Abstract
Aristolochia, belonging to the family Aristolochiaceae, has immense ecological significance due to its large size and huge geographic distribution. In the context of dealing with a genus with a huge number of species like Aristolochia, these markers come in handy to precisely identify a particular species and enumerate the genetic diversity. Also, certain species of Aristolochia are economically important due to the presence of secondary metabolites and vast use in traditional and modern medicine. But, the presence of profitable biochemical constituents in Aristolochia is very low and the breeding process of the plant is highly dependable on pollinators. Hence, identifying different biotechnological approaches to fasten the reproductive cycle of Aristolochia and increase the secondary metabolites is of great interest to the researchers. In this study, a comprehensive review has been established on different types of morphological/anatomical markers (starch grains with “Maltese cross”), phytochemical markers (aristolochic acid, triterpenoid, aristolactam etc.) and genetic markers (ISSR, SSR, DNA bar-coding) for various Aristolochia spp. We have also discussed the applications of different biotechnological tools in Aristolochia spp. which include discrete approaches to promote in vitro germination, in vitro shooting, root induction, somatic embryogenesis, synthetic seed production, acclimatization and hardening and sustainable production of secondary metabolites. In a nutshell, the present review is a first of kind approach to comprehensively demonstrate the genetic diversity studies and biotechnological aspects in Aristolochia spp.
Key points
• Insights into the in vitro propagation of Aristolochia spp.
• In vitro production and optimization of secondary metabolites.
• Assessment of genetic diversity by molecular markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aristolochia is a large genus of flowering plants having a large geographical distribution almost all over the world in various diverse climatic situations. The members are often called birthwort, due to the resemblance of the flower with the birth cannel (Ansari et al. 2021; Qin et al. 2021). Aristolochia is considered to be toxic due to the presence of aristolochic acids (AAs) which causes chronic renal failure, urothelial malignancies and tubulointerstitial fibrosis together termed as aristolochic acid nephropathy (AAN) in both humans and mice (Jelaković et al. 2019 Ji et al. 2021). Consumption of food containing AA through traditional medicine or ingestion of food that is environmentally contaminated with AA can cause progressive renal interstitial fibrosis that frequently leads to AAN. AA intoxication leads to haemodynamic abnormalities by causing an increase in endothelin and reduction in renal nitric oxide levels. Due to such an imbalance in vasoactive factors, vasoconstriction happens to lead to hypoxia, tubular injury, inflammation and ultimately fibrosis.
However, Aristolochia have widespread use in various regions. Like in Europe, Aristolochia sp. were introduced by the Greek scholar Dioscorides (first century), and aristolochic acid and its extracts isolated from Aristolochia clematitis were formerly approved to treat against eczemas, abscesses and different long-lasting dermatosis and as a stimulant for immune system (Scarborough and Fernandes 2011; Pohodina et al. 2019). The whole plant of Aristolochia is considered to hold different types of medicinal properties in Indian traditional medicine. The roots of Aristolochia are designated as anti-inflammatory, diuretic and cardiotonic due to the presence of an alkaloid, aristolochin; leaves are useful for the treatment of cholera and intermittent fever in children; and seeds are traditionally used against inflammation, biliousness and dry cough (Padhy 2021). Hence, a throughout heath risk assessment should be done before using this kind of drugs for any medicinal practices.
On the other hand, various species of Aristolochia (viz. A. longa, A. triangularis, A. bodamae, A. longa etc.). and their compounds have been reported to demonstrate profound antimicrobial properties (Pereira et al. 2018; Dalcol et al. 2021; El Omari et al. 2020; Ozen et al. 2020; Doudach et al. 2022). Therefore, in vitro cultures can be used as an exciting option to produce and characterize the antimicrobial phyto-constituents from the plant. Besides the genus is also known to host many endophytic microbes (Guevara-Araya et al. 2020, 2022) which can also be cultured in vitro for the production of secondary metabolites. Aristolochic acid (AA) has the potential inhibitory role against snake venom L-amino acid oxidase (LAAO). As AA is notorious for its genotoxic activity, its non-toxic artificial hydroxyl and chloro-derivatives can be used for such purpose (Bhattacharjee et al. 2017).Improvements in in vitro techniques offer novel strategies to the viable processing of even threatened and endangered plants and their economically and industrially promising secondary metabolites. In vitro propagation of medico-botanicals and in vitro optimization of their bioactive principles have been done in many plants for the commercial production of therapeutically active plant-based medicines. In plant tissue culture, the production of secondary metabolites has been reported from various species (Jayaprakash et al. 2021; Manokari et al. 2021; Shekhawat et al. 2021; Swamy et al. 2021). Many reports involve the use of different elicitors to increase the quantity of plant secondary metabolites (Dey et al. 2019, 2020; Nandy et al. 2021; Pandey et al. 2021; Nazir et al. 2021a). The present review encompasses an outline on the in vitro propagation and biotechnological tools as an aid for clonal propagation and production of aristolochic acid and allied compounds. In addition, for its use as multipurpose drug in traditional and modern medicines and presence of important toxins, tissue culture Aristolochia is a better way out for faster growth, low dependence on natural pollinators and to conserve its short genotype. Aristolochia is exploited as a model plant for in vitro regeneration and formation of artificial seed (Remya et al. 2013), clonal propagation (Sarma and Tanti 2017b) and somatic hybridization (Bliss et al. 2009).
A study of markers not only help to distinguish between different population and individual plant itself but also provide insights into the different genetic arrangement, phytochemical constitutes and morphological parameters of one group of plants (Kaur et al. 2019; 2021; Nazir et al. 2021b; Tikendra et al. 2021a,b). Different types of morphological, phytological and genetic markers of Aristolochia is well established to differentiate the species of this large genus. Also such markers can be useful as a genetic and breeding tool for crop development (Chesnokov et al. 2020; Desai and Tatke 2019). Therefore, methodologies for measuring genetic diversity and/or proximity in different species utilizing various markers have also been elucidated (Table 1).
Distribution
Aristolochia spp. is a genus present in the family of Aristolochiaceae which include approximately 550 species (Cai et al. 2020). All these species are distributed generally under tropic, subtropic, Mediterranean and temperate regions of the world including America, Asia and Africa (Fig. 1). Aristolochia subgenus Siphisia include 70 species in which 50 of them are found in the southern and eastern parts of Asia, while 20 of them are found in the central and northern parts of America (Do et al. 2015). Aristolochia ringens species is found in the tropical regions of America and Africa, whereas Aristolochia elegans species are native for South America, while it is exotic for North America, Africa and Asia. Vietnam’s tropical, humid and forest region favour the growth of species like Aristolochia bidoupensis (van Do et al. 2016). Aristolochia delavayiis widely distributed in southwest China along with the Jinsha river valley at an altitude of 1220–2250 m (Yu et al. 2021). In India, 18 species of the genus Aristolochia is found, in which Aristolochia maxima is found in north Western Ghats (Tilari Ghats) of Maharashtra (Pandurangan and Deepu 2018). This species is native for central and South America. Another species Aristolochia indica is found throughout tropical, subtropical and Mediterranean countries including India, Nepal and Bangladesh (Dey and De 2011; Sarma et al. 2018). In Africa, generally on the western side, Madagascar favours the growth of 11 species which include A. albida, A. baetica, A. bracteolate, A. embergeri, A. heppi, A. hockii, A. fontanesii, A. paucinervis, A. pistolochia, A. rigida and A. sempervirens (de Groot et al. 2006). There are three medicinal and endemic Aristolochia species from eastern India which have been reported namely Aristolochia indica Linn., Aristolochia saccata Wall. and Aristolochia cathcartii (Sarma and Tanti 2015). Aristolochia delavayi, another medicinal and endangered member of Aristolochiaceae, is endemic to China. They generally grow in the warm and dry areas along the Jinsha river (Yu et al., 2021).
Worldwide distribution of Aristolochia L. (source: https://www.gbif.org/species/2873978)
Botanical description
Aristolochia is the type of genus of the family Aristolochiaceae or the birthwort family having over 450 species in 3 subgenera; Aristolochia, Siphisia and Pararistolochia (Wanke et al. 2006; Yan and Ma 2019). Among them Aristolochia itself has approximately 350 species. They are generally evergreen or deciduous lianes. But some deciduous herbaceous members from Mediterranean region are also investigated (Neinhuis et al. 2005). Leaves are found to be 3–7 lobbed, most of the time cordate, 3 (Aristolochia dalyi) — 7 nerved (Gonzalez 1998). In Aristolochia longissima and Aristolochia ornithorhyncha, conspicuous pellucid gland dots are present with the leaves (Jimenez et al. 2021). Flowers are irregular, either solitary or in groups and axillary in position. Perianth is colourful, tubular with various bending, generally flattened at the base but narrow near the throat and pubescent on the inner side; limbs can be either open or recurved, entire or lobbed. Stamens are 6 or more; anthers sessile adnate to the stigma or the back of style. Style is short and lobed near the apex with usually 6-locular inferior ovary. Fruit is capsule, and septicidally dehiscent; seeds are compressed (Britannica, T. Editors of Encyclopaedia, 2013).
In some instances, various authors showed their disagreement with these common morphological features of the different species of such vast genus like Aristolochia. Like, González and Stevenson had interpreted the perianth of Aristolochiaas a trimerous calyx based on morphology, position, development and juxtaposition with the closely related taxa (González and Stevenson 2000) after studying 42 species.
Adams et al. have done a comparative study between 4 closely related species of Aristolochia from all over the world namely A. californica, endemic to California, A. macrophylla and A. tomentosa of eastern USA and A. manshuriens of eastern Asia based on seed characters (like seed mass, surface area wings, state and shape of embryo). It was found that though having similar morphological characteristics of the embryo (linear and underdeveloped), those features are statically different which assign them into 4 separate species (Adams et al. 2005).
Aristolochia was found to have a profound trapping mechanism for pollination in case of in proterogynous flowers. Experiments on 6 Mediterranean species have shown during female flowering stage an array of well-developed trichomes present on the underlying surface of the flower which ensure the entry of the pollinators into the flower but inhibit their exit. But after the flowers enter the stages of anthesis, the inner surface characters of the flower modify to enable the insect to escape (Oelschlägel et al. 2009).
Genetic diversity assessment
Morphological markers
Morphological markers are visual phenotypic characters of a plant that is particular to the certain species or genus. But the main disadvantage of these markers is that they are few in number and always influenced by environmental factors and covers of the entire genome (Chesnokov et al. 2020).
Sudhakaran has investigated the root anatomy of Aristolochia indica through pharmacognostic profiling and found the transverse section having a circular outline with tissue organization of thin-walled cork cells, small cortex and inner cortical cells interspersed with groups of stone cells. Secondary xylem was separated to form narrow strips. Wide medullary rays had greater quantities of parenchyma with ray cells having deposition of starch. Solitary vessels are occluded with tyloses and starch grains with ‘Maltese cross’. He suggested these specific anatomical characters might be an authentic marker of this taxon (Sudhakaran 2014).
Phytochemical markers
Phytochemical markers are chemical constituents made up of single or group of herbal drugs with a well-defined chemical structure. These constituents may or may not possess any medicinal characteristics but are useful for quality control of plant-based drug or formulation (Desai and Tatke 2019). The genus contains a number of constituents which can be utilized as important phytochemical markers (Fig. 2).
2D and 3D chemical structures of the bioactive compounds of Aristolochia spp.: a Aristolochic acid I, b Aristolochic acid II, c Aristololactam II, d Ishwarol, e Ishwarone, f Aristolochene (source: www.PubChem.com)
Aristolochia baetica is a wild member of Aristolochia from Morocco whose roots have been used against cancer from a long time ago. The root aqueous extract of A. baetica was subjected to preliminary qualitative phytochemical screening to find out about the phyto-organic component behind their biological activities. The presence of polyphenol, alkaloids, flavonoids, saponins and tannins has been observed (Bourhia et al. 2019). Similar study also was done on the methanolic root extract of A. bracteolata which has also shown the presence of alkaloids, glycosides, saponins, starch and protein. High performance thin-layer chromatography fingerprinting study revealed the occurrence of aristololochic acid and triterpenoid as distinctive phytochemical markers of the particular plant (Avchar er al., 2021).
In a study to explore the phytochemical constituents of Aristolochia moupinensis and Aristolochia cathcartii, 8 aristolactams and 5 aristolochic acid derivatives were isolated from A. moupinensis, whereas 6 aristolactams and 3 aristolochic acids were isolated from A. cathcartii (both were isolated from whole herb). Aristolactam I, aristolactam AII, aristolochic acid A and aristolochic acid BII were found from both of the species. Aristolactam-type alkaloids and aristolochic acid derivatives were widely spread in all the plant members of genus Aristolochia. Aristololactam is an intermediate in the biosynthetic pathway for aristolochic acid. The aristolochic acids in the Aristolochia possessed a unique chemical structure and are responsible for the same biological property of Aristolochia species. Hence, aristololactam-type alkaloids and aristolochic acids derivatives might be hypothesized to be unique for the particular genus, thus could be used as potential phytochemical markers for the genus (Zhang et al. 2016).
Eight aristololactam-type alkaloids and seven aristolochic acid derivatives were isolated from the whole plant of A. tagala. Among them sauristolactam and 7-methoxyaristololactam IV are very unique to the species. Sauristolactam is not found in any other Aristolochia species and though the occurrence of 7-methoxyaristololactam was observed in some of the Asian members of Aristolochia, it is quite rare. Hence, both of them could be used as chemotaxonomic or phytochemical marker of the species (Liu and Zhang 2020).
After analysing the essential oils from the roots of 10 Aristolochia species by GC–MS, 75 compounds were found. Multivariate analysis of those chemicals from roots classifies the 10 species into 4 morphological groups based on principal component analysis. The groups were identified by principal component 1 (monoterpenes, like a-thujene) and principal component 3 (sesquiterpenes, such as germacrene A (52), c-elemene (39) and b-gurjunene) (Francisco et al. 2008).
-
Group 1: A. arcuata, A. chamissonis, A. lagesiana, A. melastoma and A. pubescens.
-
Group 2: A. gigantean (highest positive PC1).
-
Group 3: A. elegans (highest positive PC3).
-
Group 4: A. esperanzae, A. galeata, and A. malmeana.
Molecular markers
ISSR
Inter-simple sequence repeats (ISSR) is a molecular technique which helps in the determination of multi-locus marker (repetitive sequence present in genome of organism) by the help of PCR by using microsatellite sequence as primers. The amplicons produced by this technique help in the study of evolutionary history, genetic diversity and gene mapping of closely related species. Forty-five Passion fruit (Passiflora sp.) accession was selected to check against eighteen ISSR primers. The result obtained from the mean Shannon–Weaver diversity index was 0.32 which represents a good diversity in the selected Passiflora germplasm by the ISSR markers (Santos et al. 2011). In another study, 23 mango germplasm accessions were collected from Guangxi province, China and checked against 18 ISSR primers. The result showed out of 156 bands, 87 are polymorphic. It can be concluded among the other cultivars, the genetic similarities in Xiang Ya mango type and their progenies are very high (Luo et al. 2011).
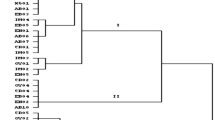
To determine the genetic variation of Aristolochia species, 8 ISSR primers are used for the 4 species from Assam. These species are A. indica, A. cathcartii, A. saccata and A. tagala. It is found that A. indica and A. cathcartii (Cluster-I) are 62% similar in terms of genetic variation while A. saccate (Cluster-II) is 28% similar with Cluster-I species, while A. tagala (Cluster-III) is 22% similar with A. saccate. Nine out of 66 PCR amplicon’s bands of these four species are similar indicating that they are sharing common evolutionary history. Remaining bands are the proof for the genetic divergence present in the species and showing polymorphism in their character. Dendrogram of these 4 species also shows the same genetic similarity for A. indica and A. cathcartii, while the other two species are not much closely related due to divergence in the genes (Sarma and Tanti 2017a).
SSR
Simple sequence repeat is a microsatellite DNA repeat found in genome having a high rate of polymorphism and widely distributed within the eukaryotic organisms, and the number of repeat is very specific and varies between organisms and helps in the assessment of genetic diversity between closely related organisms having minimal characteristic differences. Twenty-nine cucumbers (Cucumis sativus L.) accessions were selected to estimate the genetic diversity by comparing 13 genomic microsatellites (gSSR) and 16 expressed sequence tag (EST)-SSR (eSSR) markers. The dendrogram produced individually from the results of both of these markers shows similarity in the position of most of the cucumber germplasm. Comparing the data from eSSR markers, independent sub-clusters can be identified containing five germplasms. They all are resistant to downy mildew concluding a probable connection between those eSSR markers and disease resistance of plants (Hu et al. 2011).
SSR analysis is performed for the analysis of genetic diversity and difference in genetic makeup of the Aristolochia delavayi species with respect to its wild type. Fifteen pairs of microsatellite SSR primers are used for 193 individuals from ten natural populations. Through AMOVA (analysis of molecular variance), 68.4% genetic diversity is seen within the population, whereas 31.6% genetic diversity is seen among the population of Aristolochia delavayi. High genetic diversity within the population is due to outcrossing within the population by sexual reproduction or due to the retention of genetic resources. Restriction of gene flow may cause the less genetic diversity among the population which restricts the reproductive abilities of plants.
Since Aristolochia delavayi is found along the Jinsha River and warm and dry areas of China, so this kind of geographical habitat restricts the exchange of gene among the species. Another reason is that pollination of this species mainly depends on the family Ceratopogonidae and Chironomidae pollinator which are less efficient and have weak flying abilities and warm and dry climate does not favour the dispersal of seed due to which gene flow is restricted within the species and cause major genetic diversity within the population (Yu et al. 2021).
DNA barcoding
DNA barcoding is the technique in which short sequence of DNA or organelle DNA is used to identify and help in the comparison of genetic diversities among species. The genes which are used as DNA barcode include cytochrome c oxidase I (COI or COX1) coding gene present within mitochondrial DNA, internal transcribed spacer (ITS) rRNA etc. due to their less variation in intraspecific level compared to interspecific level in species. Four plastid coding genes (rpoB, rpoC1, rbcL and matK) and 3 noncoding spacers (atpF-atpH, psbK-psbI and trnH-psbA) based on the chloroplast genome sequence of Lemna minor as proposed by the CBOL (Consortium for the Barcode of Life) were used to distinguish 97 accessions representing 31 species of Lemnaceae (aquatic monocots). It can be concluded that among other genes chosen in the study, the atpF-atpH noncoding spacer could be used as a universal DNA barcoding marker for species-level identification for Lemmaceae based on reliable amplification, straightforward sequence alignment and rates of DNA variation between species and within species (Wang et al. 2010).
Recent studies on DNA barcoding use rbcL, matK, ITS2 and trnH-psbA as markers to identify the genetic variability and polymorphism within the 11 species of Aristolochia from Thailand. This evaluates the highest variations which are found in ITS2 (28.98%) region of DNA followed by rnH-psbA (11.56%), matK (11.15%) and rbcL (3.29%) in all 11 species of Aristolochia (Dechbumroong et al. 2018).
Achievements made in Aristolochia through modern biotechnological tools
In vitro germination
In vitro germination is an easier method to propagate for conservation and economic purposes (Table 2). The in vitro germination of Cannabis sativa seeds has faced some difficulties regarding uniformity and germination time due to issues in the standardization of disinfection procedures. A recent study has been mediated by using the generalized regression neural network (GRNN) to assess the type and concentration of disinfectants and the time of immersion for in vitro germination of the seeds. The results showed that treating the seeds with 4.6% sodium hypochlorite along with 0.008% hydrogen peroxide for 16.81 min manifested the best results with a 0% contamination rate and 100% germination after scarification within 1 week (Pepe et al. 2021).
The presence of scanty endosperm and linear, small embryo of Aristolochia tagala makes the germination of the seeds under normal conditions inconvenient (Biswas et al. 2007). Therefore, these factors have driven the utilization of different techniques of in vitro germination in order to enhance the viability of seeds and propagation rate. A report by Krishnan et al. (2019) suggested that exposure of seeds presoaked in warm water (50 °C) for 5 min to a germinator not only amplified the germination percentage but also successfully reduced the number of days taken for germination. The percentage of germination, however, was found to be greater in contrast to presoaked seeds exposed to an open room. According to Bhat et al. (2020), presoaking of seeds with warm water tends to stimulate the softening of seed coat and promotes rapid protrusion of tip of radicle upon attaining maturity. GA3, KNO3 and thiourea treatment at varying concentrations was found to have a pronounced effect on the rate of germination and effectively reducing the time taken for germination. Thiourea, a potential agent of in vitro germination, elevates endogenous cytokinin levels to alleviate inhibition on seed coat.
In vitro shooting
Morphogenesis from callus culture using a wide array of explants including adventitious shoot, nodes, internodes and leaves is beneficial for developing multiple clones of endangered medicinal plants. In vitro organogenesis can be manipulated by regulating the concentration of plant growth regulators (PGRs) specifically auxin and cytokinin. Though cytokinin along with auxin has a positive impact on shoot proliferation from callus, higher concentration of BAP (benzyl amino purine) and KIN (kinetin) can counteract this activity. It is also essential to utilize phloroglucinol to support in vitro shooting since accumulation of phenolic compounds may interfere with the normal phenomena and turn the callus black. In a study conducted on rice to standardize a reproducible and highly efficient plant regeneration protocol, the highest shoot regeneration was observed on the Murashige and Skoog (MS) medium supplemented with 2.0 mg/L benzylaminopurine, 0.5 mg/L 1-naphthaleneacetic acid, 500 mg/L proline and 500 mg/L glutamine in the callus obtained from MS medium complemented with 2.0 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 500 mg/L proline and 500 mg/L glutamine (Pawar et al. 2015).
The most effective result of shoot organogenesis in Aristolochia from callus culture was recorded with BAP in combination with NAA. Media of 2 µM BAP, 1 µM NAA (1-naphthalene acetic acid) and 10 µM PG in MS (Murashige and Skoog) or MS media fortified with 2,4-D (2,4-dichlorophenoxyacetic acid) and KIN provided prominent results (Remya et al. 2013). The capability of NAA to initiate shoot organogenesis was put forward by Biswas et al. (2007), where 79% explants cultured showed successful shooting with 2 mg/L BAP and 0.5 mg/L NAA in A. tagala. In A. saccarta and A. cathcartii, 3–4 mg/L BAP and 0.5–1 mg/L NAA demonstrated the highest mean shoot length of 4.02–4.34 and the number of shoot in the range of 3.4–6.2 (Sarma and Tanti 2017a, b). Remya et al. (2016) suggested for elongation of shoot from apical bud explant, it is convenient to supplement MS media with GA3. This report validated that BAP with KIN is even a fruitful alternative for in vitro shoot formation along with 0.1% charcoal fortification. BAP and spermidine, a polyamine, synergistically assisted the proliferation of shoot from nodal explant-derived calli (Dey et al. 2020).
Root induction
Root organogenesis from a wide variety of explants under precisely suitable culture conditions are dependent upon the type and concentration of auxin present in the medium. Germplasm conservation and reduplication Artemisia sp. for research and therapeutic purposes through biotechnological interventions have been greatly studied for the past decade. Recently, to establish a protocol for micropropagation of Artemisia annua L, the optimum rooting was observed on the Murashige and Skoog (MS) medium supplemented with 0.1 mg/L IBA in leaf-derived calluses (Zayova et al. 2020).
According to Sebastinraj and Siddique (2011), Sarma and Tanti (2017a, b) and Biswas et al. (2007), IBA (indole-3-butyric acid) is most appropriate for in vitro rooting of Aristolochia in contrast to NAA and IAA (indoleacetic acid). On one hand, Sarma and Tanti (2017a, b) reported 0.5 mg/L IBA exhibited surpassing effect on a number of rootlets and mean height of roots than NAA in A. saccarta. Remya et al. (2016), on the other hand, observed efficient in vitro rooting from apical bud explants of A. tagala on exposure to an amalgamation of IAA, BAP and KIN. Manjula et al. (1997) stated MS media and low ionic strength White media supplemented with precise concentration of IBA is effectual enough to initiate rhizogenesis and lateral root formation. In vitro rooting potency is also dependent upon the type of explant used. It was recorded by Pattar and Jayaraj (2012) that 80% rooting and greater root length were available in shoots from leaf-derived calli, but shoots from nodal calli displayed 95% rooting. In both cases, rooting was evident when MS media were supplemented with 0.8 mg/L NAA. The effect of other secondary metabolites on the in vitro rooting was put forward by Dey et al. (2020). They proved the fact how polyamine like spermidine (0.5 mM) along with IAA (1 mg/L) in MS media scaled up the rooting and lateral root development.
Somatic embryogenesis
This technology for in vitro propagation of endangered plants has been of immense focus on account of low propagation rate by seeds. The potential of somatic embryogenesis to regenerate plantlets is considered to be far more efficient than adventitious shoot, leaves and apical bud organogenesis. The in vitro propagation of elite palms through somatic embryogenesis is found to be advantageous for the high degree of variability, which will exist among improved progenies. By using such technologies, oil production from oil palm can be increased up to many degrees (Soh et al. 2011). For this context, somatic embryos of Elaeis guineensis Jacq (oil palm) were obtained after culturing the thin cell layer sections from the base of the explants in Murashige and Skoog (MS) medium supplemented with 450 µM picloram and 2,4-dichlorophenoxyacetic acid (2,4-D) with 3.0% sucrose, 500 mg /L glutamine, and 0.3 g /L activated charcoal and gelled with 2.5 g /L Phytagel for 12 weeks (Scherwinski-Pereira et al. 2010.
Very few reports have till now suggested methods to develop somatic embryo directly or in directly. Successful somatic embryo formation can be carried out by nodal explants cultured in different concentrations of 2-isopentenyl-adenine (2-iP) and benzylaminopurine (BAP) in combination with naphthaleneacetic acid (NAA) which permitted callus induction. A. saccata and A. cathcartii low concentrations of NAA positively influenced somatic embryo development, and there has been 88.3–96.6% success rate of regeneration of shoot from explants (Sarma and Tanti 2017b). A. indicus-directed callus induction up to 90% in 1:2 of NAA and BAP, respectively (Siddique et al. 2006). It was also observed in some cases cytokinin (Kinetin, BAP) and auxin (NAA, IAA) can be used in 1:2 ratio.
Synthetic seed production
Artificial seeds usually harbour the somatic embryo or explants from mother plant and are an excellent alternative to zygotic embryos as well as conventional plant breeding techniques. Plant species which have a drawback in terms of seed viability, inconvenience faced in vegetative propagation and storage can successfully propagate via synthetic seeds (Rihan et al. 2017). They help in the formation of multiple clones of the target plant thus preserving their genetical identity. To develop an efficient protocol for the production of the synthetic seeds of Rhinacanthus nasutus for their faster multiplication and isolation of Rhinacanthin-C, Rhinacanthin-D and Rhinacanthin-N, young healthy cotyledon explants were grown on MS medium supplemented with 4 mg/L 2, 4-D and 0.5 mg/L IBA to develop an embryonic callus. Those calluses are further cultured for 45 days in half-strength MS medium supplemented with 4.0 mg/L indole-3-butyric acid (IBA) to produce several somatic embryos. Somatic embryos at the torpedo stage were suspended in a matrix of MS medium supplemented with sodium alginate (3% W/V), and then dropped into the 100 mM calcium chloride (CaCl2·2H2O) solution to generate the synthetic seeds. The optimum growth ability of the synthetic seed was evaluated on MS medium with 0.2 mg/L gibberellic acid (GA3) (Cheruvathur et al. 2013).
Remya et al. (2013) proposed a protocol for developing artificial seeds in commercially important plant A. tagala. In vitro shoot and nodal explants cultured in MS media were allowed to form the bead like seeds using different concentrations of sodium alginate and 1% CaCl2. Most appropriate results were obtained at an intermediate concentration of sodium alginate, and maximum shoot formation was observed when these synthetic seeds were made to propagate in MS media supplemented with 3 µM BAP and 0.5 µM KIN.
Acclimatization and hardening of plants
Acclimatization and hardening are indispensable in order to ensure high chances of survival of in vitro plantlets. Direct transfer of the in vitro plants to the field can be detrimental. In order to overcome this drawback, certain approaches have been made. Sarma and Tanti (2017a, b) reported in vitro grown plantlets could be hardened by 1% w/v bavistine and irrigation with 0.5 × MS inorganic salts consecutively for 7 days. Acclimatization of plantlets was attained by exposing plantlets to aseptic culture room under controlled photoperiod and temperature for 2 weeks. Furthermore, the plantlets were exposed gradually to sunlight for acclimatization and were maintained in a garden. In A. bracteolata, 95% survival rate of in vitro plants were confirmed when acclimatized to soil, farmyard and garden soil manure in the ratio of 1:1:1 (Sebastinraj and Sidique 2011). Survival rate of 80% has been reported in A. tagala by Remya et al. (2016) when plantlets were acclimatized with vermicute and soil mixture (1:1). A. indica was seen to exhibit approx. 95% survival rate when in vitro plantlets were hardened with White media and vermicute and transferred to greenhouse conditions (Manjula et al. 1997). Biswas et al (2007) also confirmed 80% survival rate when in vitro plantlets were hardened and acclimatized in plastic pots with 1:1 soil and manure. Survival rate of 100% of in vitro plants were noted when they were hardened with autoclaved soil, sand, soilrite and acclimatized by transferring to large pots, where soil and soilrite were in the ratio of 2:1 (Dey et al. 2020). Figure 3 presents various in vitro tools and biotechnological aspects for plant propagation, regeneration and flowering.
Biosynthesis and regulation of secondary metabolites
Aristolochia sp. is considered to be an essential plant owing to the wide range of pharmaceutically active secondary metabolites available in different parts of the plant. Terpenoids, steroids and phenolic compounds like lignans, coumarins; alkaloids like berberine, aristolochic acid, aristolactams, isoquinolines and benzylisoquinoline are major categories of components extracted from roots, leaves and stem (Kuo et al. 2012). β-Sitosterol and stigmasterol were two major steroids extracted from different plant parts of Aristolochia sp. Terpenoids are beneficial for its anti-inflammatory, antibacterial, antiviral and antirheumatic effects and have neutralizing potency against haemorrhagic effect (Dey and De 2011). Aristolochic acid has antisnake venom properties, but recently some reports have highlighted carcinogenic and nephrotoxic effects (Dey et al. 2020).
Biosynthesis of terpenoids
Comprehensive study of the terpenoid content of Aristolochia sp. has demonstrated the prevalence of sesquiterpenoids, monoterpenoids and deterpenoids from the plant extracts. Among these, sesquiterpene hydrocarbons like ishwarane, ishwarone, aristolochene, ishwarol, aristolactone, cadinanes, aristolanes, beta caryophyllene, germacranes and bicyclogermacranes are present in considerable amounts. Pacheco et al. (2009) verified the presence of diterpenoid and its derivative compounds from Aristolochia sp. by isolating abundant kaurene, cledorane, labdane and assigned structures by 13C-NMR. Aristolin, another terpenoid isolated, was found to be an ester of aristolochic acid and a diterpenoident-kauran-16-β, 17-diol (Kuo et al. 2012). Preistap et al. (2002) confirmed sesquiterpenes were more abundant, as compared to monoterpenes in leaves and stems. Ishwarone, the tricyclic precursor molecule of ishwarane, undergoes retrosynthetic removal of C8 resulting in the formation of octalone. Methylation of octalone resulted in the formation of decalone which in turn synthesized ishwarane via tertiary alcohols and octalin.
Biosynthesis of alkaloids
Since aristolochic acids are usually derived from benzylisoquinolinealakoids (BIA), having clear knowledge of their biosynthetic pathway is of utmost importance, as this data would be indispensable for having clarity regarding biosynthetic pathway of aristolochic acids. Cui et al. (2022) elucidated the BIA synthesis pathway using Aristolochia contorta as experimental model by genome-wide analysis and transcriptomic analysis. Tyrosine derivatives act as a precursor molecule in the pathway along with dopamine and 4 hydroxyphenylacetaldehyde which undergoes condensation in the presence of enzymes coded by NCS 70, 71 to form(S)-norcoclaurine. This product is catalysed by enzymatic activity of 6 OMT, CNMT, NMCH 4′OMT 67, 72–76 to yield s-reticuline as intermediate product. S-reticulline ultimately is responsible for synthesis of alkaloids like berberine.
Sustainable production of secondary metabolites by in vitro techniques
Owing to high demand of pharmaceutically active secondary metabolites in the drug industry, sustainable in vitro production of plants is becoming a necessity. One such instance is enhancing naturally low-calorie sweeteners (Steviol glycosides) obtained from Stevia rebaudiana. After culturing the 3-week-old in vitro plantlets on liquid woody plant medium (WPM) supplemented with 100 µM methyl jasmonate (MeJA) for 2 weeks, a 17-fold increase in stevioside production has been observed in comparison to the control plant (plantlet is grown without any elicitors) (Bayraktar et al. 2018). Also, hairy roots of Nicotiana tabacum L. cv. Petit Havana SR1 do not produce geraniol naturally. But after the genetic manipulation to express a plastid-targeted geraniol synthase gene isolated from Valeriana officinalis L. (VoGES) mediated by Agrobacterium rhizogenes, it was observed that the hairy roots can produce geraniol ranging from 13.7 to 31.3 μg/g dry weight ( Ritala et al. 2014).
Massive exploitation of this perennial herb has eventually led to its classification as a critically endangered species. This approach is an effective conservational technique which is the sole solution to the exuberant harvesting of Aristolochia sp. from Western Ghats and Assam where it is endemic to. As of now, there are limited reports with respect to in vitro production on account of less comprehensible findings regarding the metabolic pathways involved. Certain works have also supported the fact that growth culture conditions and presence or absence of phytohormones precisely regulated the synthesis of secondary metabolites from explants.
Remya et al. (2016) reported the extraction of ishwarane, a tetracyclic sesquiterpene from in vitro plant leaves developed from apical bud explants. This particular method of in vitro regeneration of plantlets also confirmed the isolation of phenols, flavonoids, terpenoids and fatty acids in a sustainable quantity. Leaf-derived callus failed to report the absence of ishwarane but confirmed the presence of certain bioactive compounds unique to this in vitro approach. Alkaloid berberine was successfully isolated by Remya et al. (2016) by both in vitro techniques.
It is evident from many reports how culture media enriched with plant growth regulators, secondary metabolites can implement in vitro shooting, rooting, callus induction and multiplication. They are capable enough to simultaneously upgrade the synthesis of bioactive metabolites in in vitro plants. Dey et al. (2020) validated in vitro regeneration of A. indica to isolate aristolochic acid and analysed its endogenous level in in vitro shoots and roots. In this species of Aristolochia sp., nodal explant and apical shoot buds were cultured in SH media and were enriched with varying concentrations of polyamines (0.5 mM–1 mM) like putrescine, spermidine, spermine along with plant growth regulators auxin (IAA — 1.5 mg/L, IBA —1 mg/L) and cytokinin (KIN — 2 mg/L, BAP — 1.5 mg/L) to induce calli, direct shoot organogenesis and multiplied axillary shoot formation. Their HPLC study of the metabolite from in vitro regenerated plant extracts as well as mother plant illuminated the fact that aristolochic acid was higher in in vitro roots than plants grown under natural field conditions thus corroborating the pivotal role of polyamines in augmenting the concentration of aristolochic acid in combination with phytohormones.
Conclusions and future prospective
To combat the increasing demands of Aristolochia phytochemicals, sustainable conservational strategies are needed to be implemented. Several biotechnological interventions are already being performed to preserve the germplasms and produce economically important phytochemicals of these genera. Studies elucidating the use of different markers (morphological, biochemical, molecular) to identify and assess variability among several species of Aristolochia are of prime use. This review is a small effort to gather the existing information and state the knowledge gaps for future works.
References
Adams CA, Baskin JM, Baskin CC (2005) Comparative morphology of seeds of four closely related species of Aristolochia subgenus Siphisia (Aristolochiaceae, Piperales). Bot J Linn Soc 148(4):433–436
Ansari MD, Sofi G, Hamiduddin H, Ahmad H, Basri R, Alam A (2021) Optimization of the whole extract of Zarawand Mudaharaj (Aristolochia rotunda L.) root by response surface methodology (RSM). CELLMED 11(3):15
Avchar BK (2021) Phytochemical analysis of Aristolochia bracteolata lam root extract. Bioinfolet 18(2):226–7
Bayraktar M, Naziri E, Karabey F, Akgun IH, Bedir E, GÜREL A, (2018) Enhancement of stevioside production by using the biotechnological approach in vitro culture of Stevia rebaudiana. Int Second Metabolite 5(4):362–374
Bhat R, Shetty GR, Rajasekharan PE, Pooja DA, Ganapathi M, Nadukeri S (2020) Effect of pre-sowing seed treatments on Aristolochia tagala Cham: a threatened medicinal plant of South India. Int J Curr Microbiol App Sci 9(10):1803–1808
Bhattacharjee P, Bera I, Chakraborty S, Ghoshal N, Bhattacharyya D (2017) Aristolochic acid and its derivatives as inhibitors of snake venom L-amino acid oxidase. Toxicon 138:1–7
Biswas A, Bari MA, Roy M, Bhadra SK (2007) In vitro regeneration of Aristolochia tagala Champ. a rare medicinal plant of Chittagong hill tracts. J BioSci 15:63–67
Bliss BJ, Landherr L, DePamphilis CW, Ma H, Hu Y, Maximova SN (2009) Regeneration and plantlet development from somatic tissues of Aristolochia fimbriata. Plant Cell Tissue Organ Cult 98(1):105–114
Bourhia M, Haj Said A A, Chaanoun A, El Gueddari F, Naamane A, Benbacer L, & Khlil N (2019) Phytochemical screening and toxicological study of Aristolochia baetica linn roots: histopathological and biochemical evidence. J Toxicol 2019
Britannica T Editors of Encyclopaedia (2013, September 29). Aristolochia. Encyclopedia Britannica. https://www.britannica.com/plant/wild-ginger
Cai L, He DM, Huang YS, Dao ZL (2020) Aristolochia wenshanensis, a new species of Aristolochiaceae from karst region in southeastern Yunnan. China Taiwania 65(1):41–46
Cheruvathur MK, Kumar GK, Thomas TD (2013) Somatic embryogenesis and synthetic seed production in Rhinacanthus nasutus (L.) Kurz. Plant Cell Tissue and Organ Culture (PCTOC) 113(1):63–71
Chesnokov YV, Kosolapov VM, Savchenko IV (2020) Morphological genetic markers in plants. Russ J Genet 56(12):1406–1415
Cui X, Meng F, Pan X, Qiu X, Zhang S, Li C, Lu S (2022) Chromosome-level genome assembly of Aristolochia contorta provides insights into the biosynthesis of benzylisoquinoline alkaloids and aristolochic acids. Hortic Res 5:9
Dalcol II, Pereira AO, Paz LH, Benetti G, Siqueira FS, Campos M, Ethur EM, Morel AF (2021) Aristolochia triangularis Cham stems and leaves essential oils and their antimicrobial and antimycobacterial effects. The Natur Prod J 11(2):200–206
De Groot H, Wanke S, Neinhuis C (2006) Revision of the genus Aristolochia (Aristolochiaceae) in Africa, Madagascar and adjacent islands. Bot J Linn Soc 151(2):219–238
Debelle FD, Vanherweghem JL, Nortier JL (2008) Aristolochic acid nephropathy: a worldwide problem. Kidney Int 74(2):158–169
Dechbumroong P, Aumnouypol S, Denduangboripant J, Sukrong S (2018) DNA barcoding of Aristolochia plants and development of species-specific multiplex PCR to aid HPTLC in ascertainment of Aristolochia herbal materials. PLoS ONE 13(8):e0202625
Desai S, Tatke P (2019) Phytochemical markers: classification, applications and isolation. Curr Pharm Des 25(22):2491–2498
Dey A, De JN (2011) Aristolochia indica L.: a review. Asian Journal of Plant Sciences 10:108–116
Dey A, Hazra AK, Nongdam P, Nandy S, Tikendra L, Mukherjee A, Banerjee S, Mukherjee S, Pandey DK (2019) Enhanced bacoside content in polyamine treated in-vitro raised Bacopa monnieri (L.) Wettst. South Afr J Bot 123:259–269
Dey A, Nandy S, Nongdam P, Tikendra L, Mukherjee A, Mukherjee S, Pandey DK (2020) Methyl jasmonate and salicylic acid elicit indole alkaloid production and modulate antioxidant defence and biocidal properties in Rauvolfia serpentina Benth. ex Kurz. in vitro cultures. South Afr J Bot 135:1–7
Dey A, Nongdam P, Nandy S, Mukherjee S, Mukherjee A, Tikendra L, Hazra AK, Pandey DK (2021a) Polyamine elicited aristolochic acid production in in vitro clonally fidel Aristolochia indica L.: an ISSR and RAPD markers and HPTLC based study. S Afr J Bot 140:326–335
Dey A, Hazra AK, Mukherjee A, Nandy S, Pandey DK (2021b) Chemotaxonomy of the ethnic antidote Aristolochia indica for aristolochic acid content: implications of anti-phospholipase activity and genotoxicity study. J Ethnopharmacol 266:113416
Do TV, Luu TH, Wanke S, Neinhuis C (2015) Three new species and three new records of Aristolochia subgenus Siphisia from Vietnam including a key to the Asian species. Syst Bot 40(3):671–691
Dos Santos LF, de Oliveira EJ, dos Santos SA, de Carvalho FM, Costa JL, Pádua JG (2011) ISSR markers as a tool for the assessment of genetic diversity in Passiflora. Biochem Genet 49(7):540–554
Doudach L, Al-Mijalli SH, Abdallah EM, Mrabti HN, Chibani F, Faouzi ME (2022) Antibacterial evaluation of the roots of Moroccan Aristolochia longa against referenced Gram-positive and Gram-negative Bacteria. Advancements in Life Sci 9(1):116–121
Francisco CS, Messiano GB, Lopes LM, Tininis AG, de Oliveira JE, Capellari L Jr (2008) Classification of Aristolochia species based on GC–MS and chemometric analyses of essential oils. Phytochemistry 69(1):168–175
González F (1998) Two new species of Aristolochia (Aristolochiaceae) from Brazil and Peru. Brittonia 50(1):5–10
González F, Stevenson DW (2000) Perianth development and systematics of Aristolochia. Flora 195(4):370–391
Guevara-Araya MJ, Vilo C, Urzúa A, González-Teuber M (2020) Differences in community composition of endophytic fungi between above-and below-ground tissues of Aristolochia chilensis in an arid ecosystem. Rev Chil Hist Nat 93(1):1–9
Guevara-Araya MJ, Escobedo VM, Palma-Onetto V, González-Teuber M (2022) Changes in diversity and community composition of root endophytic fungi associated with Aristolochia chilensis along an aridity gradient in the Atacama Desert. Plants 11(11):1511
Hu J, Wang L, Li J (2011) Comparison of genomic SSR and EST-SSR markers for estimating genetic diversity in cucumber. Biol Plant 55(3):577–580
Jadot I, Declèves AE, Nortier J, Caron N (2017) An integrated view of aristolochic acid nephropathy: update of the literature. Int J Mol Sci 18(2):297
Jayaprakash K, Manokari M, Badhepuri MK, Raj MC, Dey A, Shekhawat MS (2021) Influence of meta-topolin on in vitro propagation and foliar micro-morpho-anatomical developments of Oxystelma esculentum (Lf) Sm. PCTOC 147(2):325–337
Jelaković B, Dika Ž, Arlt VM, Stiborova M, Pavlović NM, Nikolić J, Colet JM, Vanherweghem JL, Nortier JL (2019) Balkan endemic nephropathy and the causative role of aristolochic acid. Semin Nephrol 39(3):284–296
Ji H, Hu J, Zhang G, Song J, Zhou X, Guo D (2021) Aristolochic acid nephropathy: a scientometric analysis of literature published from 1971 to 2019. Medicine 100(27)
Jimenez JE, Fernández RA, Blanco MA (2021) Two new species of Aristolochia series Thyrsicae (Aristolochiaceae) from southern Central America, with comments on morphologically similar species. Phytotaxa 520:169–183
Johnson M, Kalaiarasi V, Sivaraman A, Janakiraman N, Babu A, Narayani M (2014) Phytochemical and antibacterial studies on Aristolochia tagala Cham. World J Pharm Res 3(2):2172–2178
Kaur P, Pandey DK, Gupta RC, Kumar V, Dwivedi P, Sanyal R, Dey A (2021) Biotechnological interventions and genetic diversity assessment in Swertia sp.: a myriad source of valuable secondary metabolites. Appl Microbiol Biotechnol 105:4427–4451
Kaur P, Pandey DK, Gupta RC, Dey A (2019) Assessment of genetic diversity among different population of five Swertia species by using molecular and phytochemical markers. Ind Crop Prod:111569
Krishnan SG, Dan M, Kumar ES (2019) Studies on the seed germination and seedling morphology of Aristolochia tagala Cham (Aristolochiaceae) from the Western Ghats India. Int J Res Anal Rev 6(2):620–5
Kuo PC, Li YC, Wu TS (2012) Chemical constituents and pharmacology of the Aristolochia (馬兜鈴 mădōu ling) species. J Tradit Complement Med 2(4):249–266
Lago JH, Kato MJ (2007) 3 α, 4 α-Epoxy-2-piperidone, a new minor derivative from leaves of Piper crassinervium Kunth (Piperaceae). Nat Prod Res 21(10):910–914
Liu R, Zhang HC (2020) Chemical constituents from Aristolochia tagala and their chemotaxonomic significance. Biochem Syst Ecol 90:104037
Luo C, He XH, Chen H, Ou SJ, Gao MP, Brown JS, Tondo CT, Schnell RJ (2011) Genetic diversity of mango cultivars estimated using SCoT and ISSR markers. Biochem Syst Ecol 39(4–6):676–684
Manjula S, Thomas A, Daniel B, Nair GM (1997) In vitro plant regeneration of Aristolochia indica through axillary shoot multiplication and organogenesis. Plant Cell Tissue Organ Cult 51(2):145–148
Manokari M, Priyadharshini S, Jogam P, Dey A, Shekhawat MS (2021) Meta-topolin and liquid medium mediated enhanced micropropagation via ex vitro rooting in Vanilla planifolia Jacks. ex Andrews. Plant Cell Tissue Organ Cul 146:69–82
Nandy S, Hazra AK, Pandey DK, Ray P, Dey A (2021) Elicitation of industrially promising vanillin type aromatic compound 2-hydroxy 4-methoxy benzaldehyde (MBAlD) yield in the in-vitro raised medicinal crop Hemidesmus indicus (L) R Br by methyl jasmonate and salicylic acid. Ind Crops Prod 164:113375
Nazir R, Gupta S, Dey A, Kumar V, Yousuf M, Hussain S, Dwivedi P, Pandey DK (2021a) In vitro propagation and assessment of genetic fidelity in Dioscorea deltoidea, a potent diosgenin yielding endangered plant. South Afr J Bot 140:349–355
Nazir R, Kumar V, Gupta S, Dwivedi P, Pandey DK, Dey A (2021b) Biotechnological strategies for the sustainable production of diosgenin from Dioscorea spp. Appl Microbio Biotech 105:569–585
Neinhuis C, Wanke S, Hilu KW, Müller K, Borsch T (2005) Phylogeny of Aristolochiaceae based on parsimony, likelihood, and Bayesian analyses of trnL-trnF sequences. Plant Syst Evol 250:7–26
Oelschlägel B, Gorb S, Wanke S, Neinhuis C (2009) Structure and biomechanics of trapping flower trichomes and their role in the pollination biology of Aristolochia plants (Aristolochiaceae). New Phytol 184(4):988–1002
El Omari N, Akkaoui S, El Blidi O, Ghchime R, Bouyahya A, Kharbach M, Yagoubi M, Balahbib A, Chokairi O, Barkiyou M 2020 HPLC DAD/TOF MS chemical compounds analysis and evaluation of antibacterial activity of Aristolochia longa root extracts. Natural Product Communications. 15(8):1934578X20932753
Ozen T, Bora N, Yenigun S, Korkmaz H (2020) An investigation of chemical content, enzyme inhibitory propert, antioxidant and antibacterial activity of Aristolochia bodamae Dingler (develiotu) (Aristolochiaceae) root extracts from Samsun. Turkey Flavour and Fragrance J 35(3):270–283
Pacheco AG, Machado de Oliveira P, Piló-Veloso D, de Carvalho F, Alcântara A (2009) 13C-NMR data of diterpenes isolated from Aristolochia species. Molecules 14(3):1245–1262
Padhy GK (2021) A review of Aristolochia indica: ethnomedicinal uses, phytochemistry, pharmacological and toxicological effects. Current Traditional Medicine 7(3):372–386
Pandey DK, Konjengbam M, Dwivedi P, Kaur P, Kumar V, Ray D, Ray P, Nazir R, Kaur H, Parida S, Dey A (2021) Biotechnological interventions of in vitro propagation and production of valuable secondary metabolites in Stevia rebaudiana. Appl Microbio Biotech 105:8593–8614
Pandurangan AG, Deepu S (2018) Aristolochia maxima (Aristolochiaceae): a new record for India. Rheedea 28(2):108–110
Pattar PV, Jayaraj M (2012) In vitro regeneration of plantlets from leaf and nodal explants of Aristolochia indica L.an important threatened medicinal plant. Asian Pac J Trop Biomed 2(2):S488–93
Pawar B, Prashant KA, Bahurupe J, Jadhav A, Anil KA, Pawar S (2015) Proline and glutamine improve in vitro callus induction and subsequent shooting in rice. Rice Sci 22(6):283–289
Pepe M, Hesami M, Jones AM (2021) Machine learning-mediated development and optimization of disinfection protocol and scarification method for improved in vitro germination of cannabis seeds. Plants 10(11):2397
Pereira AO, Avila JM, do Carmo G, Siqueira FS, Campos MM, Back DF, Morel AF, Dalcol II, (2018) Chemical composition, antimicrobial and antimycobacterial activities of Aristolochia triangularis Cham. from Brazil. Ind Crops Prod 121:461–467
Pohodina L, Burda N, Kyslychenko V (2019) Fatty acids composition study of birthwort Dutchman’s pipe (Aristolochia clematitis L.) herb and root. Norwegian J Dev Int Sci 31–1:53–57
Priestap HA, van Baren CM, Leo Lira PD, Prado HJ, Neugebauer M, Mayer R, Bandoni AL (2002) Essential oils from aerial parts of Aristolochia gibertii Hook. Flavour Frag J 17(1):69–71
Qin L, Hu Y, Wang J, Wang X, Zhao R, Shan H, Li K, Xu P, Wu H, Yan X, Liu L (2021) Insights into angiosperm evolution, floral development and chemical biosynthesis from the Aristolochia fimbriata genome. Nat Plants 7(9):1239–1253
Remya M, Narmatha Bai V, Mutharaian VN (2013) In vitro regeneration of Aristolochia tagala and production of artificial seeds. Biol Plant 57(2):210–218
Remya M, Bai VN, Murugesan S, Mutharaian V (2016) Changes in bioactive components of Aristolochia tagala. Cham, a rare species of medicinal importance during its in vitro development through direct regeneration. bioRxiv 037028
Rihan HZ, Kareem F, El-Mahrouk ME, Fuller MP (2017) Artificial seeds (principle, aspects and applications). Agronomy 7(4):71
Ritala A, Dong L, Imseng N, Seppänen-Laakso T, Vasilev N, van der Krol S, Rischer H, Maaheimo H, Virkki A, Brändli J, Schillberg S (2014) Evaluation of tobacco hairy roots for the production of geraniol the first committed step in terpenoid indole alkaloid pathway. J Biotech 176:20–8
Sarma B, Tanti B (2015) Karyomorphology of three species of Aristolochia–rare and endemic medicinal plants of Assam, India. Caryologia 68(2):154–158
Sarma B, Tanti B (2017a) Analysis of genetic diversity of certain species of Aristolochia using ISSR-based molecular markers. Curr Life Sci 3(4):47–53
Sarma B, Tanti B (2017b) In vitro regeneration of plantlets from nodal explants of Aristolochia saccata and Aristolochia cathcartii. Eur J Biol Res 7(3):191–201
Sarma B, Baruah PS, Tanti B (2018) Habitat distribution modeling for reintroduction and conservation of Aristolochia indica La threatened medicinal plant in Assam India. J Threatened Taxa 10(11):12531–7
Scarborough J, Fernandes A (2011) Ancient medicinal use of Aristolochia: birthwort’s tradition and toxicity. Pharm Hist 53(1):3–21
Scherwinski-Pereira JE, da Guedes RS, Fermino PC, Silva TL, Costa FH (2010) Somatic embryogenesis and plant regeneration in oil palm using the thin cell layer technique. In Vitro Cell Dev Biology-Plant 46(4):378–385
Sebastinraj J, Sidique KI (2011) In vitro rapid clonal propagation of Aristolochia bracteolata Lam (Aristolochiaceae) a valuable medicinal plant. World J Agri Sci 7(6):653–8
Shah SN, Husaini AM, Shirin F (2013) Micropropagation of the Indian Birthwort Arsitolochia indica L. Int J Biotechnol 4(6):86–92
Shekhawat MS, Mehta SR, Manokari M, Priyadharshini S, Badhepuri MK, Jogam P, Dey A, Rajput BS (2021) Morpho-anatomical and physiological changes of Indian sandalwood (Santalum album L.) plantlets in ex vitro conditions to support successful acclimatization for plant mass production. Plant Cell Tissue Organ Cul 147:423–435
Shibutani S, Dong H, Suzuki N, Ueda S, Miller F, Grollman AP (2007) Selective toxicity of aristolochic acids I and II. Drug Metabol Dispos 35(7):1217–1222
Siddique NA, Bari MA, Pervin MM, Nahar N, Banu LA, Paul KK, Kabir MH, Huda AK, Ferdaus KM, Hossin MJ (2006) Plant regeneration from axillary shoots derived callus in Aristolochia indica Linn. an endangered medicinal plant in Bangladesh. Pak J Biol Sci 9:1320–1323
Soh AC, Wong G, Tan CC, Chew PS, Chong SP, Ho YW, Wong CK, Choo CN, Nor Azura H, Kumar K (2011) Commercial-scale propagation and planting of elite oil palm clones: research and development towards realization. J Oil Palm Res 23:935–952
Sudhakaran MV (2014) Pharmacognostic and HPTLC Fingerprint profile of the root of Aristolochia indica Linn and quantification of the marker compound. EuroJ Med Plant 4(9):1113–24
Swamy MK, Nath S, Paul S, Jha NK, Purushotham B, Dey Rohit KC, A, (2021) Biotechnology of camptothecin production in Nothapodytes nimmonian Ophiorrhiza sp and Camptotheca acuminata. Appl Microbio Biotech 105:9089–9102
Tikendra L, Potshangbam AM, Amom T, Dey A, Nongdam P (2021a) Understanding the genetic diversity and population structure of Dendrobium chrysotoxum Lindl.-an endangered medicinal orchid and implication for its conservation. South Afr J Bot 138:364–376
Tikendra L, Potshangbam AM, Dey A, Devi TR, Sahoo MR, Nongdam P (2021b) RAPD, ISSR, and SCoT markers based genetic stability assessment of micropropagated Dendrobium fimbriatum Lindl. var. oculatum Hk. f.-an important endangered orchid. Physiol Mol Biol Plant 27:341–357
Van Do T, Wanke S, Neinhuis C (2016) Aristolochia bidoupensis sp nov from southern Vietnam. Nord J Bot 34(5):513–6
Wang W, Wu Y, Yan Y, Ermakova M, Kerstetter R, Messing J (2010) DNA barcoding of the Lemnaceae, a family of aquatic monocots. BMC Plant Biol 10(1):1–1
Wanke S, González F, Neinhuis C (2006) Systematics of pipevines: combining morphological and fast-evolving molecular characters to investigate the relationships within subfamily Aristolochioideae (Aristolochiaceae). Int J Plant Sci 167(6):1215–1227
Warrier, PK (1993) Indian medicinal plants: a compendium of 500 species (vol. 5). Orient Blackswan
Wu TS, Damu AG, Su CR, Kuo PC (2004) Terpenoids of Aristolochia and their biological activities. Nat Prod Rep 21(5):594–624
Yan YJ, Ma JS (2019) Reinstatement of Isotrema, a new generic delimitation of Aristolochia subgen Siphisia (Aristolochiaceae). Phytotaxa 401(1):001–023
Yu YL, Wang HC, Yu ZX, Schinnerl J, Tang R, Geng YP, Chen G (2021) Genetic diversity and structure of the endemic and endangered species Aristolochia delavayi growing along the Jinsha River. Plant Div 43(3):225–233
Zayova E, Nedev T, Petrova D, Zhiponova M, Kapchina V, Chaneva G (2020) Tissue culture applications of Artemisia annua L callus for indirect organogenesis and production phytochemical. Plant Tissue Culture Biotech 30(1):97–106
Zhang HC, Liu R, An ZP, Li H, Zhang R, Zhou F (2016) Aristolactam-type alkaloids and aristolochic acids from Aristolochia moupinensis and Aristolochia cathcartii. Biochem Syst Ecol 65:198–201
Author information
Authors and Affiliations
Contributions
SN, NG, MTAA and SP conceptualized the designed review. AM, MTP and AM revised the primary draft; AVG, MHR and MK conducted the literature survey. R and MG contributed figures and analytical tools. SP and AD analysed and finalized the manuscript. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nath, S., Ghosh, N., Ansari, T.A. et al. Genetic diversity assessment and biotechnological aspects in Aristolochia spp.. Appl Microbiol Biotechnol 106, 6397–6412 (2022). https://doi.org/10.1007/s00253-022-12152-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12152-1