Abstract
Application of filamentous fungi for the production of commercial enzymes such as amylase, cellulase, or xylanase is on the rise due to the increasing demand to degrade several complex carbohydrates as raw material for biotechnological processes. Also, protein production by fungi for food and feed gains importance. In any case, the protein production involves both cellular synthesis and secretion outside of the cell. Unfortunately, the secretion of proteins or enzymes can be hampered due to accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER) as a result of too high synthesis of enzymes or (heterologous) protein expression. To cope with this ER stress, the cell generates a response known as unfolded protein response (UPR). Even though this mechanism should re-establish the protein homeostasis equivalent to a cell under non-stress conditions, the enzyme expression might still suffer from repression under secretory stress (RESS). Among eukaryotes, Saccharomyces cerevisiae is the only fungus, which is studied quite extensively to unravel the UPR pathway. Several homologs of the proteins involved in this signal transduction cascade are also found in filamentous fungi. Since RESS seems to be absent in S. cerevisiae and was only reported in Trichoderma reesei in the presence of folding and glycosylation inhibitors such as dithiothreitol and tunicamycin, more in-depth study about this mechanism, specifically in filamentous fungi, is the need of the hour. Hence, this review article gives an overview on both, protein secretion and associated stress responses in fungi.
Key points
• Enzymes produced by filamentous fungi are crucial in industrial processes
• UPR mechanism is conserved among many fungi, but mediated by different proteins
• RESS is not fully understood or studied in industrially relevant filamentous fungi
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filamentous fungi have become a remarkable part of industrial biotechnology since the nineteenth century. The first patent in the field in 1896 was made by Dr. Jokichi Takamine for the production of digestive enzymes by Aspergillus oryzae (Bennett and Baker 2008). In 1942, Anne Sheafe Miller suffering from a Streptococcus infection was saved through penicillin synthesized by Penicillium chrysogenum, which was discovered in 1929 by Alexander Fleming. Basically, in the first half of the twentieth century, industrial bioprocesses used fungi for the production of organic acids and antibiotics. Later, researchers developed advanced fungal systems for the production of many other primary and secondary metabolites such as vitamins and alkaloids (Baker et al. 2008; Grigoriev et al. 2011). It is estimated that fungi are used today to make more than 700 commercial products. For instance, citric acid produced on an industrial scale by A. niger is used in food, soft drinks, cosmetics, and leather manufacture. Fumaric acid used in making alkyd and wetting agents is produced by Rhizopus nigricans. A plant growth hormone, gibberellic acid, is derived from Fusarium moniliforme. Eremothecium ashbyi is cultivated for the production of riboflavin (vitamin B2) as a vitamin supplement fat (Patel et al. 2016).
Many fungi naturally secrete a variety of enzymes in order to breakdown complex substrates into easily metabolizable mono- or oligomers or to dissolve solid substrates. Importantly, enzyme-based products and solutions are used in over 40 industry sectors such as household care, bioenergy, agriculture, animal health, and food. Food application of enzymes includes bakery, brewery, juice, wine, dairy, and oil/fats (Patel et al. 2016). Enzymes have been exploited industrially on such a vast scale due to their good performance under a wide range of physical and chemical conditions. They have several advantages over conventional methods (like chemical synthesis) such as reduced process time, cost-effectiveness, low energy input, non-toxicity, greater efficiency, higher-quality products, and eco-friendly characteristics (Gurung et al. 2013; Kamini et al. 1999; Singh et al. 2016). Application of Aspergillus species is of utmost importance for dairy industry as they produce acid proteinase and lipase for milk coagulation used to improve the quality of cheese (Vishwanatha et al. 2010; Neelakantan et al. 1999). Enzymes like xylanase, glucose oxidase, and protease produced by A. niger and A. oryzae play an important role in dough conditioning and strengthening, which improves the structure of bread (Saxena et al. 2001). The beverage industry employs enzymes like pectinase, amylase, cellulase, naringinase, and laccase from a number of filamentous fungi such as A. oryzae, A. niger, T. atroviride, Cochiobolus miyabeanus, and Trametes versicolor for processes such as depectinization, fruit liquefaction, debittering, and to synthesize aromatic aldehydes (Yamasaki et al. 1964; El-Zalaki and Hamza 1979; Singh et al. 2016; Ito and Takiguchi 1970; Fritz-Langhals and Kunath 1998). Furthermore, Aspergillus sp. are also employed in detergent industry to produce certain enzymes that have the capability to remove stains consisting of protein, carbohydrates, and lipids (Souza 2010; Vishwanatha et al. 2010). Leather industry utilizes enzymes like amylase, neutral protease, and lipase for fiber splitting, dehairing, soaking, and degreasing of the raw material (Souza et al. 2015; Singh et al. 2016).
According to the European Industry Association of Manufacturers and Formulators of Enzyme Products (AMFEP), the list of commercial enzymes for food, feed, and technical applications includes 243 enzymes manufactured by cultivation of microorganisms. Furthermore, over 300 food enzyme dossiers are under evaluation by the European Food Safety Authority (EFSA) and the EU FIAP (Food Improvement Agents Package) regulatory frame. Importantly, most of the commercial enzymes used in the food industry are manufactured using fungal hosts (Arnau et al. 2020). Most fungal production strains that the major enzyme companies use are recombinant, and the enzymes are mostly produced in heterologous hosts, primarily in Aspergilli and Trichoderma reesei. Though numerous attempts are made to improve the used fungal strains for enhanced production of the target enzymes and their activity, the information about the secretion mechanism and related stress is very meager among filamentous fungi (compare Supplementary Table S1). Therefore, this review article aims to shed light on the available information about protein secretion and potentially associated secretory stress that can be responsible for limiting the maximum enzyme secretion in industrially employed filamentous fungi.
Protein secretion
Commonly, native proteins are expressed and secreted by at least 1000 times more efficient than heterologously expressed proteins in filamentous fungi (Sakekar et al. 2021). This fact led researchers to investigate the single attributes of fungal protein expression and secretion mechanisms (Sun and Su 2019). The complex and regulated process of protein production consists of three phases, synthesis, modification, and secretion. This review focuses on secretion events in filamentous fungi. To give an overview, secretion starts from translocation of the polypeptide to the ER lumen, where it undergoes folding and some post-translational modifications (e.g., glycosylation). These polypeptides are further transported to the Golgi apparatus for further modifications, followed by transport to the plasma membrane and secretion.
Although this process seems to be simple at first a glance, several steps are crucial in their details. The classical pathway for protein secretion is described in S. cerevisiae. It begins with targeted translocation of a polypeptide to the ER through either the signal recognition particle (SRP)-dependent or the SRP-independent mechanism. In the SRP-dependent mechanism, the secretory signal sequence (N-terminal 15–36 amino acids sequence) associated with the nascent polypeptide chain is recognized by SRPs. These SRPs direct the polypeptide to the cytosolic side of the ER where it binds to the peptide translocation complex (translocon). Here, the translocation through the ER membrane occurs co-translationally. After dissociation of the SRPs, further elongation of the peptide is carried out inside the ER lumen until the protein synthesis is completed. Then, the signal sequence is removed by a signal peptidase (Sec11, Spc1, Scp2, and Scp3) and addition of glycolipids and sugars (Antonin et al. 2000) in the ER lumen. Whereas in SRP-independent mechanism, proteins are targeted to the ER post-translationally (Conesa et al. 2001). In both mechanisms, the growing polypeptide chain interacts with cytosolic chaperones (e.g., HSP70) and co-chaperones, which subsequently engages with translocase/SEC complex (e.g., Sec62, Sec72, Sec73) in fungi and ortholog of translocon (Sec61, Sbh1 and Sss1) in S. cerevisiae (Romisch 1999), to enter the ER lumen (Sakekar et al. 2021). Among these, Sec61 is reported to be expressed in higher amounts during UPR, highlighting its crucial role in protein secretion (Kautto et al. 2013). Once the protein enters the ER lumen, it needs to be folded and matured to obtain its native form to become functional. This process is assisted by helper proteins named chaperones and foldases. Chaperones prevent non-productive protein–protein interactions by transiently and non-covalent bonding between non-native proteins, thus promoting correct folding (Gething and Sambrook 1992). Foldases catalyze covalent changes, such as disulfide bond formation, or proline isomerization, which are essential, but slow, and often rate-limiting steps in protein synthesis.
Now the correctly folded proteins exit the ER and enter the Golgi apparatus or a Golgi-like structure, as the classical dictyosome organization of the Golgi apparatus is not commonly present in filamentous fungi (Markham 1995). Once the vesicle arrives at the Golgi complex (Cis compartment), a tethering complex interacts with Sar1 and gets inactivated, which destabilizes the COPII coat. This reaction is called docking reaction. It generates the SNARE complex, which consists of four subunits associated with both, the vesicle and the Golgi membrane, hence facilitating the membrane fusion (Jahn and Scheller 2006; Kim et al. 2006). This membrane fusion and docking reaction are under tight regulation of the monomeric G-protein of the Rab family, Ypt1 (Novick and Zerial 1997). In the Golgi apparatus, further modifications like N- and O-glycosylation are highly conserved in fungal extracellular proteins, among which oligomannose N- and O-glycans are predominant ones (Archer and Peberdy 1997). Maras et al. (1999) have also reported the presence of glucose, sulfate, phosphate, and galactose on the linked glycans. Once the modifications in the Golgi compartment are complete, proteins are targeted either to the plasma membrane for secretion or to the vacuole.
A whole genome gene deletion strain library of Neurospora crassa was generated to understand the factors involved in secretion of enzymes with the focus on secretion of proteins through the fungal hyphae. The first mature leading hyphal segment of N. crassa has been well described with four regions; in apical (region I), sub-apical (regions II–IV), (Riquelme et al. 2002) and the protein secretion route that exists behind the apex is independent of the Spitzenkörper (Fajardo-Somera et al. 2013). This mechanism of protein secretion was presumed to be similar as the one of S. cerevisiae (Idiris et al. 2010; Shoji et al. 2014). The following chapter describes the knowledge about protein secretion mechanism in three industrially important filamentous fungi.
Protein secretion mechanism in model and/or industrially employed filamentous fungi
Aspergillus oryzae
In 1950s, α-amylase, historically known as Taka-amylase A from Takadiastase, encoded by three similar genes amyA/B/C, was isolated from A. oryzae (Fischer and De Montmollin 1951; Akabori et al. 1954). Due to the highest expression of amyB among the amyA/B/C genes, it was used to investigate and understand the molecular mechanism of protein secretion at the cell biology level (Tada et al. 1989; Nemoto et al. 2012). Secretory proteins harbor a signal peptide at the N-terminus targeted toward ER. And from ER, these proteins are transported to the plasma membrane via the Golgi apparatus by vesicular trafficking and eventually secreted outside the cell. While passing through ER and Golgi, the secretory proteins are modified with N- and/or O-glycan chains to provide the stability and localization (Goto 2007; Deshpande et al. 2008). In the ER lumen, during calnexin/calreticulin cycle, high-mannose oligosaccharide and a polysaccharide derivative (Glc3Man9GlcNAc2) are attached to the asparagine residue of glycoproteins and glucose moieties are removed by glucosidases I and II (Fig 1a). This step acts as quality control system of the glycoproteins before transporting them to the Golgi apparatus, as studied in A. oryzae (Watanabe et al. 2009, 2007). Thereafter, a sequential cleavage of mannose moieties by two 1,2-α-mannosidases (ManE and FmanIB) occurs at the ER and the Golgi apparatus as well, also studied in A. oryzae (Yoshida et al. 2000; Akao et al. 2006, 2012). The remaining Man6GlcNAc2 moiety is a secretory form of the N-glycan chain (Fig. 1b). Here, the single GlcNAc moiety is predicted to be important to maintain the proper protein structure and function (Kasajima et al. 2006).
An overview of protein secretion and post-translational modifications in A. oryzae. A SRP (black triangles)-mediated polypeptide transfer into ER via translocon/SEC complex and alnexin/calreticulin cycle; Glc3Man9GlcNAc2 (Glc, glucose; Man, mannose; GlcNAc, N-acetylglucosamine) are attached to the asparagine (Asn) residue of glycoproteins and the glucose moiety is removed by glucosidases I and II in the ER. B Sequential cleavage of mannose moieties by two 1,2-α-mannosidases in the Golgi complex generate the secretory form of proteins. C tER- and D Erd2p-mediated retrieval of proteins from ER and Golgi, respectively, for subsequent secretion through the hyphal tip (green line)
Several studies over decades using state-of-the-art approaches like in vivo imaging with fluorescent proteins revealed that secretory proteins are present at the apical vesical cluster, known as Spitzenkörper, and are secreted via hyphal tips (Masai et al. 2003; Kimura et al. 2010). For septum-directed secretion a different molecular machinery is employed, which is only dependent on microtubule cytoskeleton, in contrast to hyphal tip secretion, which is dependent on actin and microtubule cytoskeleton (Hayakawa et al. 2011).
The intracellular dynamics of secretory proteins begins with correct folding in the ER lumen with the help of chaperon proteins such as BipA (binding protein). In Maruyama et al. 2006, Maruyama and co-worker reported crowded, mesh-like structures with dynamic mobility in the tip region of hyphal cells in A. oryzae. The study of vesicular trafficking pathway revealed a site of ER membrane, called transitional ER (tER), with punctate localization toward hyphal tip. So, the tER, in addition to lectin-like receptors AoVip36 and AoEmp47, localized to ER-Golgi are responsible for retaining secretory proteins in the ER and Golgi (Fig. 1c) (Kimura et al. 2010; Hoang et al. 2015). Further, an ortholog of Erd2p (S. cerevisiae) in A. oryzae is essential for retrieving and secretion of proteins from the Golgi apparatus (Liu et al. 2014). Now the proteins are encapsulated and transported properly, wherein vesicule-SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) and target-SNAREs (t-SNAREs) play an important role. A complex consisting of three t-SNAREs and one v-SNARE facilitates the fusion between the membrane of secretory vesicle containing protein and the membrane of the target organelle or plasma membrane (Fig. 1d) (Rothman and Warren 1994).
Another interesting observation was that although all the proteins bearing signal peptides are destined to be secreted from the hyphal tip, certain proteins, such as the acyl-CoA binding protein AoAcb2 (Acb1 ortholog in S. cerevisiae), lack these signaling peptides and undergo a process called unconventional protein secretion (Malhotra 2013; Zhang and Schekman 2013).
Aspergillus niger
An important factor equivalent to the small GTP-binding protein Sar1p in S. cerevisiae was cloned and characterized in A. niger. It was reported to affect the protein secretion pathway, to be precise, the transport from the ER to the Golgi apparatus (Nakano et al. 1988; Nakano and Muramatsu 1989). The A. niger secretion-associated and Ras-related (sarA) gene has five introns and the encoded protein shares 70–80% identity with Sar1. Complementing S. cerevisiae sar1 and sec12 mutants with expression vectors containing A. niger sarA cDNA confirmed its functional homology. Another study using site-directed mutagenesis of A. niger sarA (D29G, E109K, D29G/E109K) resulted in a thermosensitive dominant-negative phenotype, differing from S. cerevisiae, in which similar mutations yielded a thermosensitive phenotype. This suggests that the sarA gene plays an essential role in A. niger (Veldhuisen et al. 1997).
Trichoderma reesei
Again, the steps involved in the secretion mechanism remain conserved in T. reesei and begin with the translocation of the polypeptide containing a hydrophobic signal sequence at the N-terminus into the ER lumen (Kottmeier et al. 2011). A ternary complex is formed during translation that includes the nascent protein, the mRNA, and the ribosome. This ternary complex is further directed to the ER surface via the SRP, which recognizes the signal peptide. At the surface the peptide is transported to the ER lumen through a protein channel across the ER membrane, called translocon (Corsi and Schekman 1996). The translocon in T. reesei consists of three subunits: Sec62, Sec72, and Sec73 (Saloheimo and Pakula 2012), that are orthologs of Sec61, Sbh1, and Sss1 present in S. cerevisiae (Romisch 1999). Further assistance is provided by chaperones such as Kar2, Mpd1, Mpd2, Eug1, Cpr5, and Cne1, to give the polypeptide chain its native structure (Normington et al. 1989; Frigerio and Pelham 1993; Parlati et al. 1995; Kimura et al. 2004; Pakula et al. 2003). In the ER of yeasts, during post-translational modifications, proteins are allocated with N-linked and/or O-linked glycans (Kruszewska et al. 2008; Mora-Montes et al. 2009) and such modifications were observed on secreted glycoproteins of Trichoderma as well. The modifications for O-linked glycans present in glycoproteins of Trichoderma include either glucose and galactose or phosphorylated or sulfonated mannobiosides and mannotriosides (Kruszewska et al. 2008). When N-linked glycan is present in the secreted glycoproteins, the N-linked glycan core possess phosphorylated mannoses as small outer chains (Kruszewska et al. 2008). These modifications are crucial for the secretory proteins as the deletion of pmt1 (encoding a relevant protein for modifications like O-glycosylation, but not N-glycosylation) in T. reesei leads to the loss of a significant amount of secretory proteins (Górka-Nieć et al. 2008; Zembek et al. 2011). The next step, the packaging of proteins in coat protein complex II (COPII)-coated vesicles to facilitate the transport from the ER to the Golgi apparatus (Hernández-Chávez et al. 2014), is well described in yeast. A bioinformatics analysis of Trichoderma genomes has shown the presence of the genes to generate COPII-coated vesicles for this kind of anterograde transport from ER to Golgi complex (Saloheimo and Pakula 2012). But the steps following COPII-coated vesicle formation remain to be determined in filamentous fungi. The gene-encoding functional equivalents of the small GTP-binding protein Sar1p in S. cerevisiae, which was reported to affect the transport from the ER to the Golgi apparatus (Nakano et al. 1988; Nakano and Muramatsu 1989), were cloned and characterized in T. reesei. The T. reesei gene sar1 has four introns, and the first intron is positioned similarly as the single S. cerevisiae sar1 intron. The encoded protein of T. reesei shares 70–80% identity with Sar1 from S. cerevisiae. Complementing S. cerevisiae sar1 and sec12 mutants with expression vectors containing T. reesei sar1 cDNA confirmed their functional homology (Veldhuisen et al. 1997).
Again, once the modifications in the Golgi apparatus are complete, the final protein with secretory N-glycan chain, proper protein structure, and function is trafficked toward the cell wall of mycelium or hyphae. In T. reesei, genes snc1, sso1, and sso2 have been isolated and characterized. Among which products of sso1 and snc1 were found in subapical areas of the hyphal plasma membrane instead of apical region along with vesicle-containing Snc1 localized within the Spitzenkörper (Valkonen et al. 2007). Additionally, a complex containing Snc1 and Sso1 was also detected in the plasma membrane and compartments of the subapical region, while the complexes of Snc1 and Sso2 were found exclusively in growing apical compartments (Valkonen et al. 2007).
In 2016 another study by Nykänen and co-worker shed light on the structure of the early secretory pathway in the T. reesei hyphae, comparing a wild-type strain (QM6a), a cellulase-overexpressing strain (Rut-C30), and a Rut-C30 strain overexpressing a BiP1-VenusYFP fusion protein. Using various microscopic techniques, the researchers observed differences in the organization of the ER among the three strains after 24 h of growth in both cellulase-inducing and non-inducing media. The wild type showed distinct ER subdomains, including ER whorls and autophagy vacuoles, while Rut-C30 strains displayed parallel tubular/cisternal ER with fewer autophagy vacuoles and increased localization of the recombinant BiP1-VenusYFP fusion protein. The findings suggest that these structural differences are inherent traits rather than responses to protein overload in the high-secreting strain (Nykänen et al. 2016). Hence, one needs to consider the inherent properties of the particular strain when producing heterologous proteins and to not solely focus on protein overload or misfold as one of the reasons for any decrease in protein secretion.
In support of the above statement, a study by Nykänen and co-worker in 1997 was the first to explore how a fungus simultaneously produces and secretes both a native protein (the cellobiohydrolase I (CBHI)) and a foreign/heterologous protein (the barley endopeptidase B (EPB)). They found that the locations where these proteins are made (translational sites) match where their genetic instructions are read (transcriptional locations). However, the native CBHI is produced and secreted throughout the mycelium, while the foreign EPB is only secreted via specific parts, i.e., at apical and subapical cells. This suggests that CBHI might have a signal-promoting effective secretion. Comparing the transcript levels of the immunoreactive EPB and the native CBHI revealed that the translation efficiency of the recombinant mRNA was quite high. Therefore, the low yields of the secreted recombinant EPB cannot be attributed to translation issues. The study also discovered that the efficiency of producing the foreign EPB is reasonably high, so low yields are likely due to factors like degradation or incomplete processing rather than translation problems. Further research would be required for understanding how EPB is processed in the fungus (Nykänen et al. 1997). Hence, there is a still a need to study more in detail how the heterologous proteins are processed in filamentous fungi.
ER stress
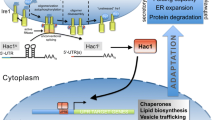
Unfolding or misfolding of proteins accounts for a significant threat to all living cells. Proteins can be unfolded or misfolded in several subcellular compartments such as cytoplasm, peroxisomes, and mitochondria. But the risk of protein misfolding is particularly acute in the ER. Here, newly synthesized secretory and transmembrane proteins are transformed into proper tertiary structures. Even though eukaryotic cells have efficient quality control systems, which evolved over time to prevent incompletely folded molecules, the accumulation of misfolded proteins in the ER effects detrimentally the function and/or localization of approximately one-third of all cellular proteins. There are three different mechanistic levels to deal with the arise of unfolded proteins, namely, transcriptional induction, translational attenuation, and degradation (Mori 2000). The coordination between these mechanisms improves the efficiency of folding, processing, and exporting of secretory proteins. Additionally, it also removes the fraction of polypeptides that failed to fold and are responsible for reduced flow of proteins into the ER compartment. The intracellular signaling pathway, common from yeast to human, starts in the ER and ends at nucleus, is called UPR. The UPR target genes mostly encode for chaperons (Bip/Grp78 and Grp94) and enzymes involved in protein folding in the ER (protein disulfide isomerase encoded by pdi1 and peptidyl-prolyl cis-trans isomerase). The present literature depicts that the target genes also include numerous proteins involved in several steps or stages of the secretion pathway (Travers et al. 2000). The UPR itself includes three signaling pathways mediated either by IRE1 (inositol-requiring enzyme 1), PERK (protein kinase R-like ER kinase), or ATF6 (Activating transcription factor 6) that plays a vital role in re-establishing protein homeostasis of the cell. Among these, the Ire1 pathway is studied most extensively as it is the only one present in S. cerevisiae. It involves spliceosome-independent splicing of hac1 and xbp1 mRNA in yeast or mammalian cell, respectively. Recent evidence suggests that the unspliced mRNA in mammals is actually translated, and a hydrophobic segment in the nascent protein helps to target the mRNA to the ER membrane, thereby facilitating its splicing (Yanagitani et al. 2009). On the contrary, the long intron of 252 nt in the S. cerevisiae Hac1 blocks the translation of the unspliced mRNA by forming a stem-loop structure. This stem-loop interacts with the 5’UTR of the unspliced Hac1 mRNA, thereby stalling ribosomes (Rüegsegger et al. 2001). The removal of this intron by Ire1-mediated splicing releases the translation block, allowing the spliced mRNA to be translated.
Several pieces of evidence have been reported on the use of transgenic UPR strains in biotechnology industry to improve enzyme production. By expressing the activated form of the transcription factor HacA, induction significantly increased the production of T. versicolor laccase by up to sevenfold and bovine preprochymosin by up to 2.8-fold in this biotechnologically important fungus (Valkonen et al. 2003b). As additional examples, overexpressing the transcription factor Hac1 in A. awamori resulted in a 7- and 2.8-fold increased production of laccase and bovine prechymotrypsin is reported, respectively (Valkonen et al. 2003b). Further, disrupting the autophagy-related gene aoatg15 in A. oryzae resulted in a threefold increase in bovine chymosin secretion (Yoon et al. 2013). The following section describes the understanding of UPR and the impact of UPR on production processes in industrially employed filamentous fungi.
Examples of UPR and RESS in model and /or industrially employed filamentous fungi
Aspergillus fumigatus
The A. fumigatus UPR initiates with the proximal ER stress sensor IreA, comprising a similar domain organization as the yeast Ire1 (Feng et al. 2011). However, the target mRNA, hacAu, contains a short, 20 nucleotides (nt)-long intron (Richie et al. 2009) in contrast to the 252 nt-long intron in the corresponding hac1 mRNA in S. cerevisiae. The hacAu intron is similar in length to what has been already described in mammals, Candida albicans, Caenorhabditis elegans, and some filamentous fungi. Due to its small size, i.e., only 20 nt in HacAu, it is considered not to be involved in blocking 5′UTR of the unspliced hacAu mRNA (Calfon et al. 2002; Wimalasena et al. 2008; Saloheimo et al. 2003; Mulder et al. 2004). Another prominent difference between the yeast and A. fumigatus UPR is the size of the reading frame shift caused by removal of the unconventional intron in the hac1/hacA mRNAs. In A. fumigatus, the hacAu mRNA encodes a protein of 433 amino acids. The removal of the unconventional intron results in a replacement of a C-terminus domain of 220 amino acids of the HacAu protein with a 129 amino acids C-terminus domain. It was observed that high levels of exogenous ER stress (e.g., DTT) triggered the signaling through IreA-HacA pathway. This results in a signal transduction cascade influencing solely the secretory pathway elements, such as protein folding, ER translocation, vesicular trafficking, ER glycosylation, and ER degradation (Feng et al. 2011). However, in the absence of any exogenously induced ER stress, the transcriptional response of the UPR was more pronounced and more diverse in function, suggesting that the UPR is constantly modifying the output of the pathway in proportion to the stress level, even during normal growth. It is speculated that the challenge of delivering cell wall and membrane components to rapidly growing hyphal tips of fungi creates fluctuations in ER stress requiring dynamic changes in UPR activity to maintain ER homeostasis. Krishnan and co-worker made a surprising observation while studying UPR in A. fumigatus: a large fraction of differentially expressed genes in the absence of external ER stress were dependent on IreA, but not on HacA. This further suggests that IreA controls dual signaling circuits that can be both HacA dependent and HacA independent, particularly in this organism (Krishnan and Askew 2014).
Aspergillus niger
Examining gene regulation under secretion stress through genome-wide expression analysis has provided a comprehensive understanding of the same (Sims et al. 2005; Arvas et al. 2006; Guillemette et al. 2007; Carvalho et al. 2012; Kwon et al. 2012). For example, a core set of 40 genes crucial for high-level glucoamylase expression in A. niger was identified (Kwon et al. 2012). During ER stress induced by DTT and overexpression of human tissue plasminogen activator (t-PA) in A. niger, 25 genes exhibited similar upregulation (Guillemette et al. 2007).
Based on the importance of HacA in protein secretion, Valkonen and co-worker introduced a new approach to improve foreign-protein production in contrast to commonly used method of overexpression of specific chaperones and foldases. By expressing the activated form of the transcription factor HacA, they could achieve constitutive induction of the UPR pathway in A. niger var. awamori. To understand the regulatory scope of UPR, authors examined the mRNA levels of new A. niger var. awamori genes involved in various secretory functions and their findings revealed both similarities and differences compared to studies in S. cerevisiae. (Valkonen et al. 2003b). While there is an increase in the production of a native protein (invertase) in yeast, no such beneficial effect was observed in A. niger var. awamori on the production of native proteins. This discrepancy may be attributed to the lower secretion capacity of yeast, where subtle adjustments in the secretory machinery significantly impact the production of native proteins. Additionally, differences in the regulation of the secretory machinery between the two organisms could also contribute to this variation (Valkonen et al. 2003b). Many approaches have been employed to enhance protein production and secretion in A. niger based on this literature. Constitutive expression of active HacA seems adequate to reprogram the ER and downstream components of the secretory pathway. And this observation aligns with findings in S. cerevisiae (Valkonen et al. 2003a; Breinig et al. 2006) and Pichia pastoris (Vogl et al. 2014), as well as the filamentous fungus A. niger (Valkonen et al. 2003b), where constitutive activation of the UPR positively impacted heterologous protein production. Overexpressing UPR-related genes like sstC, hacA, gptA, pdiA, ostA, cnxA, or eroA, and deleting genes encoding ER-associated protein degradation (ERAD) components such as hrdC, derA, or doaA, also increased the production and secretion of heterologous proteins in A. niger (Sagt et al. 2008). Alongside UPR and ER stress-related genes, altering ERAD by deleting the factor DoaA and elevating oligosaccharyltransferase SttC (Yan and Lennarz 2002) in A. niger has been reported to increase the β-glucuronidase yield (Jacobs et al. 2009), given their involvement in glycosylation of secretory proteins. The investigation of the A. niger gene derA reveals that its deletion can not only enhance protein production but also impact cell growth (Carvalho et al. 2011; Richie et al. 2011). This highlights that the performance of protein secretion, regulated by UPR and ERAD, can be dependent on the host.
Neurospora crassa
In N. crassa, ER stress response is studied for induction and secretion of lignocellulose-degrading enzymes (Fan et al. 2015). Under ER stress conditions, 766 genes were found upregulated, of which 223 genes were regulated by the key component of UPR, i.e., IRE-1, and 186 genes by HAC-1. A total of 249 genes appeared to be pivotal for resistance against ER stress. The authors also analyzed the transcription factor (TF) network that putatively coordinates the signal flow and gene expression during the ER stress response and cellulase synthesis. Under such conditions, 33 TFs were upregulated including the UPR regulator HAC-1 and CPC-1 (a regulator associated with amino acid biosynthesis). HAC-1 was found to act as an important factor for secretion of lignocellulose-degrading enzymes, while it does not mediate the RESS feedback loop in N. crassa. When there is stress in the ER, the mRNA of hac-1 undergoes a splicing reaction (removal of 23 nt intron), thereby changing its open reading frame. Disrupting the N. crassa hac-1 gene revealed that it is essential not only for activating the UPR but also for proper growth of the fungus when exposed to chemicals causing ER stress. When N. crassa was subjected to grow on cellulose, which requires the secretion of many enzymes and consequently, the UPR and ER function become very important, then growth is significantly affected without hac-1. However, growth on hemicellulose, another substrate demanding enzyme secretion, is not affected in the mutant, suggesting that overall secretion is not altered without hac-1 (Montenegro-Montero et al. 2015). Further, CPC-1 may play a critical role in N. crassa ER stress response and cellulase secretion, although its regulation does not seem to be controlled by the IRE-1/HAC-1-mediated UPR cascade (Fan et al. 2015).
A homolog of the TF Ace1 (a cellulase repressor) in T. reesei and StzA (a stress responsive factor) in A. nidulans was identified as NCU09333 in N. crassa (Aro et al. 2003; Chilton et al. 2008). This TF is upregulated during ER stress; however, its role seems to be rather a stress responsive factor as it had limited effect on cellulase secretion. The expression level of the carbon catabolite repression regulator CRE-1 (NCU08807) in N. crassa was also found to be upregulated during ER stress. Based on experimental evidence, authors suggested that the carbon catabolite repression pathway might cross-talk with the ER stress response (Fan et al. 2015).
RESS in N. crassa was shown to be independent of the IRE-1/HAC-1-mediated UPR transduction cascade. RESS-mediated repression of cellulase genes was likely to be a direct result of the down-regulation of the two regulators CLR-2 and XLR-1. To support this assumption, the expression levels of some carbohydrate transporter genes belonging to the CLR-2 and XLR-1 regulons in N. crassa, i.e., CDT-1 (NCU00801) and LAT-1 (NCU02188), were evaluated. Both genes had a decrease in expression level; however, how it is triggered in the presence of ER stress remains to be investigated (Fan et al. 2015). Also, understanding the enzyme secretion pathway in N. crassa, wherein HAC-1 does not play the crucial role, will widen the knowledge about fungal mechanisms for breaking down plant cell walls, which in turn could have impact on industrial biotechnology (Montenegro-Montero et al. 2015).
Trichoderma reesei
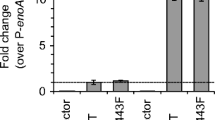
In case of T. reesei, the mechanism to cope up with ER stress remains conserved. Here also unfolded proteins bind to the ER chaperone Bip1. This hinders the binding of Bip1 to the transmembrane protein kinase Ire1. Ire1 activates itself by homodimerization and autophosphorylation and splices the mRNA of the transcription factor Hac1 (Fig 2). In T. reesei a short intron of 20 bp is spliced from the Hac1 mRNA and additionally, a long part at the 5′-flanking region (Saloheimo et al. 2003). The truncation of the 5′-flanking region (250 nt) can be mediated either by ribonuclease cleavage or by transcription from a different start site. It was suggested that preferably the latter is the case in T. reesei because the region is extremely CT rich, which is a typical feature for a transcription start site (Gurr 1987). T. reesei Hac1 mRNA has two upstream ORFs encoding 18 and 2 amino acids. The longer one is absent from the mRNA during the induction of UPR. The presence of 2 amino acids in the 250 nt-truncated Hac1 indicates its non-significant effect on Hac1 mRNA translation. Hac1 upregulates expression of chaperones, proteases, and vesicle trafficking genes (Fig. 2) that work together to remove the unfolded proteins from the ER and re-establish the balance in the protein secretion pathway (Travers et al. 2000). In a study in T. reesei, the results from the combined analysis of the expressed sequence tag (EST) collection of T. reesei subjected to different stress conditions confirmed the induction of Bip1 and Pdi1, characterized as UPR-related genes (Saloheimo et al. 2003; Pakula et al. 2003). Furthermore, ESTs corresponding to at least 457 genes were found to be putatively induced during secretion stress in T. reesei. A specific EST homolog present in T. reesei corresponds to cpc gene in filamentous fungi or to Gcn4 of S. cerevisiae, which regulates amino acid biosynthesis (Arvas et al. 2006). However, the key difference is their activation under amino acid deprivation conditions. Gcn4 is regulated at the translational level, whereas Cpc proteins are found to be controlled at both the translational and transcriptional level (Wanke et al. 1997; Paluh et al. 1988; Albrecht et al. 1998). The corresponding Cpc1 targets in T. reesei, that are induced or affected under UPR, are the glt1, arg1, and aro1 genes (Arvas et al. 2006). In addition, two more crucial genes related to protein secretion, Ypt1/YptA and Nsf1/NsfA, were described in T. reesei and A. niger var. awamori. These genes encode for the Rab protein and the general fusion factor, respectively. Both show high conservation with their counterparts in S. cerevisiae and mammals. The T. reesei ypt1 gene resembles the effect observed in a yeast Ypt1p (Ypt1 protein) deletion study. Transcriptional regulation of T. reesei genes involved in protein trafficking (ypt1, nsf1, and sar1) was examined using the protein-folding inhibitor DTT and the protein-trafficking inhibitor brefeldin A. DTT induced nsf1 and pdi1, sar1 mRNA increased under strong UPR induction, and ypt1 mRNA did not show a clear increase. Brefeldin A strongly induced pdi1 and other intracellular trafficking genes, suggesting a potential transcriptional activation of the entire secretory pathway of T. reesei in response to protein accumulation stress. This is in contrast to S. cerevisiae, in which only a small subset of the secretory pathway-encoding genes responds to the UPR (Saloheimo et al. 2004). Another comparative study of the homologs of the S. cerevisiae proteins Ire1 and Ptc2 in T. reesei resulted in the confirmation of the same functional activity in both organisms. The T. reesei Ire1 protein displays inherent kinase activity, which was demonstrated in vitro by an autophosphorylation assay. When Ire1 was overexpressed in a T. reesei strain producing a foreign protein (laccase 1 from Phlebia radiata), an up-regulation of the UPR pathway was observed. by detecting elevated expression levels of UPR target genes such as bip1 and pdi1 (Valkonen et al. 2003a).
Also, elevating the expression of genes encoding protein disulfide isomerase (Pdi) or Kar2/Bip was demonstrated to increase the production of certain foreign proteins in both S. cerevisiae and filamentous fungi (Punt et al. 1998). This study illustrated that inducing the UPR by overexpressing S. cerevisiae hac1 and T. reesei hac1 in S. cerevisiae enhanced the production of both foreign and native proteins (Valkonen et al. 2003a). In T. reesei, overexpressing bip1 and hac1 led to a 1.5- and 1.8-fold increase in the secretion of heterologous glucose oxidase (Wu et al. 2017). Furthermore, hacA overexpression in A. niger var. awamori stimulated the expression of two foreign proteins, T. versicolor laccase1 and calf preprochymosin (Valkonen et al. 2003b; Valkonen et al. 2004). In addition, Pakula et al. observed a transcriptional down-regulation of secreted protein-encoding genes under secretion stress in T. reesei. This response was termed as RESS, which was also described in A. niger and A. nidulans (Al-Sheikh et al. 2004; Sims et al. 2005). However, how RESS is triggered during UPR and whether it acts instead of or additionally to regulated Ire1-dependent pathway is unknown. A previous study indicated that elevated Cre1 expression levels cause perturbations in intracellular glucose homeostasis under ER stress conditions. And this observation raises the possibility that metabolic repression caused by internal fluctuations of intracellular nutritional cues (e.g., simple sugars or free amino acids) might have an impact on RESS, although more data need to be acquired to support this hypothesis (Fan et al. 2015).
Outlook
A recent study using S. cerevisiae strains with W303 background (an allelic variant of mip1, which increases petite frequency and lacks a functional copy of the RNA-binding protein and translational repressor Ssd1) revealed that the nucleosome spacing–enzyme Isw1 (ATP-dependent chromatin remodeler), which promotes the accessibility of chromatin and exports nuclear-retained mRNPs (Babour et al. 2016), is critical to fine tune the UPR homeostasis feedback loop. It binds to the 3′UTR of the hac1 transcript and limits its nuclear export resulting into cytoplasmic splicing (Matabishi-Bibi et al. 2022). This raises the questions firstly, about its presence in industrially important filamentous fungi, and secondly, whether it has any effect on RESS and eventually on the secretion of hydrolytic enzyme either at transcriptional or translational level.
To conclude, protein secretion is a crucial and complex process in filamentous fungi and the ER plays a central role in protein synthesis, folding, and secretion. However, the high demand for protein production can lead to ER stress, triggering the UPR. The UPR is a multifaceted adaptive mechanism that aims to restore ER homeostasis and ensure correct protein folding and secretion. It involves the upregulation of chaperones, foldases, and protein degradation. But the systematic information on ER stress at a cellular level is explored in only three model systems, mammalian cells (human B cells) (Dombroski et al. 2010), Drosophila lines (Chow et al. 2013), and knock-out mutants of S. cerevisiae (Jonikas et al. 2009). The information on ER stress along with the protein secretion pathway in industrially employed filamentous fungi (particularly enzyme producers) points to a lack of knowledge on a) association between protein secretion and ER stress, b) how antioxidants could reduce ER stress in relation with oxidative stress, and c) RESS, which might be a crucial problem for heterologous or overexpression of enzymes produced at industrial scale. The latter point is important because there are some fungi, which seem to either do not have or have Hac1-independent RESS mechanisms, like A. fumigatus and N. crassa, while others, like T. reesei, do. Hence, it would be beneficial to investigate more industrially employed fungi for this mechanism to reveal the precise connection to UPR that might allow to modify or inhibit RESS.
References
Akabori S, Ikenaka T, Hagihara B (1954) Isolation of crystalline taka-amylase A from “Takadiastase Sankyo”. J Biochem 41(5):577–582. http://www.jbsoc.or.jp/
Akao T, Yahara A, Sakamoto K, Yamada O, Akita O, Yoshida T (2012) Lack of endoplasmic reticulum 1, 2-α-mannosidase activity that trims N-glycan Man9GlcNAc2 to Man8GlcNAc2 isomer B in a manE gene disruptant of Aspergillus oryzae. J Biosci Bioeng 113(4):438–441. https://doi.org/10.1016/j.jbiosc.2011.11.015
Akao T, Yamaguchi M, Yahara A, Yoshiuchi K, Fujita H, Yamada O, Akita O, Ohmachi T, Asada Y, Yoshida T (2006) Cloning and expression of 1, 2-α-mannosidase gene (fmanIB) from filamentous fungus Aspergillus oryzae: in vivo visualization of the FmanIBp-GFP fusion protein. Biosci Biotechnol Biochem 70(2):471–479. https://doi.org/10.1271/bbb.70.471
Albrecht G, Mo HU, Hoffmann B, Reusser U, Braus GH (1998) Monitoring the Gcn4 protein-mediated response in the yeast Saccharomyces cerevisiae. JBC 273(21):12696–12702. https://doi.org/10.1074/jbc.273.21.12696
Al-Sheikh H, Watson AJ, Lacey GA, Punt PJ, MacKenzie DA, Jeenes DJ, Pakula T, Penttilä M, Alcocer MJ, Archer DB (2004) Endoplasmic reticulum stress leads to the selective transcriptional downregulation of the glucoamylase gene in Aspergillus niger. Mol Microbiol 53(6):1731–1742. https://doi.org/10.1111/j.1365-2958.2004.04236.x
Antonin W, Meyer HA, Hartmann E (2000) Interactions between Spc2p and other components of the endoplasmic reticulum translocation sites of the yeast Saccharomyces cerevisiae. JBC 275(44):34068–34072. https://doi.org/10.1074/jbc.M006126200
Archer DB, Peberdy JF (1997) The molecular biology of secreted enzyme production by fungi. Crit Rev Biotechnol 17:273–306. https://doi.org/10.3109/07388559709146616
Arnau J, Yaver D, Hjort CM (2020) Strategies and challenges for the development of industrial enzymes using fungal cell factories. In: Nevalainen H (ed) Grand challenges in fungal biotechnology. Springer, pp 179–210. https://doi.org/10.1007/978-3-030-29541-7_7
Aro N, Ilmén M, Saloheimo A, Penttilä M (2003) ACEI of Trichoderma reesei is a repressor of cellulase and xylanase expression. AEM 69(1):56–65. https://doi.org/10.1128/AEM.69.1.56-65.2003
Arvas M, Pakula T, Lanthaler K, Saloheimo M, Valkonen M, Suortti T, Robson G, Penttilä M (2006) Common features and interesting differences in transcriptional responses to secretion stress in the fungi Trichoderma reesei and Saccharomyces cerevisiae. BMC Genom 7(1):1–8. https://doi.org/10.1186/1471-2164-7-32
Babour A, Shen Q, Dos-Santos J, Murray S, Gay A, Challal D, Fasken M, Palancade B, Corbett A, Libri D, Mellor J (2016) The chromatin remodeler ISW1 is a quality control factor that surveys nuclear mRNP biogenesis. Cell 167(5):1201–1214. https://doi.org/10.1016/j.cell.2016.10.048
Baker SE, Thykaer J, Adney WS, Brettin T, Brockman F, Dhaeseleer P, Martinez A, Miller R, Rokhsar D, Schadt CW, Torok T, Tuskan GA, Benett J, Berka R, Briggs S, Heitman J, Taylor J, Turgeon G, Werner-Washburne M, Himmel M (2008) Fungal genome sequencing and bioenergy. Fungal Biol Rev 22:1–5. https://doi.org/10.1016/j.fbr.2008.03.001
Bennett JW, Baker SE (2008) An overview of the genus Aspergillus. In: Goldman GH, Osmani SA (eds) The Aspergilli: genomics, medical aspects, biotechnology, and research methods. CRC Press, Taylor & Francis Group, BR FL, pp 3–13. https://doi.org/10.1201/9781420008517.sec1
Breinig F, Diehl B, Rau S, Zimmer C, Schwab H, Schmitt MJ (2006) Cell surface expression of bacterial esterase A by Saccharomyces cerevisiae and its enhancement by constitutive activation of the cellular unfolded protein response. Appl Environ Microbiol 72:7140–7147. https://doi.org/10.1128/AEM.00503-06
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415(6867):92–96. https://doi.org/10.1038/415092a
Carvalho ND, Arentshorst M, Kooistra R, Stam H, Sagt CM, van den Hondel CA, Ram AF (2011) Effects of a defective ERAD pathway on growth and heterologous protein production in Aspergillus niger. Appl Microbiol Biotechnol 89:357–373. https://doi.org/10.1007/s00253-010-2916-5
Carvalho ND, Jorgensen TR, Arentshorst M, Nitsche BM, van den Hondel CA, Archer DB, Ram AF (2012) Genome-wide expression analysis upon constitutive activation of the HacA bZIP transcription factor in Aspergillus niger reveals a coordinated cellular response to counteract ER stress. BMC Genom 13:350. https://doi.org/10.1186/1471-2164-13-350
Chilton IJ, Delaney CE, Barham-Morris J, Fincham DA, Hooley P, Whitehead MP (2008) The Aspergillus nidulans stress response transcription factor StzA is ascomycete-specific and shows species-specific polymorphisms in the C-terminal region. Mycol Res 112(12):1435–1446. https://doi.org/10.1016/j.mycres.2008.06.028
Chow CY, Wolfner MF, Clark AG (2013) Using natural variation in Drosophila to discover previously unknown endoplasmic reticulum stress genes. PNAS 110(22):9013–9018. https://doi.org/10.1073/pnas.1307125110
Conesa A, Punt PJ, van Luijk N, van den Hondel CA (2001) The secretion pathway in filamentous fungi: a biotechnological view. Fungal Genet Biol 33(3):155–171. https://doi.org/10.1006/fgbi.2001.1276
Corsi AK, Schekman R (1996) Mechanism of polypeptide translocation into the endoplasmic reticulum. JBC 271(48):30299–30302. https://doi.org/10.1074/jbc.271.48.30299
Deshpande N, Wilkins MR, Packer N, Nevalainen H (2008) Protein glycosylation pathways in filamentous fungi. Glycobiology 18(8):626–637. https://doi.org/10.1093/glycob/cwn044
Dombroski BA, Nayak RR, Ewens KG, Ankener W, Cheung VG, Spielman RS (2010) Gene expression and genetic variation in response to endoplasmic reticulum stress in human cells. AJHG 86(5):719–729. https://doi.org/10.1016/j.ajhg.2010.03.017
El-Zalaki ME, Hamza M (1979) Edible mushrooms as producers of amylases. Food Chem 4:203–211. https://doi.org/10.1016/0308-8146(79)90005-0
Fajardo-Somera RA, Bowman B, Riquelme M (2013) The plasma membrane proton pump PMA-1 is incorporated into distal parts of the hyphae independently of the Spitzenkörper in Neurospora crassa. Eukaryot Cell 12:1097e1105. https://doi.org/10.1128/ec.00328-12
Fan F, Ma G, Li J, Liu Q, Benz JP, Tian C, Ma Y (2015) Genome-wide analysis of the endoplasmic reticulum stress response during lignocellulase production in Neurospora crassa. Biotechnol Biofuel 8(1):1–7. https://doi.org/10.1186/s13068-015-0248-5
Feng X, Krishnan K, Richie DL, Aimanianda V, Hartl L, Grahl N, Powers-Fletcher MV, Zhang M, Fuller KK, Nierman WC, Lu LJ (2011) HacA-independent functions of the ER stress sensor IreA synergize with the canonical UPR to influence virulence traits in Aspergillus fumigatus. PLoS Pathog 7(10):e1002330. https://doi.org/10.1371/journal.ppat.1002330
Fischer EH, De Montmollin R (1951) Crystallization of the α-amylase of Aspergillus oryzae. Nature 168(4275):606–607. https://doi.org/10.1038/168606a0
Frigerio G, Pelham HR (1993) A Saccharomyces cerevisiae cyclophilin resident in the endoplasmic reticulum. J Mol Biol 233(1):183–188. https://doi.org/10.1006/jmbi.1993.1497
Fritz-Langhals E, Kunath B (1998) Synthesis of aromatic aldehydes by laccase-mediator assisted oxidation. Tetrahedron Lett 39:5955–5956. https://doi.org/10.1016/S0040-4039(98)01215-5
Gething MJ, Sambrook J (1992) Protein folding in the cell. Nature 355:33–44. https://doi.org/10.1038/355033a0
Górka-Nieć W, Pniewski M, Kania A, Perlińska-Lenart U, Palamarczyk G, Kruszewska JS (2008) Disruption of Trichoderma reesei gene encoding protein O-mannosyltransferase I results in a decrease of the enzyme activity and alteration of cell wall composition. Acta Biochim Pol 55(2):251–259. https://doi.org/10.18388/abp.2008_3072
Goto M (2007) Protein O-glycosylation in fungi: diverse structures and multiple functions. Biosci Biotechnol Biochem 71(6):1415–1427. https://doi.org/10.1271/bbb.70080
Grigoriev IV, Cullen D, Goodwin SB, Hibbett D, Jeffries TW, Kubicek CP, Kuske C, Magnuson JK, Martin F, Spatafora JW, Tsang A (2011) Fueling the future with fungal genomics. Mycology 2:192–209. https://doi.org/10.1080/21501203.2011.584577
Guillemette T, van Peij N, Goosen T, Lanthaler K, Robson GD, van den Hondel CA, Stam H, Archer DB (2007) Genomic analysis of the secretion stress response in the enzyme-producing cell factory Aspergillus niger. BMC Genom 8:158. https://doi.org/10.1186/1471-2164-8-158
Gurr SJ (1987) The structure and organization of nuclear genes of filamentous fungi. Gene structure in eukaryotic microbes, pp 93–139. https://books.google.at/books?hl=en&lr=&id=8me4mMg2p2sC&oi=fnd&pg=PA396&dq=Gurr+SJ+(1987)+The+structure+and+organization+of+nuclear+genes+of+filamentous+fungi.+Gene+structure+in+eukaryotic+microbes.+93-139.&ots=_1vdvF3XiR&sig=0a1SlSKhx90ytrCyJgY9Bc-8r4I&redir_esc=y#v=onepage&q=Gurr%20SJ%20(1987)%20The%20structure%20and%20organization%20of%20nuclear%20genes%20of%20filamentous%20fungi.%20Gene%20structure%2139.&f=false
Gurung N, Ray S, Bose S, Rai V (2013) A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. Biomed Res Int. https://doi.org/10.1155/2013/329121
Hayakawa Y, Ishikawa E, Shoji JY, Nakano H, Kitamoto K (2011) Septum-directed secretion in the filamentous fungus Aspergillus oryzae. Mol Microbiol 81(1):40–55. https://doi.org/10.1111/j.1365-2958.2011.07700.x
Hernández-Chávez MJ, González-Hernández RJ, Trujillo-Esquivel JE, Hernández-Cervantes A, Mora-Montes HM (2014) The secretory pathway in the filamentous fungus Trichoderma. In: Biotechnology and biology of Trichoderma. Elsevier, pp 115–121. https://doi.org/10.1016/B978-0-444-59576-8.00009-6
Hoang HD, Maruyama JI, Kitamoto K (2015) Modulating endoplasmic reticulum-Golgi cargo receptors for improving secretion of carrier-fused heterologous proteins in the filamentous fungus Aspergillus oryzae. AEM 81(2):533–543. https://doi.org/10.1128/AEM.02133-14
Idiris A, Tohda H, Kumagai H, Takegawa K (2010) Engineering of protein secretion in yeast: strategies and impact on protein production. Appl Microbiol Biotechnol 86:403e417. https://doi.org/10.1007/s00253-010-2447-0
Ito T, Takiguchi Y (1970) Naringinase production by Cochiobolus miyabeanus. Jpn Patent 7:875
Jacobs DI, Olsthoorn MM, Maillet I, Akeroyd M, Breestraat S, Donkers S, van der Hoeven RA, van den Hondel CA, Kooistra R, Lapointe T, Menke H, Meulenberg R, Misset M, Muller WH, van Peij NN, Ram A, Rodriguez S, Roelofs MS, Roubos JA et al (2009) Effective lead selection for improved protein production in Aspergillus niger based on integrated genomics. Fungal Genet Biol 46(Suppl 1):S141–S152. https://doi.org/10.1016/j.fgb.2008.08.012
Jahn R, Scheller RH (2006) SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol 7(9):631–643. https://doi.org/10.1038/nrm2002
Jonikas MC, Collins SR, Denic V, Oh E, Quan EM, Schmid V, Weibezahn J, Schwappach B, Walter P, Weissman JS, Schuldiner M (2009) Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 323(5922):1693–1697. https://doi.org/10.1126/science.1167
Kamini N, Hemachander C, Mala JGS, Puvanakrishnan R (1999) Microbial enzyme technology as an alternative to conventional chemicals in leather industry. Curr Sci 77:80–86 https://www.jstor.org/stable/24102916
Kasajima Y, Yamaguchi M, Hirai N, Ohmachi T, Yoshida T (2006) In vivo expression of UDP-N-acetylglucosamine: α-3-D-mannoside β-1, 2-N-acetylglucosaminyltransferase I (GnT-1) in Aspergillus oryzae and effects on the sugar chain of α-amylase. Biosci Biotechnol Biochem 70(11):2662–2668. https://doi.org/10.1271/bbb.60265
Kautto L, Grinyer J, Paulsen I, Tetu S, Pillai A, Pardiwalla S, Sezerman U, Akcapinar GB, Bergquist P, Te’o J, Nevalainen H (2013) Stress effects caused by the expression of a mutant cellobiohydrolase I and proteasome inhibition in Trichoderma reesei Rut-C30. New Biotechnol 30(2):183–191. https://doi.org/10.1016/j.nbt.2012.07.005
Kim YG, Raunser S, Munger C, Wagner J, Song YL, Cygler M, Walz T, Oh BH, Sacher M (2006) The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell 127(4):817–830. https://doi.org/10.1016/j.cell.2006.09.029
Kimura S, Maruyama JI, Watanabe T, Ito Y, Arioka M, Kitamoto K (2010) In vivo imaging of endoplasmic reticulum and distribution of mutant α-amylase in Aspergillus oryzae. Fungal Genet Biol 47(12):1044–1054. https://doi.org/10.1016/j.fgb.2010.09.003
Kimura T, Hosoda Y, Kitamura Y, Nakamura H, Horibe T, Kikuchi M (2004) Functional differences between human and yeast protein disulfide isomerase family proteins. BBRC 320(2):359–365. https://doi.org/10.1016/j.bbrc.2004.05.178
Kottmeier K, Ostermann K, Bley T, Rödel G (2011) Hydrophobin signal sequence mediates efficient secretion of recombinant proteins in Pichia pastoris. Appl Microbiol Biotechnol 91:133–141. https://doi.org/10.1007/s00253-011-3246-y
Krishnan K, Askew DS (2014) The fungal UPR: a regulatory hub for virulence traits in the mold pathogen Aspergillus fumigatus. Virulence 5(2):334–340. https://doi.org/10.4161/viru.26571
Kruszewska JS, Perlińska-Lenart U, Górka-Nieć W, Orłowski J, Zembek P, Palamarczyk G (2008) Alterations in protein secretion caused by metabolic engineering of glycosylation pathways in fungi. Acta Biochim Pol 55(3):447–456. https://doi.org/10.18388/abp.2008_3050
Kwon MJ, Jorgensen TR, Nitsche BM, Arentshorst M, Park J, Ram AF, Meyer V (2012) The transcriptomic fingerprint of glucoamylase over-expression in Aspergillus niger. BMC Genomics 13:701. https://doi.org/10.1186/1471-2164-13-701
Liu L, Feizi A, Österlund T, Hjort C, Nielsen J (2014) Genome-scale analysis of the high-efficient protein secretion system of Aspergillus oryzae. BMC Syst Biol 8(1):1–3. https://doi.org/10.1186/1752-0509-8-73
Malhotra V (2013) Unconventional protein secretion: an evolving mechanism. EMBO J 32(12):1660–1664. https://doi.org/10.1038/emboj.2013.104
Maras M, van Die I, Contreras R, van den Hondel CA (1999) Filamentous fungi as production organisms for glycoproteins of biomedical interest. Glycoconj J 16:99–107. https://doi.org/10.1007/978-1-4615-5257-4_2
Markham P (1995) Organelles of filamentous fungi. In: The growing fungus. Springer, pp 75–98. https://doi.org/10.1007/978-0-585-27576-5_5
Maruyama JI, Kikuchi S, Kitamoto K (2006) Differential distribution of the endoplasmic reticulum network as visualized by the BipA–EGFP fusion protein in hyphal compartments across the septum of the filamentous fungus, Aspergillus oryzae Fungal. Genet Biol 43(9):642–654. https://doi.org/10.1016/j.fgb.2005.11.007
Masai K, Maruyama JI, Nakajima H, Kitamoto K (2003) In vivo visualization of the distribution of a secretory protein in Aspergillus oryzae hyphae using the RntA-EGFP fusion protein. Biosci Biotechnol Biochem 67(2):455–459. https://doi.org/10.1271/bbb.67.455
Matabishi-Bibi L, Challal D, Barucco M, Libri D, Babour A (2022) Termination of the unfolded protein response is guided by ER stress-induced HAC1 mRNA nuclear retention. Nat Commun 13(1):6331. https://doi.org/10.1038/s41467-022-34133-8
Montenegro-Montero A, Goity A, Larrondo LF (2015) The bZIP transcription factor HAC-1 is involved in the unfolded protein response and is necessary for growth on cellulose in Neurospora crassa. PLoS One 10(7):e0131415
Mora-Montes HM, Ponce-Noyola P, Villagómez-Castro JC, Gow NA, Flores-Carreón A, López-Romero E (2009) Protein glycosylation in Candida. Future Microbiol 4(9):1167–1183. https://doi.org/10.2217/fmb.09.88
Mori K (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101(5):451–454. https://doi.org/10.1016/S0092-8674(00)80855-7
Mulder HJ, Saloheimo M, Penttilä M, Madrid SM (2004) The transcription factor HACA mediates the unfolded protein response in Aspergillus niger, and up-regulates its own transcription. Mol Genet Genomics 271:130–140. https://doi.org/10.1007/s00438-003-0965-5
Nakano A, Brada D, Schekman R (1988) A membrane glycoprotein, Sec12p, required for protein transport from the endoplasmic reticulum to the Golgi apparatus in yeast. J Cell Biol 107:851–863. https://doi.org/10.1083/jcb.107.3.851
Nakano A, Muramatsu M (1989) A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol 109:2677–2691. https://doi.org/10.1083/jcb.109.6.2677
Neelakantan S, Mohanty A, Kaushik JK (1999) Production and use of microbial enzymes for dairy processing. Curr Sci 77:143–148 https://www.jstor.org/stable/24102922
Nemoto T, Maruyama JI, Kitamoto K (2012) Contribution ratios of amyA, amyB, amyC genes to high-level α-amylase expression in Aspergillus oryzae. Biosci Biotechnol Biochem 76(8):1477–1483. https://doi.org/10.1271/bbb.120142
Normington K, Kohno K, Kozutsumi Y, Gething MJ, Sambrook J (1989) S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell 57(7):1223–1236. https://doi.org/10.1016/0092-8674(89)90059-7
Novick P, Zerial M (1997) The diversity of Rab proteins in vesicle transport. COCEBI 9(4):496–504. https://doi.org/10.1016/S0955-0674(97)80025-7
Nykänen M, Birch D, Peterson R, Yu H, Kautto L, Gryshyna A, Te’o J, Nevalainen H (2016) Ultrastructural features of the early secretory pathway in Trichoderma reesei. Curr Genet 62:455–465. https://doi.org/10.1007/s00294-015-0555-1
Nykänen M, Saarelainen R, Raudaskoski M, Nevalainen K, Mikkonen A (1997) Expression and secretion of barley cysteine endopeptidase B and cellobiohydrolase I in Trichoderma reesei. Appl Environ Microbiol 63(12):4929–4937. https://doi.org/10.1128/aem.63.12.4929-4937.1997
Pakula TM, Laxell M, Huuskonen A, Uusitalo J, Saloheimo M, Penttilä M (2003) The effects of drugs inhibiting protein secretion in the filamentous fungus Trichoderma reesei: evidence for down-regulation of genes that encode secreted proteins in the stressed cells. J Biol Chem 278(45):45011–45020. https://doi.org/10.1074/jbc.M302372200
Paluh JL, Orbach MJ, Legerton TL, Yanofsky C (1988) The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-jun-encoded protein. PNAS 85(11):3728–3732. https://doi.org/10.1073/pnas.85.11.3728
Parlati F, Dominguez M, Bergeron JJ, Thomas DY (1995) Saccharomyces cerevisiae CNE1 encodes an endoplasmic reticulum (ER) membrane protein with sequence similarity to calnexin and calreticulin and functions as a constituent of the ER quality control apparatus (∗). J Biol Chem 270(1):244–253. https://doi.org/10.1074/jbc.270.1.244
Patel AK, Singhania RR, Pandey A (2016) Novel enzymatic processes applied to the food industry. Curr Opin Food Sci 7:64–72. https://doi.org/10.1016/j.cofs.2015.12.002
Punt PJ, van Gemeren IA, Drint-Kuijvenhoven J, Hessing JG, van Muijlwijk-Harteveld GM, Beijersbergen A, Verrips CT, van den Hondel CA (1998) Analysis of the role of the gene bipA, encoding the major endoplasmic reticulum chaperone protein in the secretion of homologous and heterologous proteins in black Aspergilli. Appl Microbiol Biotechnol 50(4):447–454. https://doi.org/10.1007/s00253-010-2916-5
Richie DL, Hartl L, Aimanianda V, Winters MS, Fuller KK, Miley MD, White S, McCarthy JW, Latgé JP, Feldmesser M, Rhodes JC (2009) A role for the unfolded protein response (UPR) in virulence and antifungal susceptibility in Aspergillus fumigatus. PLoS Pathog 5(1):e1000258. https://doi.org/10.1371/journal.ppat.1000258
Richie DL, Feng X, Hartl L, Aimanianda V, Krishnan K, Powers-Fletcher MV, Watson DS, Galande AK, White SM, Willett T, Latgé JP (2011) The virulence of the opportunistic fungal pathogen Aspergillus fumigatus requires cooperation between the endoplasmic reticulum-associated degradation pathway (ERAD) and the unfolded protein response (UPR). Virulence 2(1):12–21. https://doi.org/10.4161/viru.2.1.13345
Riquelme M, Roberson RW, McDaniel DP, Bartnicki-Garcıa S (2002) The effects of ropy-1 mutation on cytoplasmic organization and intracellular motility in mature hyphae of Neurospora crassa. Fungal Genet Biol 37:171–179. https://doi.org/10.1016/S1087-1845(02)00506-6
Romisch K (1999) Surfing the Sec61 channel: bidirectional protein translocation across the ER membrane. J Cell Sci 112(23):4185–4191. https://doi.org/10.1242/jcs.112.23.4185
Rothman JE, Warren G (1994) Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol 4(3):220–233. https://doi.org/10.1016/S0960-9822(00)00051-8
Rüegsegger U, Leber JH, Walter P (2001) Block of HAC1 mRNA translation by long-range base pairing is released by cytoplasmic splicing upon induction of the unfolded protein response. Cell 107(1):103–114. https://doi.org/10.1016/S0092-8674(01)00505-0
Sagt CMJ, Verrips CT, Muller WH, Peij VNNME (2008) Improved production of secreted proteins by filamentous fungi. Worldwide patent WO/2008/053018 A2. https://patents.google.com/patent/US8389269B2/en
Sakekar AA, Gaikwad SR, Punekar NS (2021) Protein expression and secretion by filamentous fungi. J Biosci 46:1–18. https://doi.org/10.1007/s12038-020-00120-8
Saloheimo M, Pakula TM (2012) The cargo and the transport system: secreted proteins and protein secretion in Trichoderma reesei (Hypocrea jecorina). Microbiology 158(1):46–57. https://doi.org/10.1099/mic.0.053132-0
Saloheimo M, Valkonen M, Penttilä M (2003) Activation mechanisms of the HACI-mediated unfolded protein response in filamentous fungi. Mol Microbiol 47(4):1149–1161. https://doi.org/10.1046/j.1365-2958.2003.03363.x
Saloheimo M, Wang H, Valkonen M, Vasara T, Huuskonen A, Riikonen M, Pakula T, Ward M, Penttilä M (2004) Characterization of secretory genes ypt1/yptA and nsf1/nsfA from two filamentous fungi: induction of secretory pathway genes of Trichoderma reesei under secretion stress conditions. Appl Environ Microbiol 70(1):459–467. https://doi.org/10.1128/AEM.70.1.459-467.2004
Saxena R, Gupta R, Saxena S, Gulati R (2001) Role of fungal enzymes in food processing. Appl Mycol Biotechnol 1:353–386. https://doi.org/10.1016/S1874-5334(01)80015-0
Shoji JY, Kikuma T, Kitamoto K (2014) Vesicle trafficking, organelle functions, and unconventional secretion in fungal physiology and pathogenicity. Curr Opin Microbiol 20:1e9. https://doi.org/10.1016/j.mib.2014.03.002
Sims AH, Gent ME, Lanthaler K, Dunn-Coleman NS, Oliver SG, Robson GD (2005) Transcriptome analysis of recombinant protein secretion by Aspergillus nidulans and the unfolded-protein response in vivo. AEM 71(5):2737–2747. https://doi.org/10.1128/AEM.71.5.2737-2747.2005
Singh R, Kumar M, Mittal A, Mehta PK (2016) Microbial enzymes: industrial progress in 21st century. 3 Biotech 6:174. https://doi.org/10.1007/s13205-016-0485-8
Souza PMD (2010) Application of microbial α-amylase in industry-a review. Braz J Microbiol 41:850–886. https://doi.org/10.1590/S1517-83822010000400004
Souza PMD, Bittencourt MLDA, Caprara CC, Freitas MD, Almeida RPCD, Silveira D, Fonseca YM, Ferreira Filho EX, Pessoa Junior A, Magalhães PO (2015) A biotechnology perspective of fungal proteases. Braz J Microbiol 46:337–346. https://doi.org/10.1590/S1517-838246220140359
Sun X, Su X (2019) Harnessing the knowledge of protein secretion for enhanced protein production in filamentous fungi. World J Microbiol Biotechnol 35:1–10. https://doi.org/10.1007/s11274-019-2630-0
Tada S, Iimura Y, Gomi K, Takahashi K, Hara S, Yoshizawa K (1989) Cloning and nucleotide sequence of the genomic Taka-amylase A gene of Aspergillus oryzae. Agric Biol Chem 53(3):593–599. https://doi.org/10.1080/00021369.1989.10869378
Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101(3):249–258. https://doi.org/10.1016/S0092-8674(00)80835-1
Valkonen M, Kalkman ER, Saloheimo M, Penttilaö M, Read ND, Duncan RR (2007) Spatially segregated SNARE protein interactions in living fungal cells. J Biol Chem 282(31):22775–22785. https://doi.org/10.1074/jbc.M700916200
Valkonen M, Penttilä M, Saloheimo M (2003a) Effects of inactivation and constitutive expression of the unfolded-protein response pathway on protein production in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol 69:2065–2072. https://doi.org/10.1128/AEM.69.4.2065-2072.2003
Valkonen M, Penttilä M, Saloheimo M (2004) The ire1 and ptc2 genes involved in the unfolded protein response pathway in the filamentous fungus Trichoderma reesei. Mol Gen Genomics 272:443–451. https://doi.org/10.1007/s00438-004-1070-0
Valkonen M, Ward M, Wang H, Penttilä M, Saloheimo M (2003b) Improvement of foreign-protein production in Aspergillus niger var. awamori by constitutive induction of the unfolded-protein response. Appl Environ Microbiol 69(12):6979–6986. https://doi.org/10.1128/AEM.69.12.6979-6986.2003
Veldhuisen G, Saloheimo M, Fiers MA, Punt PJ, Contreras R, Penttilä M, Van Den Hondel CAMJJ (1997) Isolation and analysis of functional homologues of the secretion-related SAR1 gene of Saccharomyces cerevisiae from Aspergillus niger and Trichoderma reesei. MGG 256:446–455
Vishwanatha KS, Rao AA, Singh SA (2010) Production and characterization of a milk-clotting enzyme from Aspergillus oryzae MTCC 5341. Appl Microbiol Biotechnol 85:1849–1859. https://doi.org/10.1007/s00253-009-2197-z
Vogl T, Thallinger GG, Zellnig G, Drew D, Cregg JM, Glieder A, Freigassner M (2014) Towards improved membrane protein production in Pichia pastoris: general and specific transcriptional response to membrane protein overexpression. New Biotechnol 31(6):538–552. https://doi.org/10.1016/j.nbt.2014.02.009
Wanke C, Eckert S, Albrecht G, Van Hartingsveldt W, Punt PJ, Van Den Hondel CA, Braus GH (1997) The Aspergillus niger GCN4 homologue, cpcA, is transcriptionally regulated and encodes an unusual leucine zipper. Mol Microbiol 23(1):23–33. https://doi.org/10.1046/j.1365-2958.1997.1741549.x
Watanabe T, Matsuo I, Maruyama JI, Kitamoto K, Ito Y (2007) Identification and characterization of an intracellular lectin, calnexin, from Aspergillus oryzae using N-glycan-conjugated beads. Biosci Biotechnol Biochem 71(11):2688–2696. https://doi.org/10.1271/bbb.70289
Watanabe T, Totani K, Matsuo I, Maruyama JI, Kitamoto K, Ito Y (2009) Genetic analysis of glucosidase II β-subunit in trimming of high-mannose-type glycans. Glycobiology 19(8):834–840. https://doi.org/10.1093/glycob/cwp061
Wimalasena TT, Enjalbert B, Guillemette T, Plumridge A, Budge S, Yin Z, Brown AJ, Archer DB (2008) Impact of the unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in the polarized growth, of Candida albicans. Fungal Genet Biol 45(9):1235–1247. https://doi.org/10.1016/j.fgb.2008.06.001
Wu Y, Sun X, Xue X, Luo H, Yao B, Xie X, Xiaoyun S (2017) Overexpressing key component genes of the secretion pathway for enhanced secretion of an Aspergillus niger glucose oxidase in Trichoderma reesei. Enzym Microb Technol 106:83–87. https://doi.org/10.1016/j.enzmictec.2017.07.007
Yamasaki M, Yasui T, Arima K (1964) Pectic enzymes in the clarification of apple juice: part I. Study on the clarification reaction in a simplified model. Agric Biol Chem 28:779–787. https://doi.org/10.1080/00021369.1964.10858304
Yan Q, Lennarz WJ (2002) Studies on the function of oligosaccharyl transferase subunits: Stt3p is directly involved in the glycosylation process. J Biol Chem 277(49):47692–47700. https://doi.org/10.1074/jbc.M208136200
Yanagitani K, Imagawa Y, Iwawaki T, Hosoda A, Saito M, Kimata Y, Kohno K (2009) Cotranslational targeting of XBP1 protein to the membrane promotes cytoplasmic splicing of its own mRNA. Mol Cell 34(2):191–200. https://doi.org/10.1016/j.molcel.2009.02.033
Yoon J, Kikuma T, Maruyama J, Kitamoto K (2013) Enhanced production of bovine chymosin by autophagy deficiency in the filamentous fungus Aspergillus oryzae. PLoS One 8:e62512. https://doi.org/10.1371/journal.pone.0062512
Yoshida T, Kato Y, Asada Y, Nakajima T (2000) Filamentous fungus Aspergillus oryzae has two types of α-1, 2-mannosidases, one of which is a microsomal enzyme that removes a single mannose residue from Man9GlcNAc2. Glycoconj J 17:745–748. https://doi.org/10.1023/A:1010984608855
Zembek P, Perlińska-Lenart U, Brunner K, Reithner B, Palamarczyk G, Mach RL, Kruszewska JS (2011) Elevated activity of dolichyl phosphate mannose synthase enhances biocontrol abilities of Trichoderma atroviride. MPMI 24(12):1522–1529. https://doi.org/10.1094/MPMI-02-11-0025
Zhang M, Schekman R (2013) Unconventional secretion, unconventional solutions. Science 340(6132):559–561. https://doi.org/10.1126/science.1234740
Funding
Open access funding provided by TU Wien (TUW). Reshma Jadhav is grateful to the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie programme grant agreement No 101034277, for financial support.
Author information
Authors and Affiliations
Contributions
RJ contributed to conception of the manuscript, performed literature study, drew the figures, and drafted the manuscript. AMA contributed to conception of the manuscript and revised the manuscript. RLM contributed to conception of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with animal or human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 81 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jadhav, R., Mach, R.L. & Mach-Aigner, A.R. Protein secretion and associated stress in industrially employed filamentous fungi. Appl Microbiol Biotechnol 108, 92 (2024). https://doi.org/10.1007/s00253-023-12985-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12985-4