Abstract
Objective
To determine normative values for volume and fat content of the gluteus medius (GMed) and minimus (GMin) muscle in healthy volunteers and to evaluate their dependence on age, gender and leg dominance.
Materials and methods
The IRB approval was obtained for this study. 80 healthy volunteers (females, 40; males, 40; age range 20–62 years), divided into four age groups, were included. Fat- and water-signal-separated MR images of the pelvis were acquired on a 3.0 T MR with a 3-point mDIXON sequence. Normalized volume and fat-signal fraction (FSF) of the GMed (ViGMed, FSFGMed) and GMin (ViGMin, FSFGMin) muscles were determined.
Results
The overall mean volumes (normalized) and FSF ± SD: ViGMed 105.13 ± 16.30 cm3; ViGMin 30.24 ± 5.15 cm3; FSFGMed 8.13 ± 1.70 % and FSFGMin 9.89 ± 2.72 %. Comparing different age subgroups within each gender no significant differences were found concerning the volumes and FSFs (except FSFGMin in male subgroup aged 20–29 versus 50–62 years, P = 0.014). Comparing FSFs differences between the two genders, only in 20–29 years subgroup, FSFGMed (P =0.003) and FSFGMin (P =0.002) were greater in female. Volume differences between the two legs were not significant (P > 0.077); FSFGMed and FSFGMin (P =0.005 for both) were significantly lower in the dominant leg in female but not in male group (P = 0.454 for FSFGMed and P = 0.643 for FSFMin).
Conclusion
No age dependency was evident for volume normative data for GMed and GMin and normative data for FSF values showed no age- or gender dependency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abductor muscles, gluteus medius (GMed) and minimus (GMin) are central in providing stability and mobility of the hips. Gluteal muscles atrophy and fatty infiltration have been often described in association with specific pathological conditions of the hip structures such as hip osteoarthritis, neuromuscular disorders, degeneration or traumatic rupture of the tendon attachments, iatrogenic injury during hip replacement surgery as well as part of a more generalized loss of muscle volume and fatty infiltration as well-known effects of aging (sarcopenia) and obesity [1–9]. These conditions are considered a major financial burden for the national health care system and the early detection and diagnosis of relevant complications may be critical in establishing the most appropriate management of these patients [8, 10].
Multiple imaging studies were recently published showing association of the afore-mentioned conditions and muscle changes [1–5, 11–14]. Pfirrmann et al. [5] found that fatty infiltration of the GMin muscle is common in asymptomatic patients following total hip arthroplasty, while fatty infiltration of the GMed muscle is almost exclusively observed in symptomatic patients. Kong et al. [12] reported that gluteus muscles atrophy and fatty infiltration represent an indirect sign of gluteal tendinopathy.
Despite these findings, most of the studies published so far, however, have not included healthy subjects. Only in a few reports was a normal population used as control group [2, 11]. Grimaldi et al. [2] showed that the GMed volume in patients with advanced hip osteoarthritis was significantly smaller on the pathological side compared to the contralateral side, but no significant volume difference was present in the control group. Engelken et al. [11] compared patients with gluteal insufficiency and healthy controls and found that, unlike the former, the healthy controls showed no fatty infiltration in the GMed muscle and only a minimal fatty infiltration in the GMin muscle. Nevertheless, these data were not collected in a systematic manner or in a large cohort. Thus, we designed a study to determine normative values for volume and fat content of the GMed and GMin muscle in a large cohort of healthy volunteers of different age subgroups (20–62 years) with normal BMI (body mass index) and to evaluate their dependence on age, gender and leg dominance.
Materials and methods
Study subjects
A total of 80 healthy volunteers (40 females; mean age 39.5 years; age range 20–62 years; 40 men; males age, 39.3 years; age range, 20–61 years) were included in this ethics board approved study and gave their written informed consent. The study was conducted between October 2011 and April 2014.
All healthy volunteers were recruited via intranet announcement in the university hospital’s online portal and by Internet announcement on the homepage of the university’s clinical trial center. Both announcements were approved by the ethics board. Inclusion criteria were: age between 20 and 62; BMI range 18.5–25 kg/m2. Volunteers were divided into four age groups aged between 20 and 29 years, 30 and 39 years, 40 and 49 years and 50 and 62 years. Each age subgroup consisted of 20 people, 10 females and 10 males. Exclusion criteria were: (1) contraindication for MR imaging; (2) previous hip and leg injury or surgery; (3) hip pain; (4) self-reported signs of hip abductors muscle insufficiency (e.g. limping); (5) myotoxic drugs usage (e.g. statins); (6) systemic, neurological, neuromuscular, musculoskeletal, endocrine or metabolic (e.g. diabetes) disorders.
All volunteers were asked to rate their sport activity according to the following levels: (1) no sport activity; (2) moderate (e.g. walking at a moderate or brisk pace for at least 1 h)/vigorous activity (e.g. hiking, jogging, playing soccer) once a week; (3) moderate/vigorous activity at least twice a week. All participants neither at the moment of the MR examination nor in the past reported personal sport history as professional athlete.
Lower limb length and leg dominance
Lower limb length was taken as the tape measure distance from the anterior superior iliac spine to the lateral malleolus on the right side. Functional leg dominance was determined with the balance recovery test. The tester pushed the subject off balance from behind by means of a nudge sufficient to cause a step and applied to the midpoint between the scapulae. We considered the leg that the subject used to recuperate balance as dominant [15].
Body fat estimation directly before MRI
We measured the barefoot standing height with a wall-mounted stadiometer (nearest 0.1 cm); bodyweight (kg) was collected via a standard scale; BMI was calculated from these two measured parameters using: weight/height2 (kg/m2). Circumference measurements were taken at the waist and hip levels and the waist/hip ratio (WHR) was calculated.
The amount of body fat was measured by two different bioelectrical impedance analysis (BIA) instruments via the electrical body resistance with foot sensor pads on a similar device of bathroom-scale (TANITA UM-018, Tanita Corp, Arlington Heights, Ill; BIA1) or with hand sensor pads on a handheld device (OMRON BF300, Omron Healthcare Ltd, Kyoto, Japan; BIA2), respectively. Both measurements were performed for each volunteer. Consistent measuring conditions were maintained for each test.
MR examination
Fat- and water-signal-separated MR images of the pelvis were performed on a clinical 3.0 T MR unit (Ingenia, Philips Healthcare, Best, The Netherlands) using a 3-point mDIXON imaging sequence with a 16 channel posterior coil and two 16 channel anterior coils for signal reception. The scanner’s dual transmit body coil was used for RF transmission. The field-of-view covered both hips from iliac crest to tuber ischiadicum of the participants in supine position with upper arms positioned along the lateral trunk. Other acquisition parameters were: sequence type, 3D FFE T1; number of echoes, 3; orientation, transverse; acquired voxel dimensions (mm), 1.0 × 1.0 × 2.0; reconstructed voxel dimensions (mm), 0.8 × 0.8 × 1.0; inter-slice gap (mm), 0.0; field of view, 560 × 300; number of sections, 210; TR, 9.4 msec; TE, 3.6, 5.3 and 7.0 msec; flip angle, 10°; number of signal averages, 2; SENSE acceleration factor (AP/SI), 2.0 and 1.5; fold-over direction, AP; water-fat shift (pixel), 0.584; receiver bandwidth (Hz pixel−1), 744.0; single series acquisition time (min), 5:55.7.
Imaging analysis
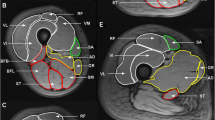
Fat and water images were used to measure muscle volume and mean fat-signal fraction (FSF) values over the whole GMed and GMin muscles, bilaterally. Gluteus muscles of each side were separately analyzed by means of a dedicated software (Myrian1; Intrasense, Paris, France) that provides a semi-automatic segmentation and automatically calculates the volume and mean pixel intensity value within the volume. A musculoskeletal radiology fellow (MM), expert in MRI data post-processing, placed the regions-of-interest (ROIs) along the edges of each muscle on each second slice of the axial water image. Care was taken to include the whole muscle and to exclude partial volume artifacts, tendinous part of the muscles and tissue outside the muscle. A validation, testing intra-observer reliability, was performed for the first ten measurements showing “almost perfect” agreement (intra-class correlation coefficient = 0.90; 95 % confidence interval, 0.78–0.95). The ROIs from the water images were exported to the fat images of the same sequence (see Fig. 1). Mean signal intensity values were collected from both, water images (Iwater) and fat images (Ifat). The FSF was obtained as follows [16]: FSF [%] = 100 × fat /(Iwater + I fat). The resulting FSF of the GMed and GMin muscles are denoted as FSFGMed and FSFGMin, respectively. The resulting volume of the GMed and GMin muscles are denoted as VGMed and VGMin, respectively. Muscle volumes were normalized to body height (muscle volume/body height2) resulting in ViGmed and ViGmin respectively.FSF and normalized volume data from both hips of each subject were averaged to mean FSFGMed, mean FSFGMin, mean ViGMed and mean ViGMin.
Water signal-only image (a) and the corresponding fat signal-only image (b) acquired in the axial plane in a 29-year-old woman. Regions of interest were traced around the margins of the GMed (green) and GMin (red) muscles in the first image (a) and then copied to the second image (b). c Coronal reconstruction obtained from fat signal-only MR images
Statistical analysis
Mean VGMed, mean VGMin, mean FSFGMed and mean FSFGMin were tested for normal distribution (Kolmogorov-Smirnov test).
The unpaired t test was used for evaluation of gender differences regarding mean ViGMed, mean ViGMin, mean FSFGMed and mean FSFGMin for the whole study cohort as well as for each age subgroup. The one-way analysis of variance was then performed to test for significant differences of mean ViGMed, mean ViGMin, mean FSFGMed and mean FSFGMin between different age subgroups and between different activity level subgroups for both genders.
Within each gender, the differences between mean ViGMed and mean ViGmin and between mean FSFGMed and mean FSFGMin were calculated with the paired Student t test. Moreover this test was performed to evaluate differences between dominant versus non-dominant leg with regard to ViGMed, ViGMin, FSFGMed and FSFGMin.
Pearson correlation coefficient (R) was used to test for correlation of mean ViGMed with mean ViGMin and mean FSFGMed with mean FSFGMin, for both genders. The R was also used to evaluate the correlation between mean ViGMed, mean ViGMin, mean FSFGMed and mean FSFGMin with the lower limb length and with the WHR and between the mean FSFGMed and FSFGMin with the BMI and the body fat percentage BIA1 and BIA2.
P values less than 0.05 were considered to indicate a significant difference. When a Bonferroni correction was applied to correct for multiple comparisons a P value less than 0.0125 was considered statistically significant. IBM SPSS software (release 17.0; SPSS, Chicago, Ill) was used for all data analyses.
Results
Dependence on age, gender and sports activity
The mean ViGMed, mean ViGMin, mean FSFGMed and mean FSFGMin values are reported in Tables 1 and 2. Mean ViGMed in males was significantly greater than in females in general (P < 0.001) and considering each age subgroup (P < 0.001 in subgroup 20–29 years, P = 0.002 in 30–39 years and P = 0.007 in 40–49 years) ,except the subgroup aged 50–59 years, which showed no significant gender difference (P = 0.040). Similarly, mean ViGMin in males was significantly greater than in females (<0.001); analyzing each age subgroup, ViGMin was significantly greater in males than in females in 20–29 and 30–39 years subgroups (P = 0.001 and P = 0.004, respectively) but no significant differences were present in the other age subgroups (P = 0.128 for subgroup 30–39 years and P = 0.030 for subgroup 40–49 years; Tables 1 and 2). Mean FSFGMed and mean FSFGMin in general, and considering each age subgroup, were not significantly different for genders (all P > 0.521), except in the subgroups aged 20–29 years; in the latter, both the values were greater in females than in males (P = 0.003 and P = 0.002 for FSFGMed and FSFGMin respectively; Tables 1 and 2; Fig. 3).
Focusing on age subgroup differences within each gender, there were no differences comparing different age subgroups with respect to mean ViGMed (P =0.049 and P =0.876 for female and male subjects, respectively), mean ViGMin (P =0.662 and P =0.670 for female and male subjects, respectively), FSFGMed (P = 0.567 and P = 0.071 for female and male subjects, respectively) and FSFGMin in the female group (P = 0.338; Figs. 2 and 3). In the male group, mean FSFGMin was significantly lower in the subgroup aged 20–29 years than in the subgroup aged 50–62 years (P = 0.014), but no significant differences were present among other age subgroups (P > 0.131).
Box plots show mean ViGMed (a) and mean ViGmin (b) in different age subgroups. Mean ViGMed in males (green) was significantly greater than in females (blue) in all age subgroups (P < 0.007) except the one aged 50–59 years (P = 0.040). Mean ViGMin in males was significantly greater than in females in 20–29 and 30–39 years subgroups (P < 0.004), but no significant differences were present in the subgroups 30–39 years (P = 0.128) and 40–49 years (P = 0.030). The boxes include the values between the 25th and 75th percentiles and the median value is represented by the line within the box. The 10th and 90th percentiles are indicated by the lines outside the boxes. Values with more than 1.5 times distance from the box limits are considered outliers 1 = 20–29 years, 2 = 30–39 years, 3 = 40–49 years, 4 = 50–62 years
Box plots show (a) mean FSFGMed and (b) mean FSFGmin in different age subgroups. Differences between male (green) and female (blue) groups were not significant for all age subgroups for both mean FSFGMed and FSFGMin (all P > 0.521) except for subgroup aged 20–29 years (P = 0.003 and P = 0.002 for mean FSFGMed and FSFGmin, respectively). The boxes include the values between the 25th and 75th percentiles and the median value is represented by the line within the box. The 10th and 90th percentiles are indicated by the lines outside the boxes. Values with more than 1.5 times distance from the box limits are considered outliers. 1 = 20–29 years, 2 = 30–39 years, 3 = 40–49 years, 4 = 50–62 years
Within each gender, the mean ViGMed was significantly greater than the mean ViGMin (P < 0.001 for both gender) and the mean FSFGMin was significantly greater than the mean FSFGMed (P < 0.001). A positive correlation was found for mean ViGMed with increasing value of mean ViGMin (R = 0.579, P < 0.001) and for mean FSFGMed with mean FSFGMin (R = 0.815, P < 0.001).
Among the volunteers, 36 (45.0 %) declared practicing no sports, 7 (8.75 %) practiced once a week and 32 (40.0 %) at least twice a week; data were not available in 5 (6.25 %) cases. With respect to mean ViGMed (P = 0.057 and P = 0.698 for female and male subjects, respectively), mean ViGMin (P = 0.914 and P = 0.865), FSFGMed (P = 0.284 and P = 0.851) and FSFGMin (P = 0.166 and P = 0.763), no differences were present comparing different activity level subgroups for each gender.
Dependence on lower limb length and leg dominance
The mean lower limb length ± SD was 98.27 ± 6.38 cm (range, 83–110 cm) for females and 103.87 ± 8.28 (range, 86–124 cm) for males. No significant correlations were found between the lower limb length and mean ViGMed (P = 0.451 for females and P = 0.807 for males), ViGMin (P = 0.662 for females and P = 0.279 for males), FSFGMed (P = 0.354 for females and P = 0.156 for males) and FSFGMin (P = 0.494 for females and P = 0.248 for males).
The left leg was dominant in 25 of 80 subjects (31.25 %) and the right in 55 of 80 subjects (68.75 %). ViGmed and ViGmin differences were not significant between the dominant and the non-dominant leg for both genders (P = 0.450 and P = 0.168 for ViGMed, and P = 0.077 and P = 0.599 for ViGmin, for females and males respectively; Table 3). Mean FSFGMed and FSFGMin were lower in the dominant leg than in the non-dominant leg but the difference was significant only in the female group (P = 0.005 for both FSFGMed and FSFGMin) and not for the male group (P = 0.454 for FSFGMed and P = 0.643 for FSFGMin; Table 3).
Dependence on estimated body fat
Values of BMI, WHR and body fat percentage BIA1 and BIA2 are shown in Table 4.The BMI showed a significant positive correlation with mean FSFGMed (R = 0.472, P = 0.002 and R = 0.414, P = 0.008 for female and male respectively) and mean FSFGMin (R = 0.352, P = 0.026 and R = 0.402, P = 0.010 for female and male respectively).
No significant correlation were found between WHR and mean ViGMed (P = 0.249 and P = 0.724, for female and male respectively), mean ViGMin (P = 0.426 and P = 0.866, for females and males respectively) and mean FSFGMed (P = 0.102 and P = 0.061 for females and males respectively). A significant correlation was found between WHR and mean FSFGMin in both genders (R = 0.325, P = 0.041 and R = 0.365, P = 0.021 for females and males respectively).
Body fat percentage BIA1 measured by the foot sensor device showed a positive correlation for both genders with mean FSFGMed (R = 0.456, P = 0.003 and R = 0.638, P < 0.001 for females and males respectively) and mean FSFGMin (R = 0.383, P = 0.015 and R = 0.684, P <0.001 for females and males respectively). Similar results with slightly stronger correlation were obtained with the handheld device (BIA2) for both genders regarding mean FSFGMed (R = 0.655, P <0.001 and R = 0.524, P = 0.001 for females and males respectively) and mean FSFGMin (R = 0.547, P <0.001 and R = 0.581, P <0.001 for females and males respectively).
Discussion
We reported the normative values for volume and fat content of the gluteus medius and gluteus minimus muscle evaluated in a large cohort of healthy volunteers of different age (20–62 years) and gender. No age dependency was evident for volume normative data for both muscles and no age- and gender dependency was observed for FSF values.
Few previous published data included healthy participants but the number of subjects was limited and inclusion criteria not homogenous, making their use inadequate as normative data [2, 11]. With aging, both, muscle atrophy and increasing infiltration of fat, are well documented and associated with reduced muscle strength and poor physical function [8, 9, 17]. Moreover, atrophy and fatty muscle infiltration are important predictors in determining functional outcome after cuff repair of the shoulder and a similar role has been hypothesized in gluteal muscles tendinopathy [1, 12, 18, 19].
MRI is considered a reliable technique for evaluating muscle quality by monitoring morphological changes such as muscular atrophy and fatty infiltration [20]. Muscle volume measurement is a time-consuming procedure which, in our case, was manually performed. Assessment of a single axial anatomical cross-sectional area is often employed as an alternative faster method for estimating muscle size but its application is limited because the cross-sectional area is not always representative of the muscle volume and the optimal level to carry out the measurement is both difficult to define and then to reproduce [21–24]. Indeed, even small differences in the selected plane for the cross-sectional area measurement may result in significant deviation during two consecutive measurements of the same muscle [24]. This was the main reason why we decided to perform the extensive way for volumetric muscle analysis in this study, despite its being time consuming. We found that mean VGmed and VGmin were not significantly different between the dominant and non-dominant leg. This observation was consistent with previous findings reported by Grimaldi et al. [2] and are of particular interest, since volume asymmetry of gluteal muscle volume is often used as parameter to establish pathological muscle atrophy, e.g. in case of tears of the abductors tendon diagnosis and in the outcome evaluation after abductors tendon repair [1, 4, 13].
Use of Dixon-based MRI to obtain fat- and water-signal only images for fat quantification of the muscle is rapidly spreading thanks to the high accuracy of the technique and the numerous advantages compared to, e.g. MR spectroscopy. The latter was often used as reference standard in the past [25–29]. Most of the previous studies relied on the visual assessment for the evaluation of the gluteal muscle fat infiltration, but this is known to hamper the detection of subtle fatty infiltration of muscle [5, 11, 13, 28, 30]. Moreover, since fatty muscle infiltration has been demonstrated to be unequally distributed within the GMed and GMin [5], the use of a fat quantification over the full volume is considered the most valid method to estimate muscle fat content. We measured mean FSF values of 9.89 ± 2.72 % (range 5.69–22.78 %) for the gluteus medius muscle and mean FSF values of 8.13 ± 1.70 % (range 5.09–13.41 %) for the gluteus minimus muscle in our healthy cohort. According to a previous study by Fischer et al. [27], these FSF values correlate with a Goutallier grading of 1, which means no fatty infiltration, apart from some fatty streaks. There exist some other studies using Dixon-based muscle MRI. In a study with patients with low back pain, the FSF measurements of the multifidus muscle ranged from 3.4 to 65.2 % with a mean FSF of 21.2 ± 14.1 % [26]. This study nicely emphasized the difference of the increased FSF in the patients with low back pain to the value of FSF in normal muscle tissue.
Previous literature on gluteus muscles suggested higher fat-signal-fractions with increasing age. However, we did not find any significant differences between the different age groups in the statistical analysis (with except of one single subgroup where the older subjects had significantly more fat content than younger subjects (i.e. male subgroup aged 20–29 years vs male subgroup aged 50–62 years for mean FSFGMin). The independent role of aging on muscle fat infiltration is not fully understood and contradictory findings reported in the literature could be attributed to the different types of muscles assessed, varying physical activity levels and different age ranges compared to our prospectively well-selected cohort of healthy subjects [8, 17, 31–33].
A significant difference was also found comparing FSFGMed and FSFGMin in the dominant versus the non-dominant leg but only in the female group. This finding may be taken into account when initial signs of muscle fat infiltration are observed, particularly when muscle volumes appear symmetrical to each other.
We also found that a significantly greater content of fat was present in the GMin than in the GMed muscle. In a previous study by Pfirrmann et al. [5], fatty infiltration of the GMin was described as common in both asymptomatic and symptomatic patients after total hip arthroplasty, whereas fatty infiltration of the GMed was seen almost exclusively in the symptomatic patients. Similarly, Engelken et al. [11] observed that no fatty infiltration of the GMed and minimal fatty infiltration of the GMin were present in healthy subjects compared to patients with gluteal muscle insufficiency who showed significant degrees of fatty infiltration of GMed as well as GMin. These previous studies and our current results suggest that presence of fatty infiltration of gluteal muscles is suggestive for gluteal muscle insufficiency, in particular when GMed fatty infiltration is evident. GMin fatty infiltration necessitates a more attentive evaluation, since in a certain degree is commonly observed also in healthy subjects. In addition to the normative gluteal muscle volume and FSF values we also evaluated their dependence on BMI and body fat percentage estimated by WHR and bioimpedance methods. Subjects included in our study had normal BMI and we found a positive (weak) correlation between the BMI and the mean FSFGMed and FSFGMin. A moderate correlation was also observed for FSFGMed and FSFGMin with WHR and the mean body fat percentage evaluated by two different bioimpedance devices. For adults, the BMI is commonly used as a simple and safe method to estimate body fat but the main limitation consists in the fact that this parameter is not able to differentiate between body mass and fat mass [34, 35]. In accordance to our findings, a previous report [22] did not identify significant BMI differences in two groups of non-obese adults with and without GMed and GMin fatty infiltration.
Several limitations of our study have to be addressed. First, our study population did not include people aged over 62 years which would represent a large portion of patients which could take advantage of normative values for volume and fat content of gluteal muscle to test for discrepancy in conditions such as pain after total hip replacement. Nevertheless, we believe that at least some of our findings such as the absence of significant gluteal muscle-volume differences between the two sides and the minimal greater fat infiltration in the non-dominant side in healthy female subjects aged less than 62 years could probably be generalized to the older population. Secondly, intra- and inter-reader variability were not assessed. A previous study has already investigated the intra-observer reliability of the same muscle volume measurements method as used in our study and excellent agreement was shown [23]. Finally, in our three-point mDIXON sequence, the fat signal is modeled as a single peak at 1.3 ppm, resulting in a slight underestimation of fat signal fraction which would have been taken better into account in a multi-peak fat modelling in a multi-point DIXON sequence [36, 37]. However, this underestimation represents a systematic error which is (1) small and (2) applies to all of our measurements and, thus, is unlikely to affect the proportional findings in this study.
In conclusion, we presented normative data for volume and FSF values for GMed and GMin muscles. No age dependency was evident for volume data for both muscles and normative data for FSF values showed no age- or gender dependency. Our normative values may serve as reference standards for future studies and may help to differentiate physiologic changes from pathologic conditions.
References
Cvitanic O, Henzie G, Skezas N, Lyons J, Minter J. MRI diagnosis of tears of the hip abductor tendons (gluteus medius and gluteus minimus). AJR Am J Roentgenol. 2004;182(1):137–43.
Grimaldi A, Richardson C, Stanton W, Durbridge G, Donnelly W, Hides J. The association between degenerative hip joint pathology and size of the gluteus medius, gluteus minimus and piriformis muscles. Man Ther. 2009;14(6):605–10.
Ten Dam L, van der Kooi AJ, Rovekamp F, Linssen WH, de Visser M. Comparing clinical data and muscle imaging of DYSF and ANO5 related muscular dystrophies. Neuromuscul Disord. 2014;24(12):1097–102.
Woodley SJ, Nicholson HD, Livingstone V, Doyle TC, Meikle GR, Macintosh JE, et al. Lateral hip pain: findings from magnetic resonance imaging and clinical examination. J Orthop Sports Phys Ther. 2008;38(6):313–28.
Pfirrmann CW, Notzli HP, Dora C, Hodler J, Zanetti M. Abductor tendons and muscles assessed at MR imaging after total hip arthroplasty in asymptomatic and symptomatic patients. Radiology. 2005;235(3):969–76.
Ikezoe T, Mori N, Nakamura M, Ichihashi N. Atrophy of the lower limbs in elderly women: is it related to walking ability? Eur J Appl Physiol. 2011;111(6):989–95.
Kiyoshige Y, Watanabe E. Fatty degeneration of gluteus minimus muscle as a predictor of falls. Arch Gerontol Geriatr. 2015;60(1):59–61.
Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14(5):362–6.
Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol Ser A Biol Med Sci. 2005;60(3):324–33.
Zhao Y, Chen SY, Lee YC, Wu N. Clinical and economic characteristics of hip fracture patients with and without muscle atrophy/weakness in the United States. Arch Osteoporos. 2013;8(1–2):127.
Engelken F, Wassilew GI, Kohlitz T, Brockhaus S, Hamm B, Perka C, et al. Assessment of fatty degeneration of the gluteal muscles in patients with THA using MRI: reliability and accuracy of the Goutallier and quartile classification systems. J Arthroplast. 2014;29(1):149–53.
Kong A, Van der Vliet A, Zadow S. MRI and US of gluteal tendinopathy in greater trochanteric pain syndrome. Eur Radiol. 2007;17(7):1772–83.
Makridis KG, Lequesne M, Bard H, Djian P. Clinical and MRI results in 67 patients operated for gluteus medius and minimus tendon tears with a median follow-up of 4.6 years. Orthop Traumatol, Surg Res. 2014;100(8):849–53.
Muller M, Tohtz S, Winkler T, Dewey M, Springer I, Perka C. MRI findings of gluteus minimus muscle damage in primary total hip arthroplasty and the influence on clinical outcome. Arch Orthop Trauma Surg. 2010;130(7):927–35.
Hoffman M, Schrader J, Applegate T, Koceja D. Unilateral postural control of the functionally dominant and nondominant extremities of healthy subjects. J Athl Train. 1998;33(4):319–22.
Cassidy FH, Yokoo T, Aganovic L, Hanna RF, Bydder M, Middleton MS, et al. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics. 2009;29(1):231–60.
Nakagawa Y, Hattori M, Harada K, Shirase R, Bando M, Okano G. Age-related changes in intramyocellular lipid in humans by in vivo H-MR spectroscopy. Gerontology. 2007;53(4):218–23.
Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83.
Lequesne M, Djian P, Vuillemin V, Mathieu P. Prospective study of refractory greater trochanter pain syndrome: MRI findings of gluteal tendon tears seen at surgery—clinical and MRI results of tendon repair. Joint Bone Spine. 2008;75(4):458–64.
Theodorou DJ, Theodorou SJ, Kakitsubata Y. Skeletal muscle disease: patterns of MRI appearances. Br J Radiol. 2012;85(1020):e1298–308.
Arokoski MH, Arokoski JP, Haara M, Kankaanpaa M, Vesterinen M, Niemitukia LH, et al. Hip muscle strength and muscle cross sectional area in men with and without hip osteoarthritis. J Rheumatol. 2002;29(10):2185–95.
Inacio M, Ryan AS, Bair WN, Prettyman M, Beamer BA, Rogers MW. Gluteal muscle composition differentiates fallers from non-fallers in community dwelling older adults. BMC Geriatr. 2014;14:37.
Marcon M, Ciritsis B, Laux C, Nanz D, Nguyen-Kim TD, Fischer MA, et al. Cross-sectional area measurements versus volumetric assessment of the quadriceps femoris muscle in patients with anterior cruciate ligament reconstructions. Eur Radiol. 2015;25(2):290–8.
Springer I, Muller M, Hamm B, Dewey M. Intra- and interobserver variability of magnetic resonance imaging for quantitative assessment of abductor and external rotator muscle changes after total hip arthroplasty. Eur J Radiol. 2012;81(5):928–33.
Alizai H, Nardo L, Karampinos DC, Joseph GB, Yap SP, Baum T, et al. Comparison of clinical semi-quantitative assessment of muscle fat infiltration with quantitative assessment using chemical shift-based water/fat separation in MR studies of the calf of post-menopausal women. Eur Radiol. 2012;22(7):1592–600.
Fischer MA, Nanz D, Shimakawa A, Schirmer T, Guggenberger R, Chhabra A, et al. Quantification of muscle fat in patients with low back pain: comparison of multi-echo MR imaging with single-voxel MR spectroscopy. Radiology. 2013;266(2):555–63.
Fischer MA, Pfirrmann CW, Espinosa N, Raptis DA, Buck FM. Dixon-based MRI for assessment of muscle-fat content in phantoms, healthy volunteers and patients with achillodynia: comparison to visual assessment of calf muscle quality. Eur Radiol. 2014;24(6):1366–75.
Karampinos DC, Baum T, Nardo L, Alizai H, Yu H, Carballido-Gamio J, et al. Characterization of the regional distribution of skeletal muscle adipose tissue in type 2 diabetes using chemical shift-based water/fat separation. J Magn Reson Imaging. 2012;35(4):899–907.
Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28(3):543–58.
Mengiardi B, Schmid MR, Boos N, Pfirrmann CW, Brunner F, Elfering A, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology. 2006;240(3):786–92.
Fischmann A, Hafner P, Fasler S, Gloor M, Bieri O, Studler U, et al. Quantitative MRI can detect subclinical disease progression in muscular dystrophy. J Neurol. 2012;259(8):1648–54.
Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J Appl Physiol (1985). 2001;90(6):2157–65.
Machann J, Thamer C, Schnoedt B, Stefan N, Stumvoll M, Haring HU, et al. Age and gender related effects on adipose tissue compartments of subjects with increased risk for type 2 diabetes: a whole body MRI/MRS study. MAGMA. 2005;18(3):128–37.
Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–39.
Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7(4):e33308.
Reeder SB, Robson PM, Yu H, Shimakawa A, Hines CD, McKenzie CA, et al. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009;29(6):1332–9.
Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, et al. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007;25(3):644–52.
Acknowledgements
We thank our physicist Dr. Roger Luechinger, PhD, for his technical support, Dr. Thi Dan Linh Nguyen-Kim for her indications concerning the analysis software, and our radiographers Nicole Aebi, Suzanne Potter and Simone Süess for performing the MR exams.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This project is supported by the University Zürich Research Program “Research Time for clinical research at the Medical Faculty of University Zurich” (Grant number: RT-13-014).
Additional information
Origin of work
Institute of Diagnostic and Interventional Radiology, University Hospital Zurich, Ramistrasse 100, 8091 Zurich, Switzerland
Glossary
- BMI
-
Body mass index
- BIA
-
Bioelectrical impedance analysis
- BIA1
-
Body fat percentage (%) measured by a BIA via the electrical body resistance with foot sensor pads on a similar device of bathroom scale (TANITA UM-018, Tanita Corp, Arlington Heights, Ill)
- BIA2
-
Body fat percentage (%) measured by a BIA via the electrical body resistance with hand sensor pads on a handheld device (OMRON BF300, Omron Healthcare Ltd, Kyoto, Japan)
- FSF
-
Fat signal fraction
- FSFGMed
-
Fat signal fraction of the gluteus medius in %
- FSFGMin
-
Fat signal fraction of the gluteus minimus in %
- GMed
-
Gluteus medius
- GMin
-
Gluteus minimus
- MRI
-
Magnetic resonance imaging
- ROI
-
Region of interest
- VGMed
-
Volume of gluteus medius in liter
- VGMin
-
Volume of gluteus minimus in liter
- ViGMed
-
Normalized volume of gluteus medius calculated from VGMed/body height2 (10−3 L/m2)
- ViGMin
-
Normalized volume of Gluteus minimus calculated from VGMin/body height2 (10−3 L/m2)
- WHR
-
Waist to hip ratio
Rights and permissions
About this article
Cite this article
Marcon, M., Berger, N., Manoliu, A. et al. Normative values for volume and fat content of the hip abductor muscles and their dependence on side, age and gender in a healthy population. Skeletal Radiol 45, 465–474 (2016). https://doi.org/10.1007/s00256-015-2325-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-015-2325-z