission of pain signals. Standard thermal radiofrequency ablation effects are limited by charring effect at the tissue-electrode interface which acts as an insulator and limits the core/destruction boundaries [15, 16]. In contrast, water-based coolant system within the cooled radiofrequency (C-RFA) probe reduces the charring effect and creates a larger zone of neurolysis [17, 18]. Given the anatomical complexity of the genicular nerve branches, a large ablation zone can account for the anatomic variability [19, 20].
Recent work has demonstrated the safety and efficacy of C-RFA as an alternative method for treating arthritic symptoms among patients with OA who failed conservative management and are not surgical candidates for joint replacement [21,22,23]. Its application, however, in the setting of symptomatic uncomplicated TKA is yet to be uncovered. The objective of this single intuition pilot study is to assess the efficacy and safety profile of C-RFA in treating symptoms such as pain and stiffness in the setting of uncomplicated TKA.
Material and methods
This was a longitudinal retrospective pilot study conducted at a tertiary academic medical center between 2019 and 2020. Institutional review board approval was obtained prior to initiation of the study. All patients included in this study were referred to interventional musculoskeletal radiology after a thorough evaluation by the orthopedic surgical services. Inclusion criteria included patients > 65 years of age status post TKA at least 2 years ago with persistent severe knee pain based on Knee Injury and Osteoarthritis Outcome Score (KOOS) and visual analog scale (VAS). These patients had no underlying hardware complications such as infection or hardware loosening to explain the underlying pain. Patients initially underwent anesthetic blocks (one block per nerve site location; one anesthetic block session per patient) of the genicular nerve branches to determine C-RFA candidacy. After adequate response to the anesthetic blocks (> 50% immediate pain relief), patients were subjected to cooled radiofrequency ablations 2–3 weeks later. Treatment response was evaluated utilizing clinically validated questionnaires (KOOS, Knee Injury and Osteoarthritis Outcome Score, and WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index) and visual analog scale (VAS) to assess pain severity, stiffness, functional activities of daily living, and use of pain medication. The higher the number for KOOS and WOMAC scores, the more satisfied (less symptomatic) the patients felt [24]. Follow-up outcome scores were collected up to 1 year after C-RFA procedure, by contacting patients via telephone and asking them the questions on the respective questionnaires.
Diagnostic genicular nerve block procedure
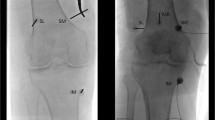
The patient was placed supine on a fluoroscopy table with the symptomatic knee(s) slightly flexed. Initially superficial local anesthesia was administered by creating a skin wheal using 2 mL of 2% lidocaine. Spinal needles were introduced at four locations to block superior lateral, superior medial, inferior medial, and suprapatellar genicular nerves under fluoroscopic guidance (Fig. 1). At each site, 1.0 mL of 2% lidocaine was injected to anesthetize each genicular nerve. Patients were assessed within 15 min of the nerve blocks by physical examination and ambulation. A positive response was considered as at least 50% of pain reduction [25].
Genicular nerve radiofrequency ablation procedure
Patient setup was like that of the diagnostic genicular nerve block procedure. When treating both knees simultaneously, one knee was positioned higher than the other to prevent significant overlay and beam artifact. Initially, superficial local anesthesia was administered by creating a skin wheal using 2 mL of 2% lidocaine at the location of the genicular nerves, followed by 50–100-mm 17-gauge (G) introducer needle at the superior lateral, superior medial, inferior medial, and suprapatellar genicular nerves under fluoroscopic guidance (Fig. 2). Through the introducer needles, 1 mL of 2% lidocaine was injected. Subsequently, 18G internally cooled, 4-mm active tip, RFA electrode (Coolief, Halyard Health, Alpharetta, GA, USA) was placed into the introducer needle, and positioning was verified with anterior posterior (AP) and lateral fluoroscopic views. Motor nerve activity was excluded with testing 2 Hz at 1 mA before initiating the ablation. Each target nerve was treated for 2 min and 30 s at a set temperature of 60 °C, which imparts a tissue temperature of 77° to 80 °C surrounding the electrode (Fig. 3).
Data collection
Demographic data for each patient, including age, gender, body mass index (BMI), analgesic medication use at presentation, laterality, and severity of symptom at presentation, was collected using electronic medical records. With verbal consent, the participant took part in a standardized survey by telephone to assess overall outcomes, complications, and current analgesic requirement 1 year after the C-RFA procedure. Treatment outcomes measured included pain severity, stiffness, and functional activities of daily living using KOOS, WOMAC, and VAS. KOOS and WOMAC questionnaires are structured to evaluate overall pain and function. They are composed of subscales including pain, function in daily living, function in sports and recreation, and quality of life. VAS score is a single-item pain scale.
Statistical analysis
Average score comparisons between the time point at baseline and at 1 year after C-RFA treatment were analyzed with the Wilcoxon signed rank test. Subgroup analyses were obtained. We also used bar plots to visually compare score averages pre and post. P values were all two sided and considered as statistically significant when p < 0.05. All analyses were performed based on available cases and conducted in the SAS version 9.4 software.
Results
Nineteen patients (5 male and 14 female) for a total of 21 knees were treated consecutively between 4/2019 and 1/2020. The average time to C-RFA treatment after TKA was 14.6 months (ranging from 8 to 16.7 months). No patients required TKA revision surgery. The mean age of patients was 70.5 years. Table 1 details the improvement in symptoms per the KOOS questionnaire, with all sub-categories displaying a statistically significant improvement 1 year after C-RFA treatment (p < 0.01). The mean total KOOS score improved significantly from baseline at 35.0 ± 14.0 to 64.2 ± 14.7 at a mean of 10.2 months after treatment (p < 0.0001), with significant improvement in mean pain score from 33.2 ± 22.2 to 75.2 ± 19.7 (p < 0.0001). Mean VAS improved with decrease from baseline at 8.30 ± 1.08 to 2.45 ± 1.76 (p < 0.0001) (Table 1). All WOMAC categories showed improvement 1 year after C-RFA treatment, including the overall mean WOMAC which increased from baseline at 36.9 ± 17.3 to 62.0 ± 15.5 (p < 0.0001) (Table 2). No major complications were encountered. No patients went on to receive re-treatment, surgical revision, or other intervention.
Opioid consumption data was collected prior to the nerve block procedure via chart review and patient interviews. Follow-up data was collected after the C-RFA procedure during the telephone survey completion. Sub-analysis was performed to investigate the use of pharmacological agents to treat the knee pain from TKA (Fig. 4). Less patients used anti-inflammatory and opioid medications to treat their knee pain after they received C-RFA treatment. Bar graphs of the KOOS and WOMAC categories are depicted in Figs. 5 and 6, respectively, showing a significant improvement in all categories 1 year after C-RFA treatment.
Discussion
This study sought to investigate the efficacy of C-RFA in managing knee pain in patients who had persistent pain status post TKA; had no underlying hardware complications, such as loosening or infection; and had failed initial medical management. It is the first study to explore C-RFA’s efficacy 1 year after the treatment in such patient population. TKAs are the most common total joint procedure in the USA with a little over 1 million primary and revision TKAs being performed from 2012 to 2019 [26]. As stated earlier, 20% of patients who receive TKA developed knee pain without underlying complications [11]. Neuroma of the infrapatellar branch of the saphenous nerve has reported in case reports after TKAs, causing anterior knee pain and stiffness in patients [27]. These can be treated with conservative management of analgesics and physical therapy but can also be treated with surgical procedures like neurolysis and knee denervation [28]. Electrocautery of the nerve end can prevent recurrence of the neuromas [27].
With the lack of long-term alternative management options, patients turn to opioids to manage the postoperative pain. New opioid prescriptions after 7 days of TKA have increased to 82% in 2012 [29]. However, patients receiving opioids after TKA had no clinically meaningful improvement in pain up to 2 months after surgery [30]. Patients undergoing TKAs were twice as likely to require filling opioid prescriptions (larger quantities and longer periods of refills) than patients receiving THA surgeries (p < 0.05) [31]. The current post-surgical treatment options only contribute to the amount of opioid prescriptions in the USA, fueling the dangerous opioid epidemic.
We found that C-RFA is effective in managing pain and improving functionality of the affected knee up to 1 year in patients who received TKAs. One study by Qudsi-Sinclair et al. conducted a double-blind, randomized clinical study that compared neurolysis with traditional RFA with local anesthetic and corticosteroid block of the superolateral, superomedial, and inferomedial genicular nerve branches [32]. In the aforementioned study, the 14 patients who received RFA treatment had TKA procedures within 6 months of the treatment. Their numeric rating scale (NRS) significantly improved from 7.07 ± 1.01 at baseline to 4.93 ± 1.98 at 12 months (p < 0.001) [32]. Walega et al. have evaluated pre-operative genicular nerve RFA prior to TKA surgery to alleviate post-operative pain, showing that there is no effect on pain or functionally post-operatively [33].
Post-operative pain management for TKA has included conservative management, like NSAIDs and opioids pre-operatively, local infiltration analgesia and spinal anesthesia intra-operatively, and minimally invasive techniques, like epidural analgesic and peripheral nerve block, post-operatively [34]. Using preemptive analgesia with celecoxib combined with tramadol/acetaminophen has proven to effectively manage movement pain up to 3 months after TKA more than the control group of multimodal analgesia (p = 0.012) [35]. Opioid use 4 months before TKA is a strong predictor of continued opioid use after surgery [36]. COX-1 and COX-2 inhibitors have also proven to help with post-operative knee pain after TKA, but not for a significant duration. Post-operative pain management can also be performed intra-operatively. Local infiltration analgesia (LIA), consisting of opioids, antibiotics, and NSAIDs, injected into the periarticular regions has been shown to effectively reduce narcotic consumption soon after TKA procedure compared to patients who received placebo infiltration [37]. LIA combined with liposomal bupivacaine (LB) has shown to improve physical function 3 months after TKA surgery [38]. Spinal anesthesia has shown to decrease hospital stay, incidence of surgical site infections, and blood transfusions [39]. These patients were only followed up for 4 weeks for complications but not for pain assessment.
Post-operative pain management options after the surgery range from conservative medical management to minimally invasive interventions, like nerve blocks. In a retrospective study by Klement et al., 184 patients received 1 or more steroid injections 5.3 months after TKA surgery, with 25% reporting clinical efficacy for more than 6 months [40]. No patients reported peri-prosthetic infection within 1 year, and only 5.6% reported minor adverse events after the steroid injection, but septic arthritis and other complications have been reported in native and replaced joints after steroid injections [41, 42]. There are many short-term options for managing knee pain after TKA surgery, but no studies have looked at the efficacy of post-operative management options following TKA over 6 months. In this study, the use of C-RFA to treat symptomatic primary degenerative OA was extrapolated to manage post-surgical pain. Our study shows that it has significant clinical applications to help manage pain and improve functional status in patients post TKA with no underlying hardware complications. If some surgeons deem revision surgeries as unnecessary for the patient, C-RFA is a useful tool to help patients cope with their pain. This study was done at a single-institution, non-randomized non-controlled, retrospective study and has limitations of treating a small number of patients with C-RFA. There were significantly more females than males, allowing for selection bias in our analysis. Future studies should include more patients to strengthen the power of the study and have more frequent and longer follow-up times. They could also compare C-RFA to other alternative methods of managing knee pain after TKA surgery.
In conclusion, our single institution study demonstrates C-RFA carries a low risk of complications and is a promising novel alternative in treating chronic pain/stiffness in the setting of uncomplicated TKA. C-RFA supersedes the time of pain relief compared to other existing conservative management options for patients and has potential to decrease usage of opioid and non-opioid pain medication in this population.
References
Cui A, et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020. p. 29–30.
Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96.
Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35.
Sharma L. Osteoarthritis of the Knee. N Engl J Med. 2021. 384(1):51–9.
DeRogatis M, et al. Non-operative treatment options for knee osteoarthritis. Ann Transl Med. 2019;7(Suppl 7):S245.
Foy CG, et al. Intensive lifestyle intervention improves physical function among obese adults with knee pain: findings from the Look AHEAD trial. Obesity (Silver Spring). 2011;19(1):83–93.
Wadden TA, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006;14(5):737–52.
Yu SP, et al. Effectiveness of knee bracing in osteoarthritis: pragmatic trial in a multidisciplinary clinic. Int J Rheum Dis. 2016;19(3):279–86.
Kompel AJ, et al. Intra-articular corticosteroid injections in the hip and knee: perhaps not as safe as we thought? Radiology. 2019;293(3):656–63.
Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3(5):2325967115581163.
Wylde V, et al. Chronic pain after total knee arthroplasty. EFORT Open Rev. 2018;3(8):461–70.
Haroutiunian S, et al. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154(1):95–102.
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet (London, England). 2006;367(9522):1618–25.
Wright A, et al. Abnormal quantitative sensory testing is associated with persistent pain one year after TKA. Clin Orthop Relat Res. 2015;473(1):246–54.
Haines DE, Verow AF. Observations on electrode-tissue interface temperature and effect on electrical impedance during radiofrequency ablation of ventricular myocardium. Circulation. 1990;82(3):1034–8.
Nath S, DiMarco JP, Haines DE. Basic aspects of radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 1994;5(10):863–76.
Bogduk N. Practice guidelines: spinal diagnostic and treatment procedures, ed. Bogduk N. San Francisco: International Spine Intervention Society. 2004.
Kapural L, Deering JP. A technological overview of cooled radiofrequency ablation and its effectiveness in the management of chronic knee pain. Pain Manag. 2020;10(3):133–40.
Franco CD, et al. Innervation of the anterior capsule of the human knee: implications for radiofrequency ablation. Reg Anesth Pain Med. 2015;40(4):363–8.
Tran J, et al. Anatomical study of the innervation of anterior knee joint capsule: implication for image-guided intervention. Reg Anesth Pain Med. 2018;43(4):407–14.
Gonzalez FM. Cooled radiofrequency genicular neurotomy. Tech Vasc Interv Radiol. 2020;23(4):100706.
Rojhani S, Qureshi Z, Chhatre A. Water-cooled radiofrequency provides pain relief, decreases disability, and improves quality of life in chronic knee osteoarthritis. Am J Phys Med Rehabil. 2017;96(1):e5–e8.
Wong PK, et al. Safety and efficacy comparison of three- vs four-needle technique in the management of moderate to severe osteoarthritis of the knee using cooled radiofrequency ablation. Skeletal Radiol. 2021;50(4):739–50.
Walker LC, et al. The WOMAC score can be reliably used to classify patient satisfaction after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2018;26(11):3333–41.
Cepeda MS, et al. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105(1–2):151–7.
Springer DB, Levine BR, Golladay GJ. Highlights of the 2020 american joint replacement registry annual report. Arthroplasty Today. 2021;9:141–2. https://doi.org/10.1016/j.artd.2021.06.004.
Kachar SM, Williams KM, Finn HA. Neuroma of the infrapatellar branch of the saphenous nerve: a cause of reversible knee stiffness after total knee arthroplasty. J Arthroplasty. 2008;23(6):927–30.
Ilfeld BM, Preciado J, Trescot AM. Novel cryoneurolysis device for the treatment of sensory and motor peripheral nerves. Expert Rev Med Dev. 2016;13(8):713–25.
Wunsch H, et al. Opioids prescribed after low-risk surgical procedures in the United States, 2004–2012. Jama. 2016;315(15):1654–7.
Shah R, et al. Opioid use and pain control after total hip and knee arthroplasty in the US, 2014 to 2017. JAMA Network Open. 2020;3(7):e2011972–e2011972.
Dwyer MK, et al. Characterization of post-operative opioid use following total joint arthroplasty. J Arthroplasty. 2018;33(3):668–72.
Qudsi-Sinclair S, et al. A comparison of genicular nerve treatment using either radiofrequency or analgesic block with corticosteroid for pain after a total knee arthroplasty: a double-blind, randomized clinical study. Pain Pract. 2017;17(5):578–88.
Walega D, et al. Radiofrequency ablation of genicular nerves prior to total knee replacement has no effect on postoperative pain outcomes: a prospective randomized sham-controlled trial with 6-month follow-up. Reg Anesth Pain Med. 2019.
Li JW, Ma YS, Xiao LK. Postoperative pain management in total knee arthroplasty. Orthop Surg. 2019;11(5):755–61.
Xu Z, et al. Preemptive analgesia by using celecoxib combined with tramadol/APAP alleviates post-operative pain of patients undergoing total knee arthroplasty. Phys Sportsmed. 2017;45(3):316–22.
Deveza LA, Hunter DJ, Van Spil WE. Too much opioid, too much harm. Osteoarthritis Cartilage. 2018;26(3):293–5.
Greimel F, et al. Matched-pair analysis of local infiltration analgesia in total knee arthroplasty: patient satisfaction and perioperative pain management in 846 cases. J Knee Surg, 2019;32(10):953–59.
Talmo CT. et al. Prospective randomized trial comparing femoral nerve block with intraoperative local anesthetic injection of liposomal bupivacaine in total knee arthroplasty. J Arthroplasty. 2018;33(11):3474–78.
Park YB, et al. Comparison of short-term complications of general and spinal anesthesia for primary unilateral total knee arthroplasty. Knee Surg Relat Res. 2017:29(2):96–103.
Klement MR, et al. Intra-articular corticosteroid injection following total knee arthroplasty: is it effective? J Arthroplasty. 2019;34(2):303–8.
Deyle, GD et al. A multicenter randomised, 1-year comparative effectiveness, parallel-group trial protocol of a physical therapy approach compared to corticosteroid injection on pain and function related to knee osteoarthritis (PTA Trial). BMJ Open. 2016;6(3):e010528.
Holland C, et al. Septic and aseptic complications of corticosteroid injections: an assessment of 278 cases reviewed by expert commissions and mediation boards from 2005 to 2009. Dtsch Arztebl Int. 2012;109(24):425–30.
Acknowledgement
The authors would like to thank Nariman Nezami, MD, University of Maryland Medical Center, Department of Radiology, 22 S. Greene St., Baltimore, MD 21201.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, F.M., Tran, A., Wong, P.KW. et al. Management of uncomplicated total knee arthroplasty chronic pain and stiffness utilizing cooled radiofrequency ablation: a single institution pilot study. Skeletal Radiol 51, 1215–1223 (2022). https://doi.org/10.1007/s00256-021-03944-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03944-z