Abstract
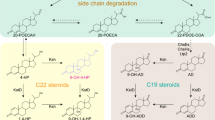
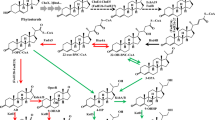
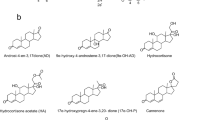
C22 steroid drug intermediates are suitable for corticosteroids synthesis, and the production of C22 steroids is unsatisfactory due to the intricate steroid metabolism. Among the C22 steroids, 21-hydroxy-20-methyl-pregna-1,4-dien-3-one (1,4-HP) could be used for Δ1-steroid drug synthesis, such as prednisolone. Nevertheless, the production of 1,4-HP remains unsatisfactory. In this study, an ideal 1,4-HP producing strain was constructed. By the knockout of 3-ketosteroid-9-hydroxylase (KshA) genes and 17β-hydroxysteroid dehydrogenase (Hsd4A) gene, the steroid nucleus degradation and the accumulation of C19 steroids in Mycolicibacterium neoaurum were blocked. The mutant strain could transform phytosterols into 1,4-HP as the main product and 21-hydroxy-20-methyl-pregna-4-ene-3-one as a by-product. Subsequently, the purity of 1,4-HP improved to 95.2% by the enhancement of 3-ketosteroid-Δ1-dehydrogenase (KSTD) activity, and the production of 1,4-HP was improved by overexpressing NADH oxidase (NOX) and catalase (KATE) genes. Consequently, the yield of 1,4-HP achieved 10.5 g/L. The molar yield and the purity of 1,4-HP were optimal so far, and the production of 1,4-HP provides a new intermediate for the pharmaceutical steroid industry.
Key points
• A third 3-ketosteroid-9-hydroxylase was identified in Mycolicibacterium neoaurum.
• An 1,4-HP producer was constructed by KshA and Hsd4A deficiency.
• The production of 1,4-HP was improved by KSTD, NOX, and KATE overexpression.








Similar content being viewed by others
Data availability
All data generated and analyzed during this study are included in this published article.
References
Capyk JK, D’Angelo I, Strynadka NC, Eltis LD (2009) Characterization of 3-ketosteroid 9alpha-hydroxylase, a rieske oxygenase in the cholesterol degradation pathway of Mycobacterium tuberculosis. J Biol Chem 284(15):9937–9946. https://doi.org/10.1074/jbc.M900719200
Capyk JK, Casabon I, Gruninger R, Strynadka NC, Eltis LD (2011) Activity of 3-ketosteroid 9 alpha-hydroxylase (KshAB) indicates cholesterol side chain and ring degradation occur simultaneously in mycobacterium tuberculosis. J Biol Chem 286(47):40717–40724. https://doi.org/10.1074/jbc.M111.289975
Ezraty B, Gennaris A, Barras F, Collet JF (2017) Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol 15(7):385–396. https://doi.org/10.1038/nrmicro.2017.26
Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral J (2003) Microbial conversion of steroid compounds: recent developments. Enzyme Microb Technol 32(6):688–705
Fernández-Cabezón L, Galán B, García JL (2018) New insights on steroid biotechnology. Front Microbiol 9:958
Josefsen KD, Nordborg A, Sletta H (2017) Bioconversion of phytosterols into androstenedione by Mycobacterium. Microbial Steroids. Springer, pp 177-197
Li H, Wang X, Zhou L, Ma Y, Yuan W, Zhang X, Shi J, Xu Z (2019) Enhancing expression of 3-ketosteroid-9alpha-hydroxylase oxygenase, an enzyme with broad substrate range and high hydroxylation ability, in Mycobacterium sp LY-1. Appl Biochem Biotechnol 187(4):1238–1254. https://doi.org/10.1007/s12010-018-2876-2
Liang L, Liu R, Wang G, Gou D, Ma J, Chen K, Jiang M, Wei P, Ouyang P (2012) Regulation of NAD(H) pool and NADH/NAD(+) ratio by overexpression of nicotinic acid phosphoribosyltransferase for succinic acid production in Escherichia coli NZN111. Enzyme Microb Technol 51(5):286–293. https://doi.org/10.1016/j.enzmictec.2012.07.011
Liu H-H, Xu L-Q, Yao K, Xiong L-B, Tao X-Y, Liu M, Wang F-Q, Wei D-Z (2018) Engineered 3-ketosteroid 9α-hydroxylases in Mycobacterium neoaurum: an efficient platform for production of steroid drugs. Appl Environ Microbiol 84(14):e02777-02717
Liu X, Zhang R, Bao Z, Yuan C, Cao H, Shi J, Sun J, Zhang B (2020) Biotransformation of phytosterols to androst-1, 4-diene-3, 17-dione by Mycobacterium sp ZFZ expressing 3-ketosteroid-δ1-dehydrogenase. Catalysts 10(6):663
Malaviya A, Gomes J (2008) Androstenedione production by biotransformation of phytosterols. Bioresource Technol 99(15):6725–6737. https://doi.org/10.1016/j.biortech.2008.01.039
Nesbitt NM, Yang X, Fontan P, Kolesnikova I, Smith I, Sampson NS, Dubnau E (2010) A thiolase of mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol. Infect Immun 78(1):275–282. https://doi.org/10.1128/IAI.00893-09
Penfield JS, Worrall LJ, Strynadka NC, Eltis LD (2014) Substrate specificities and conformational flexibility of 3-ketosteroid 9alpha-hydroxylases. J Biol Chem 289(37):25523–25536. https://doi.org/10.1074/jbc.M114.575886
Peng H, Wang Y, Jiang K, Chen X, Zhang W, Zhang Y, Deng Z, Qu X (2021) A dual role reductase from phytosterols catabolism enables the efficient production of valuable steroid precursors. Angew Chem Int Ed Engl 60(10):5414–5420. https://doi.org/10.1002/anie.202015462
Petrusma M, Dijkhuizen L, van der Geize R (2009) Rhodococcus rhodochrous DSM 43269 3-ketosteroid 9alpha-hydroxylase, a two-component iron-sulfur-containing monooxygenase with subtle steroid substrate specificity. Appl Environ Microbiol 75(16):5300–5307. https://doi.org/10.1128/AEM.00066-09
Petrusma M, Dijkhuizen L, van der Geize R (2012) Structural features in the KshA terminal oxygenase protein that determine substrate preference of 3-ketosteroid 9alpha-hydroxylase enzymes. J Bacteriol 194(1):115–121. https://doi.org/10.1128/JB.05838-11
Petrusma M, van der Geize R, Dijkhuizen L (2014) 3-Ketosteroid 9alpha-hydroxylase enzymes: rieske non-heme monooxygenases essential for bacterial steroid degradation. Antonie Van Leeuwenhoek 106(1):157–172. https://doi.org/10.1007/s10482-014-0188-2
Shao M, Zhao Y, Liu Y, Yang T, Xu M, Zhang X, Rao Z (2019) Intracellular environment improvement of Mycobacterium neoaurum for enhancing androst-1, 4-diene-3, 17-dione production by manipulating NADH and reactive oxygen species levels. Molecules 24(21):3841
Su L, Shen Y, Zhang W, Gao T, Shang Z, Wang M (2017) Cofactor engineering to regulate NAD+/NADH ratio with its application to phytosterols biotransformation. Microb Cell Factories 16(1):1–11
Su L, Xu S, Shen Y, Xia M, Ren X, Wang L, Shang Z, Wang M (2020) The sterol carrier hydroxypropyl-β-cyclodextrin enhances the metabolism of phytosterols by Mycobacterium neoaurum. Appl Environ Microbiol 86(15):e00441-00420
Sun B, Yang J, Yang S, Ye RD, Chen D, Jiang Y (2018) A CRISPR-Cpf1-assisted non-homologous end joining genome editing system of Mycobacterium smegmatis. Biotechnol J 13(9):1700588
Sun W-J, Wang L, Liu H-H, Liu Y-J, Ren Y-H, Wang F-Q, Wei D-Z (2019) Characterization and engineering control of the effects of reactive oxygen species on the conversion of sterols to steroid synthons in Mycobacterium neoaurum. Metab Eng 56:97–110
Uhia I, Galan B, Morales V, Garcia JL (2011) Initial step in the catabolism of cholesterol by Mycobacterium smegmatis mc2155. Environ Microbiol 13(4):943–959. https://doi.org/10.1111/j.1462-2920.2010.02398.x
Xu L-Q, Liu Y-J, Yao K, Liu H-H, Tao X-Y, Wang F-Q, Wei D-Z (2016) Unraveling and engineering the production of 23,24-bisnorcholenic steroids in sterol metabolism. Sci Rep 6(1):1–13
Yan M-Y, Yan H-Q, Ren G-X, Zhao J-P, Guo X-P, Sun Y-C (2017) CRISPR-Cas12a-assisted recombineering in bacteria. Appl Environ Microbiol 83(17):e00947-00917
Yuan C-Y, Ma Z-G, Zhang J-X, Liu X-C, Du G-L, Sun J-S, Shi J-P, Zhang B-G (2021) Production of 9, 21-dihydroxy-20-methyl-pregna-4-en-3-one from phytosterols in Mycobacterium neoaurum by modifying multiple genes and improving the intracellular environment. Microb Cell Factories 20(1):1–12
Zhang R, Liu X, Wang Y, Han Y, Sun J, Shi J, Zhang B (2018) Identification, function, and application of 3-ketosteroid Δ1-dehydrogenase isozymes in Mycobacterium neoaurum DSM 1381 for the production of steroidic synthons. Microb Cell Factories 17(1):1–16
Zhang R, Xu X, Cao H, Yuan C, Yuminaga Y, Zhao S, Shi J, Zhang B (2019) Purification, characterization, and application of a high activity 3-ketosteroid-Delta(1)-dehydrogenase from Mycobacterium neoaurum DSM 1381. Appl Microbiol Biotechnol 103(16):6605–6616. https://doi.org/10.1007/s00253-019-09988-5
Zhang Y, Zhou X, Yao Y, Xu Q, Shi H, Wang K, Feng W, Shen Y (2021) Coexpression of VHb and MceG genes in Mycobacterium sp strain LZ2 enhances androstenone production via immobilized repeated batch fermentation. Bioresour Technol 342:125965. https://doi.org/10.1016/j.biortech.2021.125965
Zhao A, Zhang X, Li Y, Wang Z, Lv Y, Liu J, Alam MA, Xiong W, Xu J (2021) Mycolicibacterium cell factory for the production of steroid-based drug intermediates. Biotechnol Adv 53:107860. https://doi.org/10.1016/j.biotechadv.2021.107860
Zhou X, Zhang Y, Shen Y, Zhang X, Zan Z, Xia M, Luo J, Wang M (2020) Efficient repeated batch production of androstenedione using untreated cane molasses by Mycobacterium neoaurum driven by ATP futile cycle. Bioresour Technol 309:123307. https://doi.org/10.1016/j.biortech.2020.123307
Acknowledgements
We are grateful to Dr. Yu Jiang (CAS Center for Excellence in Molecular Plant Sciences Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, Shanghai, China) and Dr. Yichen Sun (Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China) for helping us set up the Cprispr-Cpf1 system.
Funding
This work is supported by the National Key R&D Program of China (No.2017YFE0112700) and Shi Jiping Expert Workstation project of Yunnan Province in 2021.
Author information
Authors and Affiliations
Contributions
BGZ, CYY, and ZGM designed and conceived the study. CYY, ZGM, and SWH performed the research. CYY, JXZ, and XCL analyzed the data. CYY and YXL wrote the paper. YXL, BGZ, and GLD revised the paper. JSS, JPS, and GLD contributed analytical tools and supervised the project. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, C., Ma, Z., Li, Y. et al. Production of 21-hydroxy-20-methyl-pregna-1,4-dien-3-one by modifying multiple genes in Mycolicibacterium. Appl Microbiol Biotechnol 107, 1563–1574 (2023). https://doi.org/10.1007/s00253-023-12399-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12399-2