Abstract
Objectives
To describe the imaging findings of a series of myxoinflammatory fibroblastic sarcomas (MFSs) from our institution, including a case of dedifferentiated MFS and two cases with areas of high-grade tumor, in addition to typical cases of low-grade tumor. To correlate the imaging findings with the pathologic features of these tumors.
Subjects and methods
IRB approval was obtained. Retrospective search of the pathology database at our institution from 2000 to 2015 identified seven cases of MFS with available imaging. Imaging, pathology, and clinical data were reviewed.
Results
Unlike the majority of well-differentiated tumors in our series (four cases), one tumor showed dedifferentiation and two cases had areas of high-grade tumor. The dedifferentiated tumor showed peripheral post-contrast enhancement. One case with a substantial high-grade component showed osseous destruction and peripheral enhancement in the high-grade area, while the low-grade component enhanced diffusely. The second case had a small high-grade area and showed diffuse enhancement. All three of these cases had non-acral locations and lacked association with a tendon. The four cases of low-grade MFS demonstrated diffuse enhancement, were located in the distal extremities, and were associated with a tendon.
Conclusion
The imaging findings of dedifferentiated and high-grade MFS differ from the more typical low-grade tumors in that they have nonenhancing areas, a non-acral location, lack association with a tendon, and may involve bone. The radiologist should be aware that MFS represents a spectrum that includes low-grade tumors, tumors with high-grade areas, and tumors with dedifferentiation and that this spectrum presents with differing imaging features.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myxoinflammatory fibroblastic sarcoma (MFS) is a mesenchymal neoplasm that usually arises in the hands and feet. The tumor was first described in 1998 by three independent groups who noted a complex mixture of bizarre, ganglion-like cells interspersed with spindled and epithelioid cells on a background of inflamed myxoid and fibrosclerotic stroma [1–3]. Most tumors are centered in the subcutaneous tissues and grow either as a single lobulated nodule or multiple ill-defined nodules along the fibrous connective tissue of fat, fascia, or tendon sheaths [4]. This tumor is typically described as locally aggressive but with very low metastatic potential [1–3]. The largest review of 138 cases reported a 22% incidence of local recurrence and distant metastases in only 3% of cases, which involved the lymph nodes and lung [5]. Another large retrospective study of 104 cases reported a local recurrence rate of 67% and only one metastasis that appeared following multiple recurrences [4]. A recent study described 23 high-grade MFSs with metastases reported in 50% of the cases with follow-up [6]. Only three cases of MFS causing osseous destruction have been reported [7–10].
The majority of the literature is written from a pathologic or clinical perspective [1, 2, 4, 5], with only a few papers focusing on the magnetic resonance imaging (MRI) findings in small cohorts [7, 9, 11]. To our knowledge, there are no studies in the radiology literature describing the imaging appearance of dedifferentiated MFS. In this article we discuss seven cases of pathologically proven MFS from our institution, including one case of a dedifferentiated MFS, two cases with intermixed high- and low-grade tumor, and four cases of low-grade MFS with an emphasis on the imaging findings and correlation with pathology.
Subjects and methods
IRB approval
Institutional Review Board approval was obtained for this study and in accordance with the requirements of a retrospective review; informed consent was not required.
Patient selection
Cases were retrospectively identified using the pathology database at our institution with exhaustive keyword searches including dates from 1 January 2000 through 1 June 2015. Those cases with a pathologic diagnosis of myxoinflammatory fibroblastic sarcoma given at our institution were identified. The electronic medical record was then used to identify cases with available images, and only these were included in the final case list.
Imaging review
Image review was performed by three musculoskeletal radiologists (KGU, YM, CY) with 2, 11, and 13 years of experience. A standard McKesson workstation was utilized. Imaging characteristics were decided by consensus.
Pathologic review
Pathology review was performed by a soft tissue pathologist (DL) with 27 years of experience. Pathology was correlated with the imaging findings by the pathologist and two radiologists (KGU, YM).
Clinical data
Clinical data were obtained using the electronic medical record. These included demographic information, clinical presentation, and treatment history.
Results
Patient selection
Ten cases with a pathologic diagnosis of myxoinflammatory fibroblastic sarcoma given at our institution were identified. Three of ten cases did not have imaging available in our system, and these were excluded. Seven cases with available imaging were selected.
Imaging review
All available imaging was reviewed for all seven cases (Figs. 1, 2, 3, 4, 5, 6 and 7). This included ultrasound for one case (case 1), plain radiographs for two cases (cases 2 and 7), and magnetic resonance images (MRI) for all cases.
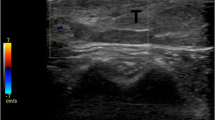
A 69-year-old male with dedifferentiated MFS of the posterior distal aspect of the right upper arm. Ultrasound images show (a) an ovoid hypoechoic mass with lobulated margins centered in the subcutaneous tissues of the distal posterior aspect of the right upper arm with slight posterior acoustic enhancement but no invasion of the underlying fascia. There was markedly increased vascularity on color Doppler imaging (b). T2-weighted MR image (T1-weighted sequences: TR/TE 398–690/10–12, T2-weighted sequences: TR/TE 3314–5877/81–88) (c) shows high signal in the mass (star). T1-weighted, fat-suppressed, post-contrast image (d) shows a lack of central post-contrast enhancement (star) but thin, linear, surrounding enhancement (arrowheads) of the soft tissues. High-power hematoxylin and eosin (H&E) stain images depict two components of the tumor. In the low-grade portions (e), cellular areas are comprised of large, scattered neoplastic cells with abundant cytoplasm and large nuclei with very prominent nucleoli mimicking Reed-Sternberg cells (arrow) amidst numerous inflammatory cells, mostly lymphocytes. (f) High-grade undifferentiated component comprised of sheets of closely spaced pleomorphic cells with marked nuclear atypia and aberrant mitotic figures (arrow), which represent the majority of the tumor. The pathologic presentation was best classified as a dedifferentiated MFS
A 60-year-old male with MFS of the left middle finger. An AP radiograph (a) shows a lucent lesion in the distal phalanx (arrowhead) with an associated pathologic fracture (arrow). The lateral radiograph (b) shows that the lesion has caused cortical destruction in the volar aspect of the phalanx (arrowhead). Sagittal T2 fat-suppressed MR images (T1-weighted sequences: TR/TE 478–615/15, T2-weighted sequences: TR/TE 3000–4050/54–60) show a high T2 signal mass (arrowhead) in the volar aspect of the distal phalanx (c). On a T1-weighted, fat-suppressed, post-contrast image (d), the majority of the mass on the volar aspect (arrowhead) has thin peripheral enhancement, with a nodular component undergoing more diffuse enhancement dorsally (arrow). The corresponding H&E stained scanning-power micrograph (e) depicts distal phalangeal osseous invasion by the tumor. The portion of the tumor situated beneath the fingernail (arrow) was low grade and rich in myxoid matrix (arrowhead), which corresponds to the nodular enhancing component in (d), while the osteoinvasive component was high grade, hypercellular, and darker in color (star), corresponding to the peripherally enhancing component in (d)
A 69-year-old female with MFS of the ulnar aspect of the left hand. An axial T2 fat-suppressed MR image (T1-weighted sequences: TR/TE 683–1233/10–11, T2-weighted sequences: TR/TE 3750–4366/110–111) (a) shows an ill-defined area of high signal along the ulnar aspect of the hand (arrowhead). An axial T1-weighted, fat-suppressed, post-contrast MR image (b) shows diffuse enhancement of the lesion (arrowhead). While much of the tumor had classic features of low-grade myxoinflammatory fibroblastic sarcoma on the H&E stained pathologic images, including (c) strands of spindle cells within pale-blue myxoid matrix and lymphocytic inflammation, other areas had high-grade features (d) such as increased cellularity, nuclear enlargement, and numerous atypical mitotic figures (arrow)
A 48-year-old female with MFS of the left thumb. An axial T2 fat-suppressed MR image (T1-weighted sequences: TR/TE 240–400/6–13, T2-weighted sequences: TR/TE 4550–5650/47–71, PD-weighted sequence: TR/TE 3200/34) (a) shows diffuse high signal within the lesion (arrowhead). On the T1 fat-suppressed post-contrast MR image (b), there is diffuse post-contrast enhancement (arrowhead). The lesion is immediately adjacent to the extensor tendon (b, arrow); there is no osseous involvement
A 66-year-old female with MFS of the right middle finger. Coronal MR images (T1-weighted sequences: TR/TE 600–686/11–20, T2-weighted sequences: TR/TE 2000–7671/30, PD-weighted sequence: TR/TE 4394/30) show the mass (a, arrowhead) on the radial aspect of the proximal phalanx with predominantly low signal on T1-weighted images but some areas of high signal (a, arrow), diffuse heterogeneous high signal on STIR images (b, arrowhead), and relatively diffuse post-contrast enhancement on T1-weighted, fat-suppressed, post-contrast images (c, arrowhead). The mass is in proximity to the proximal phalanx but without osseous involvement
A 43-year-old female with MFS of the left ankle. On MR images (T1-weighted sequences: TR/TE 433–933/10, T2-weighted sequences: TR/TE 3000–4150/47–48), the mass is comprised of multiple lobulations with low signal on sagittal T1-weighted images (a, arrowhead), high signal on sagittal STIR images (b, arrowhead), and diffuse lobular post-contrast enhancement on coronal T1 fat-suppressed post-contrast images (c, arrowheads). There is close association between the multilobulated mass and extensor tendons (c, arrows). An H&E-stained low-power micrograph (d) demonstrates the classic architectural features of myxoinflammatory fibroblastic sarcoma, consisting of a lobular arrangement of hypocellular myxoid areas (arrow) alternating with inflamed cellular areas (arrowhead). Note the infiltrative interface with adipose tissue
A 30-year-old male with MFS of the palmar aspect of the hand. Axial STIR MR image (T1-weighted sequences: TR/TE 433–933/10, T2-weighted sequences: TR/TE 3000–4150/47–48) (a) shows a lobulated mass (arrowhead) with diffuse high signal in the palmar aspect of the hand. The mass abuts multiple tendon sheaths (arrows). Sagittal T1-weighted, fat-suppressed, post-contrast image (b) shows heterogeneous but diffuse enhancement of the mass (arrowhead). A gross photograph of the amputation specimen (c) depicts a tan and glistening tumor with lobular architecture, which infiltrates and entraps adjacent adipose tissue (arrow). At high-power magnification with H&E stain (d), the myxoid areas contain abundant pale-blue extracellular matrix admixed and a sparse population of irregular cells with ample cytoplasm. Individual cells contain vacuoles of phagocytized myxoid matrix forming “pseudolipoblasts” (arrow) consistent with MFS
Ultrasound images available for case 1 showed an ovoid hypoechoic mass with lobulated margins centered in the subcutaneous tissues of the distal posterior aspect of the right upper arm (Fig. 1a). There was slight posterior acoustic enhancement but no invasion of the underlying fascia. There was markedly increased vascularity on color Doppler imaging (Fig. 1b).
Soft tissue swelling corresponding to the palpable mass with accompanying extensive osseous destruction and pathologic fracture involving the third digit distal phalanx were noted on the radiographs in case 2 (Fig. 2). Radiographs in case 7 demonstrated a nonspecific soft tissue mass without osseous involvement.
MR imaging characteristics are summarized in Table 1. On MRI, most lesions had low signal on T1-weighted images and high signal on T2-weighted images. Most notable was the variable post-contrast enhancement: Case 1 demonstrated a prominent central, nonenhancing component with predominantly linear and some nodular peripheral enhancement (Fig. 1). In case 2, there was a variable enhancement pattern with a more superficial nodular, diffusely enhancing component and a component with peripheral enhancement, which corresponded to the area of osseous involvement (Fig. 2). In cases 3–7, relatively diffuse enhancement was noted (Figs. 3, 4, 5, 6 and 7).
Pathology review
Pathology review revealed dedifferentiation in case 1. Although the majority of the tumor consisted of high-grade pleomorphic sarcoma (Fig. 1f), there were distinct areas of well-differentiated MFS consisting of myxoid lobules and fibrous zones containing numerous inflammatory cells and only scattered ganglion-like cells (Fig. 1e). There was sharp demarcation between the low- and high-grade areas consistent with dedifferentiation. Edematous fibromyxoid stroma with ectatic thin-walled vessels, capillaries, increased distance between the collagen consistent with edema, and dispersed lymphocytes were noted surrounding the periphery of the tumor capsule. There were nodules of low-grade tumor in the periphery as well with myxoid matrix, vascularity, and edema, but not to the degree of the fibromyxoid stroma. The low-grade tumor was relatively rich in myxoid matrix with edema and vascularity, while the high-grade tumor was more cellular.
In cases 2 and 3, there were areas of high-grade tumor without dedifferentiation. In case 2, pathology showed a soft tissue mass with focal areas of necrosis. The tumor was classified as an MFS of the distal extremity, grade 2/3 according to the FNCLCC grading criteria [12]. In addition to classic hypocellular myxoinflammatory areas, there were hypercellular nodules consisting of closely spaced neoplastic cells and atypical mitotic figures admixed with numerous lymphocytes and background devoid of myxoid and fibrous matrix. The high-grade hypercellular areas were mostly present within bone, while the myxoinflammatory areas were mostly in the soft tissue (Fig. 2e). Review of the pathology at our institution for case 3 confirmed a predominantly low-grade MFS with a focal area of hypercellularity and high-grade histology (Fig. 3).
In cases 4–7, a diagnosis of MFS was made at our institution, without evidence of high-grade tumor or dedifferentiation.
Clinical data
Clinical data were reviewed for each case and are summarized in Table 2. Our patient population included three males and four females with ages ranging from 30 to 69 years. The interval between the initial symptoms and patient presentation ranged from 2 weeks to 2 years; the patient with osseous destruction (case 2) had had 2 years of symptoms prior to presentation. The patients had between 4 months and 12 years of disease-free clinical and imaging follow-up. While cases 1 and 3 required re-resection because of residual tumor at presentation to our institution, there have been no known recurrences after treatment at our institution to date.
Discussion
MFS is a rare soft tissue tumor that arises most commonly but not exclusively in the distal extremities. The tumor usually occurs during the 4th or 5th decade of life, although occurrences in patients ranging from ages 4–87 years old have been reported. There is no gender predilection [5]. Most patients report a painless mass or swelling at the site of the tumor. The median size at presentation is 3 cm [13]. While MFS most often occurs in the distal extremities and the neoplasm’s name included the term “acral” until 2002 [14], up to one-third of cases occur at other sites [5]. In 2013, the World Health Organization introduced the term “atypical myxoinflammatory fibroblastic tumor” for these lesions [15]. Tumors involving the distal acral sites show a strong predilection for the dorsal aspect of the extremity [4].
The differential diagnosis of acral lesions includes giant cell tumor of the tendon sheath, glomus tumor, and epidermal inclusion cyst, among others. These entities are much more common than MFS, and while there are some distinguishing imaging features, these entities can provide a diagnostic dilemma. For example, while giant cell tumor of the tendon sheath is associated with a tendon, like most of our MFS cases, the typical low signal seen on T2-weighted images in giant cell tumors of the tendon sheath is not seen in MFS. Glomus tumor and epidermal inclusion cysts both have low signal on T1-weighted images and high signal on T2-weighted images, and glomus tumors enhance diffusely, while epidermal inclusion cysts tend to have thin peripheral enhancement. While distinctions can sometimes be made based on the location (glomus tumors in the subungual region and epidermal inclusion cysts near the skin with a tract to the surface), MFS can also involve these areas of the fingers. This emphasizes the need for caution when assigning a diagnosis to an acral lesion, and MFS should be kept in the differential diagnosis.
The six cases of pathologically - proven MFS without dedifferentiation diagnosed at our institution occurred in two males and four females between the ages of 30 and 69 years old. Presenting symptoms in all cases were variable. All of these tumors were located in the distal extremities, and sizes ranged from 1.1 to 4.9 cm in maximum dimension. On pathologic evaluation, four cases had a histologically well-differentiated appearance, while two cases had components of hypercellular high-grade tumor. One of these two cases invaded the distal phalanx, suggesting a more aggressive lesion. However, these tumors had histologically less abrupt, more gradual transitions between low- and higher-grade areas, unlike the case with frank dedifferentiation, in which there was an abrupt transition from low-grade areas to areas with dedifferentiated sarcoma. The case of dedifferentiated MFS occurred in a 69-year-old male, slightly older than the age group typically described in the literature. Unlike other cases in the current series and similar to prior reports with dedifferentiation [6], the presented case did not occur in an acral location. These varying histologic findings confirm the presence of a spectrum of MFS ranging from well-differentiated, low-grade tumors and well-differentiated, high-grade areas to dedifferentiated tumors.
Literature review of previously described MFS cases yields a variable imaging presentation [5, 7–9, 11, 16–19]. Radiographs sometimes reveal a soft tissue mass. Osseous involvement is rare and has been noted in only three cases: two with only subtle erosive changes in the digits [7, 10] and one showing extensive osseous invasion into the femur [8]. Prior reports of MRI appearances describe the tumor as ranging from a well-defined to poorly circumscribed mass. Most cases have low signal compared to skeletal muscle on T1-weighted pre-contrast images. A slight majority has high signal compared to muscle on T2-weighted images, while other cases have mixed signal on T2-weighted images [7–11, 16, 18, 19]. Low signal on T2-weighted images has not been described. More than half of the lesions show diffuse post-contrast enhancement on T1-weighted, fat-suppressed images, and most of the remaining lesions show heterogeneous post-contrast enhancement; one reported tumor showed no post-contrast enhancement [7–11, 16, 18, 19]. Half of the previously reported cases with an accompanying imaging review were described occurring in proximity to or involving a tendon sheath [7–11, 16, 18, 19].
The imaging findings of the well-differentiated MFS cases from our institution mostly followed the patterns previously described in the literature (Table 1). The well-differentiated MFS cases showed variable increased signal on T2-weighted sequences and decreased signal on T1-weighted sequences, as described in prior studies. Of note, feathery increased T1 signal was noted in one case, potentially mimicking a vascular lesion with internal fat. The morphology of these lesions included well-defined, ill-defined, and multilobular masses. Proximity to a tendon was present in a majority (n = 4) of the well-differentiated tumors. The acral distribution, proximity to tendons, and appearance led to erroneous initial diagnosis in three of the well-differentiated MFS cases (cases 3, 5, and 6) presented in this series.
The dedifferentiated tumor was notable for not being associated with a tendon on MRI, unlike the majority of other cases in our series. In addition, the dedifferentiated tumor was remarkable for predominantly peripheral enhancement with several subtle, enhancing projections in the surrounding soft tissues. The enhancement pattern in the majority of well-differentiated MFS was diffuse, without a peripheral pattern of enhancement. An exception was case 2, a well-differentiated tumor with a high-grade tumor component. In this case, there was accompanying osseous destruction with intraosseous high-grade tumor on histology, which corresponded to a peripherally-enhancing intraosseous component on MRI. The osseous involvement is an uncommon finding in MFS and is likely explained by the high-grade histology.
Correlation between the pathology and post-contrast MR images revealed that the peripheral enhancing component in case 1 correlated with pericapsular, edematous, fibromyxoid stroma with ectatic thin-walled vessels and capillaries, increased distance between collagen consistent with edema, and dispersed lymphocytes surrounding the periphery of the tumor capsule. There were low-grade tumor nodules in the periphery as well with vascularity and edema, but not to the degree of the fibromyxoid stroma. The nonenhancing area in case 1 corresponded to the dedifferentiated tumor and high-grade component, which were highly cellular in contrast to other tumor areas. Similarly, in case 2, the high-grade area that involved bone showed only thin peripheral enhancement because of its high cellularity and lack of vascularity, while the more nodular enhancing area was low - grade with increased vascularity and myxoid stroma. In case 3, the high-grade component was small, and the enhancement pattern was representative of the predominantly low-grade tumor. Thus, based on these presented MFS cases, post-contrast enhancement appears to correspond to fibromyxoid tissue or myxoid low-grade tumor, while areas without enhancement correspond to the cellular, higher-grade and/or dedifferentiated tumor. This observation may have an impact on imaging evaluation as well as planning of soft tissue sampling.
Conclusion
Myxoinflammtory fibroblastic sarcoma represents a spectrum of disease including well-differentiated, high-grade, and dedifferentiated sarcomas with variable imaging findings. Peripheral enhancement, non-acral location, and lack of association with a tendon may indicate high-grade foci or dedifferentiation. However, larger imaging studies are needed to confirm these findings. Radiologists should consider MFS in the differential diagnosis of distal extremity lesions and should be aware of the presence of a histologic spectrum as misdiagnosis of these lesions as benign entities can lead to incomplete excision.
References
Meis-Kindblom JM, Kindblom LG. Acral myxoinflammatory fibroblastic sarcoma: a low-grade tumor of the hands and feet. Am J Surg Pathol. 1998;22(8):911–24.
Montgomery EA, Devaney KO, Giordano TJ, Weiss SW. Inflammatory myxohyaline tumor of distal extremities with virocyte or Reed-Sternberg-like cells: a distinctive lesion with features simulating inflammatory conditions, Hodgkin’s disease, and various sarcomas. Mod Pathol. 1998;11(4):384–91.
Michal M. Inflammatory myxoid tumor of the soft parts with bizarre giant cells. Pathol Res Pract. 1998;194(8):529–33.
Laskin WB, Fetsch JF, Miettinen M. Myxoinflammatory fibroblastic sarcoma: a clinicopathologic analysis of 104 cases, with emphasis on predictors of outcome. Am J Surg Pathol. 2014;38(1):1–12.
Lombardi R, Jovine E, Zanini N, Salone MC, Gambarotti M, Righi A, et al. A case of lung metastasis in myxoinflammatory fibroblastic sarcoma: analytical review of one hundred and thirty eight cases. Int Orthop. 2013;37(12):2429–36.
Michal M, Kazakov DV, Hadravsky L, Kinkor Z, Kuroda N, Michal M. High-grade myxoinflammatory fibroblastic sarcoma: a report of 23 cases. Ann Diagn Pathol. 2015;19(3):157–63.
Narvaez JA, Martinez S, Dodd LG, Brigman BE. Acral myxoinflammatory fibroblastic sarcomas: MRI findings in four cases. AJR Am J Roentgenol. 2007;188(5):1302–5.
Togral G, Arikan M, Aktas E, Gungor S. Giant myxoinflammatory fibroblastic sarcoma with bone invasion: a very rare clinical entity and literature review. Chin J Cancer. 2014;33(8):406–10.
Lang JE, Dodd L, Martinez S, Brigman BE. Case reports: acral myxoinflammatory fibroblastic sarcoma: a report of five cases and literature review. Clin Orthop Relat Res. 2006;445:254–60.
Chahdi H, Damiri A, Oukabli M, Albouzidi A, Bouabid S, Lazrek K. Acral myxoinflammatory fibroblastic sarcoma. Orthop Traumatol Surg Res. 2010;96(5):597–9.
Tateishi U, Hasegawa T, Onaya H, Satake M, Arai Y, Moriyama N. Myxoinflammatory fibroblastic sarcoma: MR appearance and pathologic correlation. AJR Am J Roentgenol. 2005;184(6):1749–53.
Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchere D, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91(10):1914–26.
Hallor KH, Sciot R, Staaf J, Heidenblad M, Rydholm A, Bauer HC, et al. Two genetic pathways, t(1;10) and amplification of 3p11-12, in myxoinflammatory fibroblastic sarcoma, haemosiderotic fibrolipomatous tumour, and morphologically similar lesions. J Pathol. 2009;217(5):716–27.
Fletcher CD, Unni K, Mertens F. WHO classification of classification of tumors. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2002.
Fletcher CDM, World Health Organization. International agency for research on cancer. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013.
Ertener O, Tuna B, Akcali O, Yorukoglu K. Myxoinflammatory fibroblastic sarcoma: a case report. Acta Orthop Traumatol Turc. 2013;47(6):436–9.
Baheti AD, Tirumani SH, Rosenthal MH, Howard SA, Shinagare AB, Ramaiya NH, et al. Myxoid soft-tissue neoplasms: comprehensive update of the taxonomy and MRI features. AJR Am J Roentgenol. 2015;204(2):374–85.
Kobayashi E, Kawai A, Endo M, Suehara Y, Takeda K, Nakatani F, et al. Myxoinflammatory fibroblastic sarcoma. J Orthop Sci. 2008;13(6):566–71.
Sakaki M, Hirokawa M, Wakatsuki S, Sano T, Endo K, Fujii Y, et al. Acral myxoinflammatory fibroblastic sarcoma: a report of five cases and review of the literature. Virchows Arch. 2003;442(1):25–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
IRB exemption was obtained from our institution. All patient data were obtained using retrospective review of the medical records. No live patient interaction occurred; therefore, informed consent was not required by our IRB and was not obtained.
Rights and permissions
About this article
Cite this article
Gaetke-Udager, K., Yablon, C.M., Lucas, D.R. et al. Myxoinflammatory fibroblastic sarcoma: spectrum of disease and imaging presentation. Skeletal Radiol 45, 347–356 (2016). https://doi.org/10.1007/s00256-015-2286-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-015-2286-2