Abstract
Objective
The purpose of this study was to examine the yield of repeat CT-guided biopsy in patients with suspected infectious spondylodiscitis following an initial biopsy with negative microbiology, and to identify factors associated with successful pathogen isolation.
Materials and methods
In this retrospective study, 21 patients (12 men, 9 women; mean age, 52, range, 12–84) were identified with clinically and radiologically suspected infectious spondylodiscitis who underwent repeat biopsy following negative cultures from an initial biopsy. The microbe yield as well as demographic, clinical, and laboratory findings were reviewed and statistical analysis was performed.
Results
Repeat CT-guided biopsy isolated a causative microbe in 3/21 patients (14.3%). Younger age (p = 0.021) was significantly associated with successful microbe isolation. All three cases of successful microbe isolation occurred in patients not exposed to antibiotics (3/9 patients) whereas no successful microbe isolation occurred in patients who received antibiotics (0/12 patients); however, this difference did not reach statistical significance (p = 0.062). Gender, duration of symptoms, white blood cell count, biopsy interval, and biopsy site were not significantly associated with microbe isolation.
Conclusions
Overall microbiologic yield of repeat CT-guided biopsy for patients with suspected infectious spondylodiscitis was low at 14.3%; however, a higher yield was identified in patients who were younger in age and not exposed to pre-biopsy antibiotics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infectious spondylodiscitis is a serious and morbid disease, accounting for 2–7% of cases of osteomyelitis occurring throughout the body [1]. The majority of cases are the result of hematogenous seeding of the subchondral bone with extension to the intervertebral disc, although cases may also be the result of prior surgery or extension of an adjacent soft tissue infection [2, 3]. The diagnosis of infectious spondylodiscitis is often suspected after magnetic resonance (MR) imaging of the spine [4]. Contemporary treatment involves administration of antimicrobials tailored to the causative organism [4, 5], and the ability to isolate a specific microbe is critical to avoid incorrect antimicrobial treatment for the patient as well as to avoid unnecessary prolonged empiric broad-spectrum antibiotic therapy, which can increase antimicrobial resistance [6, 7]. Blood cultures variably yield a causative organism in 20–78% of patients [8]. In blood culture-negative patients, computed tomography (CT)-guided biopsy has emerged as the method of choice for establishing the diagnosis and isolating the causative organism [9,10,11]; however, the reported yield of image-guided biopsy ranges widely from 19 to 91% [2, 8, 11,12,13,14,15,16], with a recent meta-analysis showing a yield of 48% [17]. It is therefore not uncommon that the radiologist is consulted for repeat spinal biopsy in the setting of an initial biopsy that was unsuccessful in isolating an organism. The appropriate management of these patients is currently unclear. Although expert opinion exists suggesting either repeat CT-guided or open surgical biopsy in this setting [4, 18], that recommendation is based on the clinical importance of identifying the causative agent rather than on robust supporting literature. Some authors propose that these patients should proceed directly to open surgical biopsy [19], although surgery is more invasive than image-guided biopsy and there have been no trials comparing repeat percutaneous biopsy to surgical biopsy.
There is a paucity of data on the yield of repeat CT-guided biopsy, with no prior studies in the radiology literature and no studies using specific imaging findings as inclusion criteria. The purpose of this retrospective study is to evaluate the yield of repeat biopsy following an initial negative biopsy in a population of patients suspected of having infectious spondylodiscitis based on both clinical suspicion and specific findings suggestive of infection on pre-biopsy imaging, and to identify clinical and biopsy-related factors associated with successfully isolating an organism.
Materials and methods
This retrospective study was approved by the institutional review board and informed consent was waived. The radiology information system at a single, urban, tertiary referral academic hospital was searched from January 1995 to December 2016 for reports containing the words “infection”, “discitis”, “osteomyelitis”, or “microbiology”. The results were sorted by medical record number and procedure code. Patients with two or more biopsy procedure codes were identified. Imaging, procedure reports, and medical records were reviewed to identify patients who had undergone two or more biopsy procedures within a period of 6 months. Patients were excluded if the biopsy sites were performed at a site other than the spine, if the biopsy was performed for a noninfectious condition (most commonly malignancy), or if the first biopsy for suspected infectious spondylodiscitis yielded a causative organism.
Image review
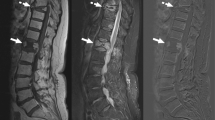
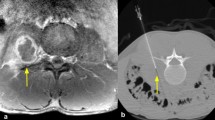
Pre-biopsy MRI or CT was available on all patients, and strict imaging criteria of findings characteristic of infectious spondylodiscitis [20, 21] were used as inclusion criteria. For MRI, these findings included vertebral bone marrow edema on T2-weighted images, bone marrow hypointensity on T1-weighted images, endplate erosion or destruction, disc hyperintensity on T2-weighted images or disc destruction, paravertebral inflammation, and (when applicable) abnormal contrast enhancement (Figs. 1a–c and 2a–c). For CT, which was utilized in three patients unable to undergo MRI, these findings included endplate erosion or destruction, disc morphologic abnormality or heterogeneity, and paravertebral inflammation. MR or CT imaging was not routinely repeated prior to repeat biopsy. Biopsy images were reviewed on each patient to determine the site biopsied, type of tissue biopsied, and side of each biopsy (Figs. 1d, e and 2e, f).
A 41-year-old male (patient 21) with suspected infectious spondylodiscitis at L3–4 with no organism isolated on initial or repeat biopsies; however, with Streptococcus parasanguinis isolated from L5-S1 6 months after the repeat biopsy. a Sagittal short tau inversion recovery image shows loss of disc height, bone marrow edema-like signal, and endplate irregularity (arrows). Edema-like signal extends into the posterior elements. b Corresponding sagittal T1-weighted image shows abnormal marrow replacement and endplate indistinctness due to erosion (arrows). c Corresponding sagittal T1-weighted, fat-saturated, post-gadolinium image shows abnormal endplate and intra-osseous enhancement as well as prevertebral enhancement (arrows). d Axial image from initial CT-guided biopsy shows core-biopsy needle sampling bone and disc material. e Axial image from repeat CT-guided biopsy performed 16 days after initial biopsy shows core-biopsy needle sampling bone and disc material at essentially the same site
A 12-year-old male (patient 3) with suspected infectious spondylodiscitis at L1–2 with Salmonella typhi isolated on repeat biopsy. a Sagittal T2-weighted, fat-saturated image shows loss of disc height, bone marrow edema-like signal, and endplate irregularity (arrows). b Corresponding sagittal T1-weighted image shows abnormal marrow replacement and endplate indistinctness due to erosion. c Corresponding sagittal T1-weighted, fat-saturated, post-gadolinium image shows abnormal endplate, disc space, and intra-osseous enhancement as well as prevertebral enhancement (arrows). d Axial T1-weighted, fat-saturated post-contrast image shows right paravertebral enhancing phlegmon (arrows), which was targeted for initial biopsy. e Axial image from initial CT-guided biopsy shows core-biopsy needle sampling paraspinal soft tissue inflammation (arrows denote enlargement of psoas muscle due to phlegmon). f Axial image from repeat CT-guided biopsy performed 19 days after initial biopsy shows core-biopsy needle within the bone and extending to disc margin
Biopsy technique
Percutaneous biopsy was performed by fellowship-trained musculoskeletal radiologists under CT guidance using standard departmental techniques. Prior to the first biopsy, a complete blood count, activated partial thromboplastin time, and prothrombin time was measured. Patients were routinely provided intravenous conscious sedation and continuous procedural monitoring was performed with pulse oximetry, cardiac tracing, and an automated cycling blood pressure cuff. Coaxial core biopsy samples were obtained in each case and the specific biopsy equipment used was dependent on the planned sampling site (bone, disc, and/or soft tissue) and preference of the radiologist, with needle sizes ranging from 13 gauge to 20 gauge used to obtain samples. Biopsy samples were submitted to microbiology in all cases, and when technically safe and feasible core biopsy samples were submitted for histopathology at the discretion of the radiologist. Multiple biopsy passes were obtained during each procedure, although the exact number of passes was not regularly recorded. The presence of any immediate procedural complication was recorded.
Microbiology
Biopsy samples were sent for aerobic and anaerobic bacterial culture, fungal culture, and mycobacterial culture. No polymerase chain reaction (PCR) assay was performed on tissue obtained from repeat CT-guided biopsy. After repeat biopsy was performed, one patient underwent open surgical biopsy after which identified Propionibacterium acnes RNA using a polymerase chain reaction assay.
Clinical data collection
For each patient, the electronic medical record or paper charts were reviewed to collect data on patient age, gender, duration of symptoms, biopsy date, level of biopsy recorded by report, microbiology results, histopathology results, the presence of antibiotic treatment prior to or following the initial biopsy, white blood cell count, ESR, and CRP.
Clinical outcome
Electronic medical record or paper chart review was performed to confirm that patients met criteria for clinically suspected infectious spondylodiscitis. This included pre-biopsy clinical suspicion for infection, a biopsy sample which did not elucidate another cause of the patient’s symptoms (such as malignancy), a post-biopsy treatment regimen including antimicrobials for suspected infectious spondylodiscitis, and post-biopsy clinical follow-up which showed improvement of clinical and imaging findings. In each case, no alternative final diagnosis was established.
Statistics
Descriptive statistics were calculated for age, gender, and biopsy yield. Correlation between categorical data (gender, antibiotic exposure, same vs. different biopsy site) and biopsy microbe yield was calculated using Fisher’s exact test. Correlation between numerical data (age, white blood cell count, biopsy interval, ESR, CRP) and biopsy yield was calculated using logistic regression. A p < 0.05 was considered statistically significant. Statistical software (JMP Pro 13.0, SAS Institute, Cary, NC, USA) was used to perform all statistical analyses.
Results
Over a 21-year period, 21 patients with suspected infectious spondylodiscitis underwent a repeat CT-guided biopsy following an initial negative biopsy (Table 1). The mean age was 53.0 years (SD ± 19.7) and 12/21 patients (57.1%) were male. The mean duration of symptoms was 80.9 days (range, 10–176; SD ± 55.7). The mean time between the first and second biopsies was 16.7 days (range, 2–79; SD ± 20.54), and the median time between biopsies was 9 days. Biopsy procedures occurred in the thoracic spine in four cases and in the lumbar spine in 17 cases. Repeat biopsy was performed at a different site at the same spinal level in 17 cases, at the same site at the same spinal level in three cases, and at an adjacent level in one patient who presented initially with contiguous two-level disease. No complications were encountered.
Culture and Gram stain results were negative for all 21 initial biopsies. Repeat biopsy isolated an organism in 3/21 cases (14.3%) (Table 1). Organisms isolated were Staphylococcus aureus (in culture), Salmonella typhi (in culture), and Gram-negative rods (on Gram stain, although culture negative). Antibiotics were tailored to the microbe isolated in each case, and antibiotics were utilized to cover suspected causative Gram-negative rod bacteria in the case of Gram stain positive but culture negative results.
In patients not receiving antibiotics, the microbe yield was 3/9 (33.3%); however, in patients who received antibiotics, the yield was 0/12 (0%). For each case in which a microbe was isolated, no antibiotics had been given prior to the first or second biopsies. This relationship did not reach statistical significance (p = 0.063, Table 2)
.
The mean age of patients with positive microbe yield from repeat biopsy was significantly lower than those with negative yield (31.3 ± 19.5 years vs. 56.6 ± 17.8 years, p = 0.021) (Table 2).
No significant association was identified between successful organism isolation and gender, duration of symptoms, white blood cell (WBC) count, biopsy interval, or same vs. different biopsy site (Table 2). The majority of patients did not have erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) drawn prior to the repeat biopsy.
As a secondary endpoint, the number of confirmed diagnoses of infectious spondylodiscitis was recorded. The diagnosis was confirmed if either culture or histopathology was positive. A diagnosis of infectious spondylodiscitis was established on initial biopsy by means of histopathology in 3/21 cases (14.3%), with all cultures negative. Repeat biopsy, yielding positive microbiology and/or histopathology in four additional patients whose results were negative on the initial biopsy, increased the overall rate of diagnosis of infectious spondylodiscitis to 7/21 cases (33.3%). Histopathology samples were not processed on 5/21 initial biopsies and 9/21 repeat biopsies, and in those cases only microbiology samples were provided. Interestingly, 2/3 patients with positive microbiologic yield upon repeat biopsy had positive histopathology on the initial biopsy.
Four patients in which no microbe was isolated from either biopsy had subsequent procedures: patient 1 underwent a third biopsy for recurrent back pain after antibiotic treatment with negative biopsy results, patient 12 underwent an open biopsy yielding Aspergillus fumigatus, patient 20 underwent open biopsy at another spinal level yielding Propionibacterium acnes RNA, and patient 21 underwent biopsy at a new level of disease yielding Streptococcus parasanguinis.
Discussion
Initial percutaneous biopsy of suspected infectious spondylodiscitis has a yield of < 50% [17]. For negative cases in which a repeat biopsy is contemplated, it is important to consider that inability to isolate the causative organism may have resulted from multiple factors including biopsy at sites devoid of living organisms, small biopsy sample size, treatment of organisms with pre-biopsy antimicrobials, low-grade infection, or inability to grow fastidious organisms [2, 10, 22,23,24]. The available data on biopsy negative patients should be interpreted in light of these considerations.
Our data show that for patients with infectious spondylodiscitis based on clinical and imaging features, a repeat biopsy identified a causative organism in 14.3% of cases. This yield is lower than the reported yield for initial biopsies, which is not unexpected given that this population of patients is likely impacted by factors or organisms that are associated with lower culture yield. In fact, three patients with negative initial and repeat biopsies were ultimately found in subsequent biopsy procedures to have infections with organisms - Streptococcus parasanguinis, Propionibacterium acnes, and Aspergillus fumigatus, which may be difficult to isolate in culture from biopsy [25,26,27]. Therefore, it may be prudent to consider adding additional testing, such as polymerase chain reaction assays, to standard culture analysis in order to maximize yield.
In a population already likely enriched for a lower diagnostic yield, it is important to identify factors that are associated with positive yield. We found a larger microbe yield in patients who had received no antibiotic therapy. In fact, no biopsy performed on a patient exposed to pre-biopsy antibiotics yielded a causative organism in our study. In this small study, this relationship did not reach statistical significance. The effect of pre-biopsy antibiotics on microbial yield in the literature is controversial. Some authors have reported a lower yield from initial biopsy in patients with suspected infectious spondylodiscitis who received pre-biopsy antibiotics [10, 11, 13, 28, 29]; however, other authors have found no difference in yield between patients who had received antibiotics and those who have not [8, 16, 30]. A recent meta-analysis [17] found a lower microbe yield from image-guided spine biopsy if patients had received antibiotics; however, the difference did not reach statistical significance and included studies were heterogeneous. None of the above studies stratified patients by the severity of infection, and it is possible that patients with severe infections may have higher bacterial load and higher microbiology yield even if treated with antibiotics [8]. Thus, if patients were treated with pre-biopsy antibiotics due to severe infection or septic shock, which is advised by expert opinion [4, 18], it is theoretically possible that microbiologic yield of a repeat biopsy may be less affected, although further studies are necessary to address this. Using our data set it would be difficult to justify percutaneous biopsy on patients who have received antibiotic therapy; however, our sample size is small and this topic remains controversial.
Some potential factors which could theoretically affect culture yield were unable to be assessed by this study. It is possible that sites devoid of living organisms were sampled. In fact, one case showing Gram-negative rods on Gram stain did not yield an organism in culture. It is possible that polymerase chain reaction assays could increase yield, although such testing was not performed in this study and has not been routinely performed in many prior studies examining microbial yield of spinal biopsy. The effect of biopsy sample size on yield could not be assessed on this study, as the number of biopsy passes was not recorded in each case.
Younger patient age was also found in our study to be associated with successful microbe isolation. The explanation for this association is unclear; however, this has been reported previously [31].
Another interesting finding in our study is the predictive effect of initial biopsy positive histopathology on repeat biopsy microbe isolation. Although only three patients were found to have positive histopathology from initial biopsy, two of those three had successful microbe isolation on repeat biopsy. It is difficult to derive any conclusions from this small number of patients and note is made that histopathology samples were not sent in all patients; however, this could be addressed in future studies.
Several prior studies have examined the yield of repeat percutaneous biopsy for infectious spondylodiscitis in the setting of an initial negative biopsy. Kim et al. [32] performed repeat fluoroscopy-guided biopsy on 16 patients ultimately proven to have infection, identifying an organism in 12.5% (2/16) of cases in which the initial biopsy was unrevealing and recommending against repeat biopsy in this setting. They report that “in principle” a biopsy was repeated if initial results were negative after 1 week while holding antibiotics, although the use of antibiotics was not specifically recorded. Gras et al. [31] performed repeat CT-guided biopsy on 33 patients with vertebral osteomyelitis, yielding positive results in 39.4% (13/33), ultimately concluding that repeat percutaneous biopsy could be an alternative to open biopsy. Patients who received prior antibiotics were excluded from their analysis and the effect of antibiotic use on repeat biopsy yield therefore could not be assessed. In our experience, it is not uncommon for a repeat biopsy request to be made in patients who had been exposed to recent antibiotics, and it was felt important to include this subset of patients in order to both evaluate the effect of antibiotics on microbe yield and to establish a study population representative of our experience in this clinical situation. Interestingly, our data show that if patients exposed to antibiotics are excluded, the yield of repeat biopsy is 33.3% (3/9), which is similar to the yield of 39.4% reported by Gras et al. Terreaux et al. [33] found the yield of repeat CT and/or fluoroscopic biopsy in ten patients to be 60% (6/10), concluding that repeat biopsy is helpful; however they did not examine the effect of antibiotics on biopsy yield. Finally, Friedman et al. [34] reported a yield for initial percutaneous biopsy of 52.5% (21/40) and considering the results of all attempted biopsies, the yield increased to 73% overall; however, details about the repeated biopsy attempts including the number of biopsies per case was not documented. The studies discussed here notably have heterogeneous inclusion criteria, with no studies documenting specific MR or CT imaging findings for inclusion criteria as utilized in the current study.
This study has several limitations. As a retrospective study, it is subject to inherent bias and potential confounding. The small sample size results in an underpowered statistical analysis. The inclusion criteria of “suspected osteomyelitis” was based on a combination of clinical and imaging parameters intended to include patients likely to have infectious spondylodiscitis; however, we acknowledge that it is not possible to confirm that infection is present in each case that is not biopsy proven. As a tertiary care center, it is common for patients to be transferred from an outside facility, and it may not be possible to confirm the presence or absence of antibiotic administration prior to arrival at our hospital. Furthermore, it would be ideal to be able to determine if the number of core samples affected biopsy yield; however, the exact number of passes was not recorded in each case. This study spans a long period of time and it is possible that the evolution of biopsy technique and equipment could affect results. Finally, this study included traditional culture-based microbiology analysis to identify pathogens, and it is possible that a higher yield may be obtained with newer and evolving molecular assays.
In conclusion, we found an overall low rate of organism isolation from repeat CT-guided biopsy in patients with suspected infectious spondylodiscitis following an initial negative biopsy. This raises questions about the broad utility of this procedure for all patients with an initial negative biopsy; however, a higher yield was identified in patients of younger age and those who had no pre-biopsy antibiotic treatment. These factors are among those to be considered when selecting patients for repeat biopsy.
References
Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM. Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine. 2006;31(23):2695–700.
Michel SCA, Pfirrmann CWA, Boos N, Hodler J. CT-guided core biopsy of subchondral bone and intervertebral space in suspected spondylodiskitis. Am J Roentgenol. 2006;186(4):977–80.
Fouquet B, Goupille P, Jattiot F, Cotty P, Lapierre F, Valat JP, et al. Discitis after lumbar disc surgery. Features of “aseptic” and “septic” forms. Spine. 1992;17(3):356–8.
Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26–46.
Yang SC, Fu TS, Chen LH, Chen WJ, Tu YK. Identifying pathogens of spondylodiscitis: percutaneous endoscopy or CT-guided biopsy. Clin Orthop Relat Res. 2008;466(12):3086–92.
Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(SUPPL. 3).
Medicine I of. The Resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment -- Workshop Summary [Internet]. Knobler SL, Lemon SM, Najafi M, Burroughs T, editors. Washington, DC: The National Academies Press; 2003. Available from: https://www.nap.edu/catalog/10651/the-resistance-phenomenon-in-microbes-and-infectious-disease-vectors-implications.
Marschall J, Bhavan KP, Olsen MA, Fraser VJ, Wright NM, Warren DK. The impact of prebiopsy antibiotics on pathogen recovery in hematogenous vertebral osteomyelitis. Clin Infect Dis. 2011;52(7):867–72.
Rimondi E, Staals EL, Errani C, Bianchi G, Casadei R, Alberghini M, et al. Percutaneous CT-guided biopsy of the spine: results of 430 biopsies. Eur Spine J. 2008;17(7):975–81.
Kim CJ, Song KH, Park WB, Kim ES, Park SW, Kim H. Bin, et al. Microbiologically and clinically diagnosed vertebral osteomyelitis: impact of prior antibiotic exposure. Antimicrob Agents Chemother. 2012;56(4):2122–4.
Enoch DA, Cargill JS, Laing R, Herbert S, Corrah TW, Brown NM. Value of CT-guided biopsy in the diagnosis of septic discitis. J Clin Pathol. 2008;61(6):750–3.
Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218(1):211–4.
Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J. 2004;80(948):607–9.
D’Agostino C, Scorzolini L, Massetti AP, Carnevalini M, D’Ettorre G, Venditti M, et al. A seven-year prospective study on spondylodiscitis: epidemiological and microbiological features. Infection. 2010;38(2):102–7.
Garg V, Kosmas C, Young PC, Togaru UK, Robbin MR. Computed tomography–guided percutaneous biopsy for vertebral osteomyelitis: a department’s experience. Neurosurg Focus. 2014;37(2):E10.
Joo EJ, Yeom JS, Ha YE, Park SY, Lee CS, Kim ES, et al. Diagnostic yield of computed tomography-guided bone biopsy and clinical outcomes of tuberculous and pyogenic spondylitis. Korean J Intern Med. 2016;31(4):762–71.
McNamara AL, Dickerson EC, Gomez-Hassan DM, Cinti SK, Srinivasan A. Yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38(10):2021–7.
Grados F, Lescure FX, Senneville E, Flipo RM, Schmit JL, Fardellone P. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine. 2007;74:133–9.
Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362(11):1022–9.
Dagirmanjian A, Schils J, McHenry MC. MR imaging of spinal infections. Magn Reson Imaging Clin N Am. 1999;7(3):525–38.
Hong SH, Choi J-Y, Lee JW, Kim NR, Choi J-A, Kang HS. MR imaging assessment of the spine: infection or an imitation? Radiographics. 2009;29(2):599–612.
Wu JS, Gorbachova T, Morrison WB, Haims AH. Imaging-guided bone biopsy for osteomyelitis: are there factors associated with positive or negative cultures? AJR Am J Roentgenol. 2007;188(6):1529–34.
Bhagat S, Mathieson C, Jandhyala R, Johnston R. Spondylodiscitis (disc space infection) associated with negative microbiological tests: comparison of outcome of suspected disc space infections to documented non-tuberculous pyogenic discitis. Br J Neurosurg. 2007;21(5):473–7.
Gillard J, Boutoille D, Varin S, Asseray N, Berthelot JM, Maugars Y. Suspected disk space infection with negative microbiological tests—report of eight cases and comparison with documented pyogenic discitis. Joint Bone Spine. 2005;72:156–62.
Doern CD, Burnham CAD. It’s not easy being green: the viridans group streptococci, with a focus on pediatric clinical manifestations. J Clin Microbiol. 2010;48:3829–35.
Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. Biomed Res Int. 2013;2013:1–10.
Sethi S, Kalra K, Siraj F, Chopra P. Aspergillus vertebral osteomyelitis in immunocompetent patients. Indian J Orthop. 2012;46(2):246.
Hassoun A, Taur YSC. Evaluation of thin needle aspiration biopsy in the diagnosis and management of vertebral osteomyelitis (VO). Int J Infect Dis. 2006;10(6):486–7.
Marco de Lucas E, González Mandly A, Gutiérrez A, Pellón R, Martín-Cuesta L, Izquierdo J, et al. CT-guided fine-needle aspiration in vertebral osteomyelitis: true usefulness of a common practice. Clin Rheumatol. 2009;28(3):315–20.
Agarwal V, Wo S, Lagemann GM, Tsay J, Delfyett WT. Image-guided percutaneous disc sampling: impact of antecedent antibiotics on yield. Clin Radiol. 2016;71(3):228–34.
Gras G, Buzele R, Parienti JJ, Debiais F, Dinh A, Dupon M, et al. Microbiological diagnosis of vertebral osteomyelitis: relevance of second percutaneous biopsy following initial negative biopsy and limited yield of post-biopsy blood cultures. Eur J Clin Microbiol Infect Dis. 2014;33(3):371–5.
Kim BJ, Lee JW, Kim SJ, Lee GY, Kang HS. Diagnostic yield of fluoroscopy-guided biopsy for infectious spondylitis. AJNR Am J Neuroradiol. 2013;34(1):233–8.
Terreaux W, Geoffroy M, Ohl X, Job L, Cart P, Eschard JP, et al. Diagnostic contribution of a second percutaneous needle biopsy in patients with spontaneous diskitis and negative blood cultures and first biopsy. Jt Bone Spine. 2016;83(6):715–9.
Friedman JA, Maher CO, Quast LM, McClelland RL, Ebersold MJ. Spontaneous disc space infections in adults. Surg Neurol. 2002;57(2):81–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Czuczman, G.J., Marrero, D.E., Huang, A.J. et al. Diagnostic yield of repeat CT-guided biopsy for suspected infectious spondylodiscitis. Skeletal Radiol 47, 1403–1410 (2018). https://doi.org/10.1007/s00256-018-2972-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-2972-y