Abstract
Myxofibrosarcoma is a malignant fibroblastic soft tissue neoplasm containing a variable amount of myxoid stroma that commonly presents as a slow-growing mass in elderly patients. The neoplasm may be superficial or deep to the muscle fascia and characteristically has an infiltrative growth pattern with a dominant or multinodular mass. We describe an unusual case of high-grade myxofibrosarcoma of the wrist and forearm that infiltrated the muscles, tendons, and wrist joint, causing bone erosions. The tumor was mistakenly diagnosed as synovitis and a chronic, erosive, inflammatory process. The diffuse nature, absence of a dominant mass, and radiographic appearance complicated the diagnosis. Although neoplasms of the synovial spaces are rare, this case demonstrates that tumors with a highly infiltrative growth pattern can mimic inflammatory synovitis and that neoplasms should be considered in the differential diagnosis when clinical and laboratory features are discordant with the imaging appearance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myxofibrosarcoma was initially described as a myxoid variant of malignant fibrous histiocytoma [1]. This sarcoma presents most commonly in the extremities of patients in their sixth to eighth decade of life, either as a solitary superficial and/or subcutaneous multinodular growth or as a mass involving the fascia and skeletal muscle [2,3,4]. The lower extremity is more often affected than the upper extremity, and approximately 10% of tumors occur in the trunk, with other locations rarely reported [1,2,3,4,5]. Superficial lesions have an infiltrative growth pattern without a distinct tumor nodule and can extend to the deep fascia, whereas deep tumors usually consist of a discrete mass [2]. Characteristic “tails” of tumor involvement may occur along the fascial planes, often making a wide margin excision difficult [6, 7].
We present a case of a high-grade myxofibrosarcoma that extensively involved the wrist and forearm with an infiltrative growth pattern occurring within the muscles, along the tendon sheaths, and within the synovium of the wrist joint. This neoplasm mimicked synovitis of a chronic inflammatory process on imaging, leading to a delay in diagnosis. To our knowledge, this growth pattern has not been previously reported for a soft tissue sarcoma.
Case report
A 79-year-old woman with a long history of Raynaud’s disease, hyperuricemia, osteoarthritis, osteoporosis, and hypertension presented with diffuse unilateral right wrist pain and swelling. Eight years earlier, she had experienced a traumatic right wrist fracture that required surgery. She recovered well but recently developed progressive loss of function in the right hand and wrist. Over the 2–3 weeks before presentation, this dysfunction had worsened so that she was unable to form a fist or perform even simple tasks such as using a fork. She noted numbness and tingling in the tips of the thumb, index finger, and long finger. The patient had no history of recent trauma or injury to the wrist and no complaints of fevers, chills, night sweats, or pain in other joints. Physical examination revealed diffuse soft tissue swelling just proximal to the wrist crease with a nodular area on the volar aspect of the radial side of the wrist. Some yellowish discoloration of the overlying skin with very mild warmth was observed. There was no significant tenderness with palpation of the swollen area, but flexion and extension of the wrist were limited. Laboratory values were normal except for minimally elevated serum calcium (10.8 mg/dL; reference range, 8.5–10.2 mg/dL) and intact parathormone (82 pg/mL; reference range, 15–65 pg/mL) levels. Her Westergren erythrocyte sedimentation rate, C-reactive protein, serum urate, creatinine, blood urea nitrogen, rheumatoid factor, anti-CCP antibody and vitamin D levels were normal. Her other joints were asymptomatic with the exception of occasional right knee pain and swelling that had been attributed to bursitis. Her medications included hydroxychloroquine, metoprolol, and meloxicam; she was also using diclofenac gel on her wrist.
Radiographs of both hands and the right wrist and noncontrast MRI of the right wrist were performed at another facility. The radiographs showed severe erosive osteoarthritis of both hands and unilateral soft tissue swelling of the right wrist (Fig. 1). There were large erosions of all of the carpal bones, the distal radius, and the distal ulna, with the largest erosions seen in the ulna, pisiform, triquetrum, hamate, and capitate. No soft tissue calcifications were seen. The noncontrast MR images of the right wrist confirmed the presence of the bone erosions and also demonstrated marked distention of the synovial spaces of the wrist and tendon sheaths. Heterogeneous signal intensity predominantly equivalent to that of muscle was seen on T1-weighted images, but heterogeneously bright signal intensity and some regions lower in signal intensity were seen on fat-suppressed proton density-weighted images (Fig. 2). The abnormality appeared to be contained within the wrist joint and the flexor tendons, with severe distention of the carpal tunnel. The proximal and distal ends of the abnormality were not included in the wrist MR study; proximally, the lesion surrounded the flexor pollicis longus tendon with extension between the pronator quadratus and flexor digitorum tendons (Fig. 2c).
Radiographs at presentation. PA view of a both hands and b oblique and c lateral views of the right wrist show severe unilateral soft tissue swelling (arrows) and erosions (arrowheads) of the right wrist involving the radius, ulna, and carpal bones (arrowheads). There is underlying erosive osteoarthritis bilaterally
MR images of the right wrist at presentation. a Axial T1-weighted and b–c fat-suppressed proton density-weighted images show severe distention of the synovial spaces of the wrist and tendon sheaths by tumor (stars) surrounding the tendons, appearing as low signal intensity on T1-weighted images and heterogeneously high signal intensity on fat-suppressed proton density-weighted images, simulating proliferative synovitis. Bone erosions (arrows) are apparent on the d coronal T1-weighted, e fat-suppressed proton density-weighted, and f sagittal T1-weighted images. The most proximal image (c) shows that the tumor encases the flexor pollicis longus tendon (arrowhead) and is interposed between the pronator quadratus and flexor digitorum tendons. The initial wrist MR images did not include the entire abnormality
Although serum inflammatory markers, rheumatoid factor and anti-CCP antibody levels were normal and no radiographic calcifications were present, the working clinical and imaging differential diagnosis was an unusual asymmetric erosive arthritis and included an atypical presentation of rheumatoid arthritis, reactive arthritis, crystalline arthropathy (gout or calcium pyrophosphate deposition), and septic arthritis caused by an unusual organism. An open surgical procedure was performed to obtain tissue specimens for histology and culture analysis. During surgery, a 15-cm incision revealed brownish semiliquefied tissue deep to the superficial fascia with other areas of “liquefied hematoma,” including a bluish mass containing thick dark red liquid. Erosions of the distal radius and ulna were confirmed. A 12 cm × 6 cm mass was removed, and a carpal tunnel release was performed to relieve an hourglass-like constriction of the median nerve. On histologic examination, the biopsy specimens were identified as probable myxofibrosarcoma.
MRI of the entire right forearm was then performed with and without intravenous contrast enhancement to determine the tumor extent (Fig. 3). These images showed extensive involvement of the entire volar forearm by an infiltrative process with edema-like muscle signal extending nearly to the elbow. A rim-enhancing nodular area within the flexor digitorum profundus muscle in the mid forearm had signal characteristics of chronic hemorrhage (not shown). The distal synovial and tenosynovial tumor regions were the only mass-like areas on MR images, and these areas showed bright, nearly homogeneous contrast enhancement (Fig. 3c, e).
MR images of the right forearm obtained after biopsy. a Sagittal short inversion time inversion recovery image shows involvement of the entire forearm, with extension of the heterogeneously high signal intensity tumor involving the muscles proximally (arrowheads) and the tendon sheaths distally (stars). b Precontrast sagittal T1-weighted image and c sagittal fat-suppressed T1-weighted image obtained after intravenous contrast administration show heterogeneous enhancement of the tumor throughout the forearm. d Precontrast axial T1-weighted non-fat-suppressed image and e axial T1-weighted fat-suppressed image obtained after intravenous contrast administration show nearly uniform enhancement of the fluid/synovitis-like signal intensity regions (stars)
An above-elbow amputation was performed, and histologic examination revealed that a wide clear surgical margin had been obtained. Surgical pathological analysis confirmed the presence of a high-grade myxofibrosarcoma. At her 15-month postoperative visit, the patient was disease free.
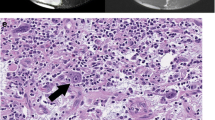
Gross pathological examination demonstrated a solid irregularly shaped white mass that extended to the deep fascia. The tumor was infiltrative and multifocal and involved the palmar aspect of the hand and forearm, associated with the fascia, the peripheral nerves, and the tendon structures. Histologically, the tumor appeared slightly nodular and predominantly myxoid (less commonly solid) with diffuse infiltration involving the dermis, subcutaneous fat, tendon, and, superficially, the skeletal muscle (Fig. 4). Curvilinear blood vessels were numerous and prominent. More solid areas resembled undifferentiated pleomorphic sarcoma, with a mitotic rate of up to 26 mitoses per 10 high-power fields. There were areas of necrosis. No significant evidence of inflammation (e.g. presence of lymphocytes or plasma cells) was seen within the tumor proper. Cytologically, individual cells displayed significant nuclear pleomorphism, with variably enlarged, elongated, and hyperchromatic to irregularly shaped nuclei; slightly eosinophilic cytoplasm; and indistinct cell margins. Large epithelioid cells with inclusion-like nucleoli, typical of myxoinflammatory fibroblastic sarcoma, were absent. Immunohistochemically, the tumor cells were negative for AE1/3, S100, SMA, and desmin. Overall, the histologic findings were characteristic of high-grade myxofibrosarcoma.
Histology with hematoxylin- and eosin-stained sections. a A low-power view (×20 original magnification) shows diffuse infiltration of the myxoid tumor into the tendon sheath (asterisk). b A slightly higher power (×40) magnification reveals the prominent arborizing and curvilinear vascular pattern with a dominant myxoid stroma. c Cytologic examination at high power (×200) demonstrates a focal area of hypercellularity with sheets and fascicles of pleomorphic spindled cells
Discussion
Myxofibrosarcoma usually presents as a painless, slow-growing palpable mass [1,2,3]. These tumors generally have a nodular appearance, with lesions ranging in size from a few millimeters to more than 25 cm (median size, approximately 7 cm); deep tumors are usually larger [1,2,3]. In an analysis of the clinicopathological features of 75 cases of myxofibrosarcoma with myxoid areas composing at least 10% of the tumor, Mentzel et al. [2] classified myxofibrosarcoma lesions into two groups: superficial (dermal/subcutaneous, 69.5% of cases) and deep (intramuscular/mainly subfascial, 30.5% of cases). Tumors superficial to muscle fascia tend to infiltrate with gelatinous or firm nodules, whereas subfascial tumors are most often single, discrete masses that frequently contain gelatinous regions. Superficial myxofibrosarcomas often spread extensively in a longitudinal manner and may involve the deep fascia; they can present with a more infiltrative pattern without a nodular appearance. On gross inspection, myxofibrosarcomas usually have a soft gray-white mucoid appearance with areas of necrosis and hemorrhage [1, 3, 8]. Histologically, myxofibrosarcomas vary in appearance from hypocellular, mainly myxoid, purely low-grade spindle cell lesions to high-grade lesions with more solid, hypercellular areas and fewer myxoid areas. In all cases, myxofibrosarcomas typically exhibit pleomorphic spindled cells [2].
The tendency of myxofibrosarcoma to spread along vascular and fascial planes is likely responsible for the reported high rates of local tumor recurrence (55–63%) [1, 2, 4, 6, 8] and positive surgical resection margins (16–29%) [5, 8,9,10]. In a study of 21 myxofibrosarcomas, 81% of the tumors demonstrated an infiltrative growth pattern; the authors therefore recommended that all regions of abnormal MR signal be resected to obtain adequate surgical margins [9]. The rate of local tumor recurrence appears to be independent of histological grade but is higher in patients with positive or close (<1 mm) surgical margins [2, 4, 5, 11]. Overall, survival at 5 years is reported to range from 61 to 77%, with lower survival rates associated with increased patient age, higher tumor grade, positive surgical margins, and increased tumor size [5, 8, 10, 11]. Metastases occur in 15–35% of patients, most commonly in the lung but occasionally in the lymph nodes, bone, viscera, muscle, and brain. Patients with a higher-grade tumor, a tumor demonstrating lower myxoid component, or an epithelioid tumor variant are more likely to develop metastatic disease [1, 5, 8, 9, 11,12,13].
Typical MR features of myxofibrosarcoma reflect the pathological growth patterns, with either a diffuse infiltrative growth pattern characterized by extensive spread along the fascial plane or a focal growth pattern demonstrating a localized mass [9]. Tumor infiltration may be much more extensive than preoperative imaging studies suggest; additionally, even when MRI shows a focal growth pattern, microscopic infiltration into the surrounding soft tissue may be found in the resected specimen [10]. On MRI, the “tail sign,” a curvilinear extension from the tumor along fascial planes, may be seen in 60–77% of primary and recurrent myxofibrosarcomas and is present in a much greater proportion of myxofibrosarcomas than in other myxoid tumors [6, 7]. The presence of the tail sign should therefore raise the possibility of myxofibrosarcoma with fascial spread of tumor; however, this sign is nonspecific and may be present in patients with other tumor types [7]. Histologically, a prominent myxoid component in the tumor is required for diagnosis. On MRI, these myxoid regions demonstrate characteristically lower signal than muscle on T1-weighted images and bright signal on water-sensitive acquisitions. After intravenous contrast administration, a variable amount of these tumors show enhancement [9].
The high-grade myxofibrosarcoma discussed here is unusual because of its involvement and dissection of the tendon and intraarticular synovial spaces. We are not aware of any reports of myxofibrosarcoma or other sarcomas demonstrating such prominent spread along the tendon sheaths or involvement of articular synovial spaces. Given the degree of tumor involvement at presentation in this case, it is unclear whether the tumor began in the muscle and extended to the tendons and joint or vice versa, although we tend to favor the former. Intraarticular soft tissue neoplasms are unusual and sarcomas are very rare, with most reported intraarticular sarcomas resulting from soft tissue extension of a neighboring bone or soft tissue neoplasm [14,15,16]. The most commonly reported intraarticular malignancies are synovial sarcoma, conventional chondrosarcoma, extraskeletal myxoid chondrosarcoma, malignant tenosynovial giant cell tumor, and epithelioid sarcoma [14]. Chebib et al. [14] reported two cases of intraarticular sarcomas: one high-grade tumor presenting as a 2.5-cm myxoid subsynovial mass and one epithelioid fibrosarcoma presenting as a multilobulated mass (images of the tumors were not presented). Myxoinflammatory fibroblastic sarcoma, also known as inflammatory myxohyaline tumor of the distal extremities [17], should also be included in the differential diagnosis of myxofibrosarcoma. Myxoinflammatory fibroblastic sarcoma is a rare tumor that usually arises from the dorsal aspects of acral sites and may involve the tendons [18]. A case series reporting the MR appearance of these tumors described the primary tumors as small (<3 cm), nodular neoplasms that abut the tendon; recurrent tumors may extend in a sheet-like pattern along the tendon sheath and mimic tenosynovitis [19]. The tumor we describe here demonstrated infiltrative, deep muscular involvement within the forearm without a dominant soft tissue mass, an appearance of myxofibrosarcoma more commonly seen when the tumor arises in the subcutaneous tissues and also unusual for other types of sarcomas. However, based solely on pathological analysis, distinguishing between these two entities is rarely difficult. Myxofibrosarcoma is virtually entirely lobulated and myxoid, with a background of curvilinear blood vessels and markedly atypical cells [2]. More solid areas may resemble undifferentiated pleomorphic sarcoma and indicate the potential for high-grade behavior. The inflammatory background typical of myxoinflammatory fibroblastic sarcoma is not observed in myxofibrosarcoma [18]. Conversely, myxoinflammatory fibroblastic sarcoma exhibits a more fibroblastic to myxofibroblastic and hyalinized stroma and contains a prominent inflammatory component that may include various proportions of lymphocytes, plasma cells, and eosinophils admixed with the tumor cells. The diagnostic lesional cells of myxoinflammatory fibroblastic sarcoma are the characteristically large epithelioid cells with inclusion-like (or Reed-Sternberg-like) nucleoli [18]. Necrosis is rarely seen in myxoinflammatory fibroblastic sarcoma but occurs more commonly in high-grade myxofibrosarcoma [2, 18]. There is no debate within the pathology community regarding the fact that myxoinflammatory fibroblastic sarcoma and myxofibrosarcoma are distinct neoplastic entities that can nearly always be easily distinguished from one another on routine histologic examination. However, at present, no immunostains specific for either entity are available. The lesion in our case was almost entirely myxoid and did not contain any inflammation within the tumor proper; additionally, no large epithelioid cells with inclusion-like nucleoli were present. Most cases of myxoinflammatory fibroblastic sarcoma exhibit a low mitotic rate (<1–2 per 10 high-power fields), unlike our case of myxofibrosarcoma, which demonstrated a mitotic rate in the more solid areas of more than 20 mitoses per 10 high-power fields.
One previous case report described a myxofibrosarcoma that infiltrated throughout the thigh without the formation of a mass or mass-like lesion, similar to the tumor described here [20]. This type of tumor infiltration may be mistaken for an inflammatory process such as fasciitis [20].
The MR images described here are very unusual for myxofibrosarcoma. The distention of the joint and tendon sheaths by heterogeneous signal intensity, presence of enhancing tissue, and presence of bone erosions are more typical of rheumatoid arthritis and other inflammatory arthritis or of chronic infection caused by an unusual organism such as a fungus or mycobacterium [21]. However, the initial presentation of severe, unilateral wrist arthritis without metacarpophalangeal involvement and with a normal anti-CCP antibody level in the eighth decade of life would be unusual for a case of rheumatoid arthritis, and serum inflammatory markers would be expected to be abnormal in the setting of infection. Although diffuse tenosynovial giant cell tumors may present with bone erosions, intraarticular masses, and tenosynovial masses, these benign neoplasms typically demonstrate heterogeneous signal intensity with low signal regions on T1-weighted, proton density-weighted, and T2-weighted MR images [22]. Additionally, extensive infiltration of the forearm muscles would not be expected in cases of inflammatory arthritis, septic joints, or tenosynovial giant cell tumors.
We have presented an unusual case of myxofibrosarcoma with extensive involvement of the forearm muscles, tendons, and wrist joint. This tumor mimicked inflammatory or septic arthritis on clinical examination and on radiographic and MR images of the wrist but demonstrated discordant laboratory findings. Although soft tissue neoplasms involving joints are rare, they should be considered in the context of discordant imaging, clinical, and laboratory findings. Biopsy should be strongly considered in these cases. Additionally, radiologists should be careful to extend the region of imaging to include the entire length of an abnormality. In our case, had the full area of forearm muscle involvement been imaged initially, neoplasm may have been suspected before the initial biopsy was performed.
References
Weiss SW, Enzinger FM. Myxoid variant of malignant fibrous histiocytoma. Cancer. 1977;39(4):1672–85.
Mentzel T, Calonje E, Wadden C, et al. Myxofibrosarcoma: clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;20(4):391–405.
Angervall L, Kindblom LG, Merck C. Myxofibrosarcoma: a study of 30 cases. Acta Pathol Microbiol Scand A. 1977;85A(2):127–40.
Huang HY, Lal P, Qin J, Brennan MF, Antonescu CR. Low-grade myxofibrosarcoma: a clinicopathologic analysis of 49 cases treated at a single institution with simultaneous assessment of the efficacy of 3-tier and 4-tier grading systems. Hum Pathol. 2004;35(5):612–21.
Sanfilippo R, Miceli R, Grosso F, et al. Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2011;18(3):720–5.
Waters B, Panicek DM, Lefkowitz RA, et al. Low-grade myxofibrosarcoma: CT and MRI patterns in recurrent disease. AJR Am J Roentgenol. 2007;188(2):193–8.
Lefkowitz RA, Landa J, Hwang S, et al. Myxofibrosarcoma: prevalence and diagnostic value of the “tail sign” on magnetic resonance imaging. Skelet Radiol. 2013;42(6):809–18.
Merck C, Angervall L, Kindblom LG, Oden A. Myxofibrosarcoma: a malignant soft tissue tumor of fibroblastic-histiocytic origin—a clinicopathologic and prognostic study of 110 cases using multivariate analysis. Acta Pathol Microbiol Immunol Scand Suppl. 1983;282:1–40.
Kaya M, Wada T, Nagoya S, et al. MRI and histological evaluation of the infiltrative growth pattern of myxofibrosarcoma. Skelet Radiol. 2008;37:1085–90.
Look Hong NJ, Hornicek FJ, Raskin KA, et al. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann Surg Oncol. 2013;20(1):80–6.
Haglund KE, Raut CP, Nascimento AF, Wang Q, George S, Baldini EH. Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int J Radiat Oncol Biol Phys. 2012;82(1):361–7.
Scoccianti G, Ranucci V, Frenos F, et al. Soft tissue myxofibrosarcoma: a clinico-pathological analysis of a series of 75 patients with emphasis on the epithelioid variant. J Surg Oncol. 2016;114(1):50–5.
Nascimento AF, Bertoni F, Fletcher CD. Epithelioid variant of myxofibrosarcoma: expanding the clinicomorphologic spectrum of myxofibrosarcoma in a series of 17 cases. Am J Surg Pathol. 2007;31(1):99–105.
Chebib I, Rosenberg AE, Fletcher CD, Rosenthal DI, Hornicek FJ, Nielsen GP. Primary intra-articular sarcoma: a clinicopathological study of 15 cases. Histopathology. 2016;69(4):614–23.
Levine BD, Motamedi K, Seeger LL. Synovial tumors and proliferative diseases. Rheum Dis Clin N Am. 2016;42(4):753–68.
Li X, Zhang Z, Latif M, Chen W, Cui J, Peng Z. Synovium as a widespread pathway to the adjacent joint in undifferentiated high-grade pleomorphic sarcoma of the tibia: a case report. Medicine (Baltimore). 2018;97(8):e9870.
Montgomery EA, Devaney KO, Giordano TJ, Weiss SW. Inflammatory myxohyaline tumor of distal extremities with virocyte or Reed-Sternberg-like cells: a distinctive lesion with features simulating inflammatory conditions, Hodgkin’s disease, and various sarcomas. Mod Pathol. 1998;11(4):384–91.
Laskin WB, Fetsch JF, Miettenen M. Myxoinflammatory fibroblastic sarcoma: a clinicopathologic analysis of 104 cases, with emphasis on predictors of outcome. Am J Surg Pathol. 2014;38(1):1–12.
Tateishi U, Hasegawa T, Onaya H, Satake M, Arai Y, Moriyama N. Myxoinflammatory fibroblastic sarcoma: MR appearance and pathologic correlation. AJR Am J Roentgenol. 2005;184(6):1749–53.
Wada T, Hasegawa T, Nagoya S, Kawaguchi S, Kaya M, Ishii S. Myxofibrosarcoma with an infiltrative growth pattern: a case report. Jpn J Clin Oncol. 2000;30(10):458–62.
Clement JP 4th, Kassarjian A, Palmer WE. Synovial inflammatory processes in the hand. Eur J Radiol. 2005;56(3):307–18.
Bravo SM, Winalski CS, Weissman BN. Pigmented villonodular synovitis. Radiol Clin N Am. 1996;34(2):311–26.
Acknowledgments
We thank Megan Griffiths for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Colak, C., Kilpatrick, S.E., Mesko, N.W. et al. Upper extremity myxofibrosarcoma mimicking an erosive inflammatory arthritis: a case report. Skeletal Radiol 48, 1643–1649 (2019). https://doi.org/10.1007/s00256-019-03217-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03217-w