Abstract
The most crucial yield constraint of pigeon pea is susceptibility to the pod borer Helicoverpa armigera, which causes extensive damage and severe economic losses every year. The Agrobacterium-mediated plumular meristem transformation technique was applied for the development of cry1Ac transgenic pigeon pea. Bioactivity of the cry1Ac gene was compared based on integration and expression driven by two promoters, the constitutive CaMV35S promoter and the green-tissue-specific ats1A promoter, in those transgenic events. The transgenic events also contained the selectable marker gene nptII flanked by loxP sites. Independent transgenic events expressing the Cre recombinase gene along with a linked bar selection marker were also developed. Integration and expression patterns of both cry1Ac and cre were confirmed through Southern and western blot analysis of T1 events. The constitutive expression of the Cry1Ac protein was found to be more effective for conferring resistant activity against H. armigera larvae in comparison to green-tissue-specific expression. Constitutively expressing Cry1Ac T1 events were crossed with Cre recombinase expressing T1 events. The crossing-based Cre/lox-mediated marker gene elimination strategy was demonstrated to generate nptII-free Cry1Ac-expressing T2 events. These events were subsequently analyzed in the T3 generation for the segregation of cre and bar genes. Five Cry1Ac-expressing T3 transgenic pigeon pea events were devoid of the nptII marker as well as cre-bar genes. H. armigera larval mortality in those marker-free T3 events was found to be 80–100%. The development of such nptII selectable marker-free Cry1Ac-expressing pigeon pea transgenics for the first time would greatly support the sustainable biotechnological breeding program for pod borer resistance in pigeon pea.
Key points
• Constitutive expression of Cry1Ac conferred complete resistance against Helicoverpa armigera
• Green-tissue-specific expression of Cry1Ac conferred partial pest resistance
• Cre/lox-mediated nptII elimination was successful in constitutively expressing Cry1Ac transgenic pigeon pea events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigeon pea (Cajanus cajan (L.) Millspaugh), popularly known as red gram (arhar) is an important pulse crop grown predominantly in developing countries of semiarid tropical and subtropical regions of the world. According to FAO (2019), pigeon pea is accepted as the sixth most important edible grain legume with worldwide production of 5.61 million tonnes per year. This has a broad influence on the overall crop yield, which is 7.9 thousand kg per hectare area, much less than the potential yield. Over the last few decades, pigeon pea production is facing global demand to meet food and nutritional security and also proving its usefulness for various purposes such as fuel, fodder, building material, etc. (Ghosh et al. 2014). Based on worldwide data, India holds the topmost position as a pigeon peaproducing country. Around 3.31 million tonnes of pigeon pea are produced annually in India, where it is the second most important pulse. As a whole, India contributes about 75% of global production (FAO 2019). Although pigeon peaharvesting areas have considerably increased, the yield has remained stagnant for the last 6 decades mainly due to the high susceptibility of the cultivars to various biotic stress (Varshney et al. 2012). Additionally, in comparison to other crops, limited efforts have been directed to generate precise knowledge on the genetic inheritance of stress-resistant traits in pigeon pea. This also affected crop yield and further limited the efficacy of crop improvement programmes (Varshney et al. 2010).
The major yield constraint of pigeon pea is the lepidopteran pest Helicoverpa armigera, which severely damages the plant growth and productivity and results in significant economic losses of over $400 million per year (Sreekanth et al. 2014). Despite several control strategies, the use of chemical pesticides has been found to be very effective for managing pests over large areas. However, extensive usage of hazardous chemicals has detrimental effects on human health and other organisms and finally resulted in environmental degradation (Mishra et al. 2018). Increased public concerns regarding the probability of destructive environmental consequences associated with these harmful pesticides prompted the search for improved methods of insect pest resistance. This situation has drawn the attention of the scientific community to adopt eco-friendly and environmentally safe technologies to improve crop protection for sustainable agriculture (Mishra et al. 2018). However, limited genetic variability in the primary gene pool coupled with poor exploitation of genetic resources is considered a major obstacle in the genetic improvement of pigeon pea (Varshney et al. 2012). Due to cross incompatibility with wild relatives and lack of genetic resistance in the gene pool, the primary objective of pigeon pea breeding to develop insect-resistant varieties has not been succeeded (Sharma et al. 2009). In recent years, modern biotechnological approaches have provided a new avenue to develop improved cultivars by overcoming severe bottlenecks associated with conventional breeding (Venkata et al. 2019). Transgenic technology has proven to be beneficial for this crop to incorporate agronomically convenient traits, which have a positive impact on yield, productivity, and nutritional security of the worldwide human population (Saxena et al. 2015). Based on recombinant DNA technology, the successful integration of several foreign genes has rendered new opportunities for the development of resistant pigeon pea cultivars with inbuilt resilience to withstand the biotic stress factors (Ghosh et al. 2014). Among them, the introduction of Bacillus thuringiensis-encoded ∂-endotoxins or Cry proteins into different crop species remained the most advantageous choice for researchers to provide good and long-term potentiality against the infestation of various lepidopteran insects (Ghosh et al. 2017). Highly specific ∂-endotoxins to a particular insect group have been justified by previous workers to forego the testing on nontarget organisms. This also facilitated its effective utilization as a biologically secured and most reliable molecular device for crop improvement (Kumar et al. 2008). Over the last few decades, limited attempts have been made in pigeon pea for successful integration of the cry gene to drive the constitutive expression of a protein that could exhibit a maximum range of insect mortality (Krishna et al. 2011; Ramu et al. 2012; Das et al. 2016; Kaur et al. 2016; Ghosh et al. 2017; Singh et al. 2018; Sarkar et al. 2021). Among them, Cry1Ac has been preferred by researchers due to its high entomotoxicity toward the lepidopteran group of insects, followed by Cry1Aa, Cry1Ab, and Cry2Aa (Perlak et al. 1990). Additionally, due to different receptor-binding potentials, using more than one Cry protein in transgenic development is the need of the future to obtain durable resistance to the lepidopteran pest (Ghosh et al. 2017). The toxicity level is directly correlated with the activity and occurrence of the gene in host plant tissue. However, the expression of such a foreign protein could be controlled by a specific promoter to manage the metabolic load in plants.
In such circumstances, the potentiality of a suitable promoter should be assessed for successful genetic transformation. The correct choice of the promoter was proven to be a crucial component in transgenic plant development through regulating the transcription in the host cell and also influencing the transgene expression level in a spatiotemporal manner (Bakhsh et al. 2011). The great majority of studies conducted in pigeon pea thus far have largely relied on the Cauliflower mosaic virus 35S (CaMV35S) constitutive promoter (Ghosh et al. 2014). This promoter is widely used in transformation technology for driving the expression of genes in almost all tissues, and also the promoter activity is independent of any environmental and developmental conditions. Alternatively, constitutive expression of transgenes, under certain conditions may interfere with normal processes in a plant and can exhibit undesirable phenotypes during plant growth and development (Potenza et al. 2004). Advancement in Agrobacterium tumefaciens-mediated foreign gene delivery system has enabled the researchers to use suitable tissue-specific promoters to adjust the transgene expression pattern. In pigeon pea, a few reports are available on the usage of tissue-specific promoters like a flower- and leaf-specific double-enhanced CaMV35S promoter (CaMV35SDE), seed-specific phaseolin, and Arabidopsis thaliana 2S2 albumin promoters to regulate the transgene expression pattern (Sharma et al. 2006; Thu et al. 2007). The use of ribulose 1,5-bisphosphate carboxylase small subunit promoters along with their chloroplast-directed transit peptides were found to be attractive candidates for obtaining high gene expression levels specifically in green tissue by targeting foreign protein into the chloroplast. If the green-tissue-specific promoter is used for the expression of Cry1Ac in pigeon pea, the accumulation of the protein in seed grains can be avoided. Additionally, the expression of Cry1Ac protein was proven to be increased around 10 to 20-fold in tobacco, under the control of A. thaliana ribulose 1,5-bisphosphate carboxylase (rubisco) small subunit (ats1A) promoter along with the transit peptide (Wong et al. 1992). The use of the ats1A promoter was proven to delineate a high level of green-tissue-specific expression of the bar gene in the cotton plants (Kumar and Timko 2004). Further, this promoter was found to be advantageous in transgenic soybean and chickpea plants for conferring complete resistance to target pests (Miklos et al. 2007; Acharjee et al. 2010). In the present report, the ats1A promoter was utilized in pigeon pea to delineate a confined expression of Cry1Ac protein mostly in the green-tissue-specific regions preferred by the lepidopteran insects.
Despite the scientific advances made in crop improvement, the presence of a selectable marker gene (SMG) has been considered a major limiting factor for the public acceptance of genetically modified (GM) crops and also hindered their commercialization (Miki and McHugh 2004). During the last few decades, several ecological and food-safety-related issues have been raised by environmentalists and consumers over the spreading of resistance markers into related wild species, bacterial strains, and non-transgenic crops (Upadhaya et al. 2010). Considering the worldwide data, it can be mentioned that transgenic products were subjected to strict regulatory measures before their commercial release. Several biosafety assessments have been done to evaluate the impact of GM crops on the environment and human nutrition. Different types of assessment methods such as allergenicity, acute oral toxicity, in vitro degradation studies, bioinformatics studies, animal dietary exposure, nutritional assessment, and also environmental risk assessment are performed following the rules of food safety authorities (Mondal et al. 2011). In addition to the gene of interest, the selectable marker gene in transgenic crops should also be evaluated by the rigid framework of biosafety assessments before commercialization (Ramessar et al. 2007). It also increases the cost of transgenic production. Therefore, the removal of nptII was found to be convenient to avoid biosafety and environmental risk assessment studies associated with the gene and marker-free transgenic plants are more acceptable for commercialization.
Additionally, after the accomplishment of each transformation, the elimination of the marker gene not only increases the public acceptability of GM crops, but it would also facilitate the pyramiding of multiple desirable genes into the same plant (Jaiwal et al. 2002; Yau et al. 2013). All these factors motivated the researchers to apply clean gene technologies for marker-free transgenic development and its subsequent application in the commercial sector (Upadhyaya et al. 2010). Several strategies were implemented for the elimination of selectable marker genes, among them the Cre/lox-mediated site-specific recombination strategy has been widely adopted in the crop system (Upadhaya et al. 2010; Bala et al. 2013). Cre recombinase recognizes the specific DNA sequences to be eliminated, which is flanked by two directly repeated asymmetric 34 bp loxP sites and induces precise recombination for successful DNA excision. In the present report, the tissue-specific and constitutive expression of Cry1Ac was compared in transgenic pigeon pea. The Cre/lox recombination strategy was utilized for the removal of the nptII marker gene from transgenic events. Further elimination of the cre gene and the bar selectable marker was successfully accomplished by genetic segregation in pod borer-resistant, T3 Cry1Ac transgenic events.
Materials and methods
Biological resources
Seeds of ICPL 87119 (Asha), a cultivar of pigeon pea, were collected from the Indian Institute of Pulses Research, Kanpur, India. The AGL1 strain (Lazo et al. 1991) of A. tumefaciens harboring binary plasmids was used for plant transformation. Bioassay experiments were performed with H. armigera larvae. Eggs of H. armigera were obtained from the National Bureau of Agricultural Insect Resources (NBAIR), Bangalore, India, in a sterile insect box.
Pigeon pea transformation
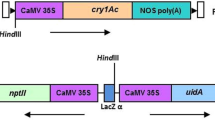
Three binary plasmid vectors, 35S-cry, ats-cry, and 35S-cre were used for the described study. These plasmids were used in Agrobacterium-mediated plumular meristem transformation to develop the Cry1Ac and Cre transgenic pigeon pea events. The synthetic cry1Ac gene (NCBI Accession No: KF630361.1) was cloned separately into the binary vector pBK11 (Chakraborti et al. 2008) to construct two new vectors 35S-cry and ats-cry. pBK11 contained the CaMV35S promoter-controlled neomycin phosphotransferase II (nptII) gene flanked by loxP sites. An EcoRI- and HindIII-digested 2.8 kb cry1Ac gene cassette driven by the CaMV35S constitutive promoter and the nos terminator was cloned into pBK11. The construct was designated as 35S-cry (~ 8.3 kb, Fig. 1a). For the development of the second vector ats-cry, BamHI- and EcoRI-digested cry1Ac gene was first blunted and then ligated into the BamHI-digested, blunted, and dephosphorylated pWM5 binary vector (Tabe et al. 1995). The resulted 5′-ats1A-transit peptide-cry1Ac-Tobssu Poly A-3′ (~ 4.0 kb) gene cassette was taken out from the pWM5 vector through EcoRI digestion and then ligated into EcoRI-digested pBK11 to develop the vector ats-cry of ~ 9.5 kb (Fig. 1b). The third binary vector 35S-cre was constructed with an I-SceI-digested cre gene cassette from pBK15 (Chakraborti et al. 2008), which was cloned into pCAMBIA3300I (Chakraborti et al. 2008) to develop a 35S-cre vector of ~ 10.6 kb size. The designated vector containing the Cre recombinase gene was under influence of the CaMV35S promoter, and the herbicide-resistant bar gene was present as a selectable marker (Fig. 1c). All the vectors were mobilized into the AGL1 strain of A. tumefaciens. Decapitated embryonic axis-based explants were utilized for Agrobacterium-mediated plumular meristem transformation as described by Ganguly et al. (2018). Agrobacterium infection followed by 250 mg l−1 cefotaxime wash of explants and subsequent transfer to the artificial soil. After 4–5 weeks, T0 plant lines were established in the soil and allowed for self-fertilization in the greenhouse to obtain T1 seeds. The untransformed control pigeon pea events were also developed under the same condition without Agrobacterium infection.
Binary vectors and analysis of T0 plants. Schematic representation of the T-DNA region of binary vectors; a constitutive promoter-driven cry1Ac, designated as 35S-cry, and b green- tissue-specific promoter-driven cry1Ac, designated as ats-cry. F1/R1 and F2/ R2 are nptII and cry1Ac specific primers, respectively, c schematic representation of the T-DNA region of the 35S-cre binary vector showing bar and cre specific primers F3/R3 and F4/R4, respectively. LB, left border of T-DNA; RB, right border of T-DNA; CaMV35S Pr, Cauliflower mosaic virus 35S promoter; ats1A Pr, Arabidopsis thaliana rubisco small subunit promoter; CaMV35S Poly A, Cauliflower mosaic virus 35S terminator; nos Poly A, nopaline synthase terminator; and Tobssu Poly A, Nicotiana tabacum small subunit terminator. d, e Representative PCR amplification of nptII-specific 700 bp and cry1Ac-specific 662 bp sequences in established Cry1Ac T0 transformants. f PCR amplification of T0 35S-cre events showing the presence of 1100 bp cre-specific amplicon. Lanes 1–3, DNA ladder, respective binary plasmid as positive and untransformed pigeon pea DNA as negative controls, respectively
Polymerase chain reaction (PCR) of pigeon pea DNA samples
Fresh young leaves of established pigeon pea transgenic events of T0, T1, T2, and T3 generations were taken for genomic DNA isolation following the cetyl trimethyl ammonium bromide (CTAB) method as described by Chakraborti et al. (2006). PCR was performed with the extracted DNA samples to determine the integration of the cry1Ac gene along with nptII selectable markers in both 35S-cry and ats-cry transgenic events. Similarly, the presence of the cre gene along with bar selectable marker was analyzed. Primers were developed to amplify nptII, cry1Ac, cre, and bar gene-specific sequences (Supplementary Table S1). PCR conditions were, 95 °C for 5 min followed by 35 cycles at 95 °C for 45 s, 57 °C for 45 s, and 72 °C for 1 min with a final extension at 72 °C for 10 min. In all PCR analyses, untransformed pigeon pea genomic DNA and plasmid DNA of respective binary vectors were taken as negative and positive controls, respectively.
Selection and establishment of T1 transgenic events
T1 seeds were obtained from 35S-cry, ats-cry, and 35S-cre T0 pigeon pea events. Kanamycin-mediated stringent selection of T1 seeds from 35S-cry and ats-cry was performed (Ganguly et al. 2017). After peeling the seed coat, overnight imbibed seeds were allowed for 100 mg l−1 kanamycin treatment for 5 h and subsequently sown onto artificial soil. Antibiotic treated and non-treated seeds of untransformed pigeon pea events were served as controls. Simultaneously, for 35S-cre events established T1 plants were screened for herbicide tolerance through a glufosinate painting assay with 2.0 mg l−1 solution of glufosinate ammonium on two out of three leaflets of each leaf.
Western blot analysis
Total soluble protein (TSP) was extracted from 1-month-old T1 and T3 Cry1Ac events as well as 35S-cre T1 events according to the method described by Ghosh et al. (2017). Protein concentration was measured (Bradford 1976). Purified Cry1Ac, obtained from the bacterial overexpression system, was used as a positive control (Ghosh et al. 2017). Around 40 μg TSP of each plant sample was separated on 12% SDS-PAGE and subsequently transferred on to HybondC membrane (Amersham Sciences, Amersham, Buckinghamshire, UK) by electro-blotting. Then, the membrane was kept in a blocking solution overnight at 4 °C. On the next day, the membrane was allowed to probe with anti-Cry1Ac or Cre polyclonal primary antibody at 1:10,000 dilution for 1 h. Then, the membrane was incubated with anti-rabbit IgG-horse radish peroxidase (HRP) conjugate (Sigma-Aldrich, USA) as a secondary antibody at 1:10,000 dilution. Chemiluminescence (ECL kit, Amersham Biosciences, Amersham, Buckinghamshire, UK) reagents were used to detect the bound secondary antibody, developed on Kodak film.
Enzyme-linked immunosorbent assay (ELISA) of soluble protein extracts
Western positive pigeon pea events of T1 and T3 generations were selected for quantification of transgenic protein through indirect ELISA from the extracted crude protein of Cry1Ac events. ELISA experiments were undertaken following the method described by Ghosh et al. (2017). Microtitre wells were coated with 20 µg of isolated soluble protein of transgenic events along with serially diluted purified Cry1Ac (5–50 ng) in ELISA coating buffer (15 mM sodium carbonate, 35 mM sodium bicarbonate, 3 mM sodium azide; pH 9.5) for 18 h at 4 °C. After washing with 1X PBST [1X phosphate-buffered saline (PBS) and 0.1% Tween-20], microtitre plates were kept for 2 h in blocking solution [PBS with 5% (w/v) bovine serum albumin (BSA)] at 37 °C. Coated protein samples were allowed to bind with anti-Cry1Ac primary antibody and HRP-conjugated anti-rabbit secondary antibody. Finally, the wells were kept in citrate buffer containing Oo-Phenylenediamine dihydrochloride (Sigma-Aldrich, St. Louis, USA) for color development. Data were recorded with a BioRad (Hercules, CA, USA) microplate reader at 415 nm. Three biological replications were used per experiment, and each experiment was performed thrice. Untransformed plant protein was used as a negative control in all experiments. All the experimental data were combined and compared through ANOVA (p = 0.05, 0.01, 0.001, 0.0001).
Southern blot hybridization
Southern hybridization experiments were carried out with transgenic events of T1, T2, and, T3 generations using both radioactive and nonradioactive methods (Ghosh et al. 2017). Forty micrograms of isolated genomic DNA was digested with restriction endonucleases and run in 0.8% (w/v) agarose gel electrophoresis and subsequently blotted onto positively charged nylon membrane following depurination, alkali denaturation, and neutralization. PCR-amplified probe sequence was labeled with [α32P] dCTP in radioactive method, whereas digoxigenin (DIG, Roche Applied Science, Indianapolis, USA) labeled probe was used for nonradioactive Southern blotting. Gel-extracted 662 bp amplicon of the cry1Ac gene obtained from 35S-cry and ats-cry binary vectors along with the 1100 bp amplicon of the cre gene from the 35S-cre plasmids served as a probe as well as one of the positive controls in the hybridization method. Digested binary plasmids and untransformed pigeon pea plant DNA were used as second positive and negative controls, respectively.
Larval mortality assay of H. armigera
Detached leaf-feeding bioassay was performed with freshly hatched 2nd instar larvae of H. armigera fed on an artificial diet (Armes et al. 1992). Leaflets of 2 to 3-month-old 35S-cry and ats-cry pigeon pea transgenic plants of T1 and T3 generations were placed in insect-breeding boxes containing 3% water agar. In every experiment, one healthy 2nd instar larva was kept in each box, and ten such boxes were taken. The survival of 2nd instar larvae was monitored at an interval of 24 h till the 7th day. Untransformed plant leaves were kept as a negative control. Each experiment was repeated three times, and the data was recorded to calculate the mean ± standard error for the percentage of larval survivability. Alternatively, weight loss of 3rd–4th instar larvae was recorded to confirm the Cry1Ac toxicity on larval growth over 13 days. A single larva was kept in one box, and five such boxes were taken per experiment. Data were recorded every 24 h by measuring the larval weight in grams. Each experiment was performed three times. All experimental data were combined and compared through ANOVA (p = 0.05, 0.01, 0.001, 0.0001).
Immunohistofluorescence localization of transgenic protein
Transverse leaflet sections of T1 Cry1Ac and Cre transgenic pigeon pea events along with untransformed negative control plants were allowed for 2 h incubation at 4 °C with 10% (v/v) trichloroacetic acid. This was followed by 3–4 times washing with 3:1 ethanol: acetic acid to remove the chlorophyll. A series of ethanol grades (90, 70, 50, and 30% (v/v) to water) were allowed to treat the leaf sections, 15 min each, and then incubated for 2 h in blocking solution [3% (w/v) BSA in 1X PBS] at room temperature. Then, leaf sections were kept in a BSA solution containing an anti-Cry1Ac/-Cre antibody (1:10,000) for 18 h at 4 °C. Then samples were washed with 1X PBS followed by 1 h incubation with anti-rabbit IgG fluorescein isothiocyanate (FITC) conjugated (1:20,000) (Sigma-Aldrich, St. Louis, USA) secondary antibody at room temperature. The treated samples were visualized under a fluorescence microscope (Axio Scope, Carl Zeiss, Jena, Germany) using an excitation filter of 450–490 nm for FITC. All the experiments were performed in triplicates using 4 single-copy transgenic events each from 35S-cry, ats-cry, and 35S-cre categories.
Histological study of leaf tissues
T1 transgenic and untransformed control leaves of pigeon pea (cv. ICPL-87119) were taken for histological study using aniline blue strain to find out anatomical differences in comparison to Nicotiana tabacum (cv. Petit Havana SR1) and A. thaliana (Col-0) leaf, according to the protocol mentioned by Schenk and Schikora (2015). All the leaf samples were treated with 1:3 acetic acid and ethanol solution for 1 h until the chlorophyll was completely removed, and the material became transparent. Then, the samples were washed with 150 mM K2HPO4 (pH 9.5) for 30 min followed by incubation for 2 h with 150 mM K2HPO4 and 0.01% aniline blue solution in a dark condition. After the treatment, the leaf samples were embedded in 50% glycerol and visualized under a fluorescence microscope using the excitation filter of 360–420 nm. All the experiments were repeated twice using 3 samples from each plant system.
Crossing of T1 pigeon pea events
Single-copy T1 35S-cry events showing 80–100% larval mortality were selected for the crossing. Ten independent 35S-cry transgenic lines were reciprocally crossed with four individual 35S-cre plants, and T2 hybrid seeds were obtained. Established hybrid pigeon pea T2 events were further evaluated to confirm the elimination of marker genes using PCR and Southern blot hybridization and subsequently analyzed for detection of protein activity through western blot and insect bioassay.
Establishment of nptII-eliminated T2 hybrids
Genomic DNA of the T2 hybrid events was extracted, and PCR analysis was carried out to determine the presence or absence of the nptII gene. The nptII negative samples were allowed for another round of polymerase chain reaction with cre, cry1Ac, and bar gene-specific primers. Finally, all nptII-eliminated transgenic events were confirmed by Southern blot analysis. Parental lines of those hybrid events were also kept as control along with the other positive and untransformed negative controls. Established Southern positive single-copy T2 transgenic plants were allowed for selfing, and T3 seeds were obtained.
Molecular analysis of T3 events to establish marker-free pigeon pea transgenics
The harvested seeds from nptII-eliminated Cry1Ac-expressing T2 hybrid events were germinated to obtain T3 plants. These plants were allowed for PCR analysis to determine the removal of the cre and bar genes, which was subsequently confirmed by Southern blot hybridization. Western blot analysis and larval mortality bioassay were performed in those marker-free transgenic events.
Results
Plumular meristem transformation and characterization of T0 transformants
Agrobacterium-mediated plumular meristem transformation was carried out in pigeon pea using 3 binary vectors, designated as 35S-cry, ats-cry, and 35S-cre (Fig. 1a, b, c). After establishment in the artificial soil, 270 Cry1Ac (35S-cry and ats-cry) and 60 35S-cre T0 events were found to be PCR positive detected through the successful amplification of transgenes (Supplementary Table S1, Fig. 1d, e, f). Binary plasmid, used for transformation, was kept as a positive control, whereas a negative control was served by untransformed plant genomic DNA in respective PCR analyses. The T1 seeds obtained from these T0 lines were taken for further analysis.
Selection of T1 progenies
Germinated T1 seeds from each Cry1Ac T0 event were screened by treating them with 100 mg l−1 kanamycin (Fig. 2a). After 3 weeks, antibiotic-resistant T1 plants showed the desired height and were found to be morphologically comparable with non-selected untransformed control plants, whereas antibiotic-treated non-transgenic T1 lines were distinctly shorter with an undeveloped root system. Fifty kanamycin-resistant Cry1Ac T1 progenies were established, which were found to be PCR positive through the successful amplification of transgenes, nptII, and cry1Ac (Fig. 2b, c).
Selection and establishment of T1 transgenics. a Germination of T1 Cry1Ac seeds treated with 100 mg l−1 kanamycin. Pot 1, non-treated untransformed events, pot 2, treated untransformed plants, and pot 3, established T1 transgenic events. b, c Representative PCR analysis of nptII-specific 700 bp and cry1Ac-specific 662 bp sequences in cry1Ac T1 plants. d Glufosinate painting assay on cre T1 plants, (i) leaflets of untransformed plant, and (ii) T1 events painted with 2.0 mg l−1 glufosinate ammonium (marked with dotted lines). e, f PCR amplification of 35S-cre events showing the presence of 440 bp bar and 1100 bp cre-specific fragments. Lanes 1–3, DNA ladder, binary plasmid as positive and untransformed pigeon pea DNA as negative controls, respectively
Glufosinate ammonium leaf painting assay was performed on T1 progenies of each 35S-cre event along with untransformed pigeon pea plants as control (Fig. 2d). Painted leaflets in the control plants exhibited necrosis after 3 days, whereas no detrimental effect of the herbicide was observed in leaflets of T1 plants. T1 progenies from 20 T0 lines were allowed for glufosinateammonium painting assay. Twenty-five T1 progenies were established after the assay. Twenty-one plants were found to be positive for the presence of a bar and cre gene-specific fragments identified through PCR (Fig. 2e, f).
Expression analyses of T1 transgenics
Western blot analysis was performed using TSP extracted from PCR-positive T1 plants of Cry1Ac (35S-cry and ats-cry) and 35S-cre events. All the PCR-positive T1 Cry1Ac events were found to express ~ 66 kDa Cry1Ac protein (Fig. 3a). Among these plants, 40 T1 events were 35S-cry, whereas 10 events were ats-cry. Purified Cry1Ac obtained from bacterial overexpression was used as a positive control. Simultaneously, ~ 33 kDa Cre protein was found to be expressed in all PCR positive 35S-cre T1 events (Fig. 3b). No such band was exhibited by untransformed negative controls.
Expression analysis of T1 transgenic events. a Representative western blot analysis of total protein extract from 35S-cry and ats-cry events; lane + ve purified Cry1Ac as the positive control; lane − ve, untransformed pigeon pea plant as the negative control. b Representative western blot analysis of 35S-cre T1 events with the untransformed plant as the negative control. c Expression level of transgenic Cry1Ac protein in 35S-cry events. d Expression level of Cry1Ac protein in ats-cry transgenic events. e Immunohistofluorescence localization of Cry1Ac in the leaflets of T1 events indicated by green fluorescence; lamina of (i) untransformed control, (ii) 35S-cry, and (iii) ats-cry leaflets; vascular bundle of (iv) untransformed control, (v) 35S-cry, and (vi) ats-cry leaflets. f Immunohistofluorescence localization of Cre recombinase protein in the transverse section of leaflets from 35S-cre events; vascular bundle of (i) untransformed control and (ii) 35S-cre leaflets; lamina of (iii) untransformed control and (iv) 35S-cre leaflets
A quantitative assessment of Cry1Ac protein accumulation in transgenic pigeon pea leaves was performed through indirect ELISA analysis. Purified Cry1Ac, obtained from bacterial overexpression, was used for serial dilution, which was required for standard curve preparation. Out of 40 selected 35S-cry transgenic events, a significantly higher (p < 0.001) level of Cry1Ac expression was observed in 14 T1 lines (22–62 μg g−1 FW (fresh weight)), and alternatively a lower amount of protein accumulation (6–28 μg g−1 FW) was noticed in all ats-cry T1 transgenic events (Fig. 3c, d). Untransformed plant protein was kept as a negative control.
Immunohistofluorescence study of T1 progenies
The spatial expression patterns of transgenic proteins in T1 transgenic events were detected through in situ immunohistofluorescence localization of leaflet tissue sections. The distinguishable accumulation of Cry1Ac protein was observed in 35S-cry and ats-cry T1 events under the control of CaMV35S and ats1A promoters, respectively (Fig. 3e). Leaflet sections of constitutive promoter-driven T1 events revealed the presence of Cry1Ac protein in the form of green fluorescence in all tissues. Alternatively, the expression of the Cry protein was found to be confined to trichomes and the outer epidermal area of the leaflet in ats1A promoter-driven Cry1Ac events. No fluorescence signal was detected in untransformed control plant tissues. Simultaneously, single-copy T1 plants of 35S-cre events demonstrated the constitutive expression pattern of Cre protein throughout the leaflet tissues (Fig. 3f). Untransformed negative control pigeon pea leaves did not show Cre protein expression.
Southern blot analysis of T1 progenies
PCR and western positive T1 transgenic events were allowed for Southern blot analysis to confirm the integration and copy number of transgenes. Genomic DNA of 35S-cry and 35S-cre transgenic lines were digested with HindIII, whereas ats-cry transgenic DNA samples were digested with BamHI restriction endonucleases. PCR-purified cry1Ac (for 35S-cry and ats-cry transgenics) and cre (for 35S-cre transgenics) sequences along with digested binary plasmids served as positive controls in Southern hybridization experiments. The hybridization signal of T1 Cry1Ac events showed the single-copy integration in the range of 4–6 kb (Fig. 4a, b, and c), and T1 pigeon pea plants of 35S-cre events exhibited single-copy insertion of the cre gene between 6 and 9 kb (Fig. 4d). Twenty-one 35S-cry (Fig. 4a, b) and 4 ats-cry T1 (Fig. 4c) events were found with single-copy integration of the cry1Ac gene, whereas 15 35S-cre events were confirmed as single-copy transgenic lines with the integration of the cre gene (Fig. 4d). Eight T1 plants of Cry1Ac events (35S-cry and ats-cry) and 3 plants of 35S-cre events exhibited double-copy insertion of the transgene. Genomic DNA of untransformed negative control plants exhibited no hybridization signal.
Southern blot analysis of established Cre/lox T1 transgenic events. a Radioactive Southern blot hybridization of HindIII-digested genomic DNA of 12 T1 35S-cry transgenic pigeon pea events. b Nonradioactive Southern blot analysis of HindIII digested genomic DNA of 14 T1 35S-cry transgenic pigeon pea events. c BamHI-digested genomic DNA of 10 ats-cry T1 transgenic events probed using the nonradioactive method. d HindIII-digested genomic DNA of 16 35S-cre T1 transgenic events probed using the nonradioactive method. Lanes + ve (1), + ve (2), + ve (3), and + ve (4) are PCR-purified 662 bp cry1Ac probe specific sequence, HindIII-digested 8.6 kb 35S-cry plasmid DNA, PCR-purified 1100 bp cre gene sequence and HindIII-digested 10.6 kb 35S-cre plasmid DNA as positive controls, respectively; lane − ve genomic DNA of the untransformed plant as the negative control
Insecticidal activity of Cry1Ac protein in T1 transgenic pigeon pea
Cry1Ac-expressing 35S-cry and ats-cry pigeon pea T1 events of both single- and double-copy integrations were used for detached leaf bioassay to study the insecticidal activity against H. armigera. The survival of 2nd instar larvae inside the breeding boxes was monitored at 24 h intervals till the 7th day. Data were compared with untransformed negative control pigeon pea events. Three 35S-cry single-copy events (222–10/1, 222–10/2, and 237–1/5) exhibited 100% mortality of the H. armigera larvae, and also more than 80% mortality was recorded in nine 35S-cry single-copy events (Fig. 5a). Alternatively, mortality in green tissue promoter-driven 8 ats-cry transgenic T1 events ranged between 40 and 60% (Fig. 5b). After 3 days of incubation, the percentage of insect mortality was found to be statistically significant in 35S-cry T1 events compared to control plants according to the analysis of variance (ANOVA, p < 0.0001). Another bioassay was conducted with 3rd to 4th instar larvae to determine the toxicity of Cry1Ac protein on larval growth. Third to fourth instar larvae fed on leaflets of 35S-cry T1 events demonstrated gradual weight loss at each 24 h interval, till the 13th day, compared to untransformed leaflets, where the larvae exhibited normal growth till maturity and developed into a pupa (Fig. 5c). Statistically, significant weight loss was noted on the 7th day (p < 0.0001). Green tissue promoter-driven Cry1Ac events did not show weight loss of the 3rd–4th instar larvae, rather a small amount of weight gain was recorded (Fig. 5d).
Insect bioassay and histological study leaf transverse sections. a, b Established T1 plants of 35S-cry and ats-cry transgenic events demonstrating 80–100% and 50–60% larval mortality, respectively. c Weight loss bioassay of 12 T1 35S-cry events in comparison to untransformed control. d Weight loss bioassay of 8 T1 ats-cry events. e Fluorescence study of leaf sections in three plant species; vascular bundles and lamina of pigeon pea (i and iv), Nicotiana tabacum (ii and v), and Arabidopsis thaliana (iii and vi), respectively
Histological analysis of pigeon pea, tobacco, and Arabidopsis leaf
The anatomy of transgenic and untransformed control pigeon pea (cv. ICPL 87119) leaf was studied in comparison to N. tabacum (cv. Petit Havana SR1) and A. thaliana (Col-0). Sections of both transformed and untransformed pigeon pea leaflets revealed that a large number of lignified cells were distributed in broad vascular bundles of midribs and veinlets. These lignified tissues showed green fluorescence of aniline blue (Fig. 5e). Alternatively, the fluorescence signal was found to be confined to relatively smaller areas in vascular bundles with a limited number of lignified cells in N. tabacum and A. thaliana leaf sections. No fluorescence signal was detected in non-lignified areas of all leaf sections. The anatomical difference between these three plant systems revealed that leaflets of pigeon pea had more lignified cells.
Molecular analysis of nptII elimination in T2 hybrids
Ten independent single-copy 35S-cry T1 events exhibiting 80–100% larval mortality were reciprocally crossed with five independent 35S-cre T1 events selected based on the western positive expression profile. T2 seeds of each hybrid event were collected separately, and independent PCR reactions were carried out using nptII-, cry1Ac-, and cre-specific primers to screen the hybrid events. Among 30 T2 plants, five events (222–10/1 × 262–1/4, 221–16/1 × 262–1/4, 239–11/1 × 262–1/4, 219–12/1 × 262–1/4, 219–45/3 × 262–1/4) were found to be negative for the nptII marker gene, whereas they successfully amplified cry1Ac, cre, and bar genes (Supplementary Fig. S1, S2a). The absence of nptII (Fig. 6a), as well as the presence of cry1Ac (Fig. 6b) genes in those T2 hybrid events, were confirmed through Southern blot analysis. 35S-cry parental lines of those hybrid events showed the presence of both nptII and cry1Ac genes. PCR-purified fragments of respective genes and HindIII-digested 8.6-bp 35S-cry binary plasmid were used as positive controls. One 35S-cry hybrid line with a non-eliminated nptII gene (219–5/1 × 262–10/1) was also taken as positive control and the untransformed plant as a negative control in those Southern blot experiments. Western blot analysis revealed the expression of Cry1Ac in those five nptII-eliminated T2 hybrid plants (data not shown).
Southern blot analysis of marker-eliminated transgenic events. a, b HindIII-digested genomic DNA of T2 events probed with nptII- and cry1Ac-specific sequences, respectively. c, d, and e HindIII digestion of nptII-eliminated T3 progeny lines probed with cre, nptII, and cry1Ac-specific sequences, respectively. Lane + ve (1) and + ve (2), + ve (3), + ve (4), and + ve (5) represent PCR purified probe sequences of the cry1Ac gene, HindIII-digested binary plasmid 35S-cry, PCR-purified probe sequence of cre gene, HindIII-digested binary plasmid 35S-cre, PCR-purified probe sequence of nptII gene, respectively, as positive controls and lane − ve represents genomic DNA of the untransformed plant as a negative control
Analyses of Cry1Ac-expressing marker-free T3 transgenic plants
T3 seeds were obtained from the self-pollinated nptII-eliminated T2 hybrid events. The established T3 progenies were allowed for another round of molecular analysis to determine the absence of cre-bar gene cassette to fulfill the goal of marker-free transgenic establishment in pigeon pea. Fifteen progenies of each nptII-eliminated T2 hybrid plant were screened by PCR analysis, and among them, 6 independent T3 events (222–10/1 × 262–1/4/2/3, 221–16/1 × 262–1/4/1/2, 221–16/1 × 262–1/4/1/9, 239–11/1 × 262–1/4/2/8, 219–12/1 × 262–1/4/2/4, 219–45/3 × 262–1/4/2/5) were found to be negative for cre and bar genes, followed by successful amplification of cry1Ac gene (Table 1, Supplementary Fig. S2b, S3). These PCR positive T3 Cry1Ac events were further confirmed by Southern blot analysis for cre, nptII, and cry1Ac genes (Fig. 6c, d, and e). Single-copy integration of the cry1Ac gene was found in the transgenic events along with the absence of nptII and cre genes. Western blot analysis exhibited the Cry1Ac expression in the abovementioned 6 marker-free events (Fig. 7a), and protein accumulation was found to be around 31–43 μg g−1 FW (Fig. 7b). These events were further allowed for insect mortality and weight loss bioassay on H. armigera larvae. The data on mortality of the 2nd instar larvae and the weight loss of the 3rd-4th instar larvae was found to be statistically identical (p > 0.05) in the marker-free T3 transgenic plants to their respective T1 parental events (Fig. 7c, d). More than 80% mortality was found in these 6 marker-free T3 plants, among them 222–10/1 × 262–1/4/2/3 exhibited 100% mortality of H. armigera (Fig. 7e, i), whereas 90% mortality was found in 221–16/1 × 262–1/4/1/9 and 239–11/1 × 262–1/4/1/8 plants (Fig. 7f, g ). All marker-free transgenic events exhibited gradual weight loss of the 3rd–4th instar larvae, at each 24 h interval till the 13th day, and statistically significant weight loss was observed at the 7th day (p < 0.0001) (Fig. 7d). Untransformed pigeon pea events were kept as a negative control in all bioassay experiments (Fig. 7h, j).
Expression analysis and bioassay of Cry1Ac protein in T3 transgenic events. a Western blot of marker-eliminated T3 events with purified Cry1Ac protein of ̴66 kDa and untransformed plant protein as positive (+ ve) and negative (− ve) controls, respectively. b Quantification of Cry1Ac protein in T3 plants to record the expression level at 32–48 μg g−1 FW. c and d Marker-free T3 events demonstrating 80 − 100% larval mortality and significant weight loss, in comparison to untransformed control, respectively. e and i Death of 2nd and 4th instar larvae, respectively, fed on the event with 100% larval mortality. f and g Death of 2nd instar larvae fed on the events with 90% larval mortality. h and j Feeding habit of 2nd and 4th instar larvae, respectively, on untransformed control leaves. The bar represents 5 mm
Discussion
Recent productivity data indicates that pigeon pea suffers from massive yield loss due to H. armigera infestations (Choudhary et al. 2013). Over the last few decades, several Cry toxins have been introduced in pigeon pea to incorporate pod borer resistance (Ghosh et al. 2017). However, in the context of safety assessment, the removal of selectable marker genes is necessary to avoid various issues regarding the commercialization of transgenic crops (Upadhyaya et al. 2010). The ever-increasing requirement for the production of improved crop varieties has expanded interest in researchers to develop marker-free transgenics, which has the potential to improve the acceptability of genetically modified products at the market level. The here described Cre/loxP recombination strategy executed through the crossing technique was found suitable concerning the plumular meristem transformation system used in this work, where T0 antibiotic selection was avoided to get a maximum number of primary transformants. The marker elimination through co-transformation using a super binary vector, as demonstrated in another legume chickpea, cannot be utilized with the present transgenic development method of pigeon pea as the co-transformation system is based on the selection of primary transformants. Although several reports are available on Cre/loxP-mediated marker-free transgenic development in model and crop plants (Dale and Ow 1991; Hoa et al. 2002; Srivastava and Ow 2003; Zhang et al. 2003; Kopertekh et al. 2004; Sreekala et al. 2005; Chakraborti et al. 2008; Sengupta et al. 2010; Bala et al. 2013; Sarkar et al. 2021), there is no published report on nptII selectable marker elimination and simultaneous Cry1Ac gene expression using the Cre/loxP system in legume crops till date.
In the majority of plant systems, transformation vectors have been developed where the foreign gene is cloned under the control of the CaMV35S constitutive promoter due to its strong expression potentiality. Alternatively, the green-tissue-specific ats1A promoter was proven to delineate a high level of tissue-specific expression of a transgene in the cotton plant compared to the expression driven by CaMV35S (Kumar et al. 2004). The present study was undertaken to verify the expression of Cry1Ac under the influence of CaMV35S and ats1A promoters independently, along with the incorporation of loxP-flanked nptII to develop marker-free transgenic pigeon pea events. The promoter activities were compared based on protein accumulation in T1 transgenic events and differential insecticidal activity against H. armigera larvae.
Although PCR positive T0 transformants obtained from A. tumefaciens-mediated plumular meristem transformation produced a sufficient amount of seeds, some T1 progenies were supposed to be non-transgenic due to the chimeric pattern of their parental events. Therefore, antibiotic-mediated stringent selection of T1 transgenic events was performed. As a result, segregation analysis could not be performed in the T1 generation, and hence no χ2 test is presented for T1 segregation. Constitutive promoter-driven Cry1Ac accumulation in T1 transgenic plants was found to be higher than green tissue promoter-controlled expression, and this constitutive expression pattern of Cry1Ac was successfully inherited in T2 and T3 generations. Among several tissue culture-based pigeon pea transformation studies, few reports are available where the expression and inheritance of Cry proteins (Cry1Ac, Cry1Ab, Cry1-EC, and Cry2Aa) were analyzed beyond the T1 generations (Surekha et al. 2005; Sharma et al. 2006; Ghosh et al. 2017). Krishna et al. (2011) reported the expression study of Cry1Ac protein through ELISA analysis from the different parts of established transgenic plants in T0 generation. Alternatively, a tissue culture-independent transformation study of pigeon pea demonstrated 3–15 μg g−1 FW of Cry1AcF protein accumulation till the T2 generation (Ramu et al. 2012). In all these studies, the Cry protein expression was driven by the CaMV35S promoter. Through a study on transgenic tobacco, the ats1A promoter together with a chloroplast transit peptide was found to exhibit a high expression of the Cry1Ac protein. The expression was reported to be around 1% of the total leaf protein (Wong et al. 1992). Another report demonstrated the development of transgenic cotton plants where the chloroplast-specific expression of Cry1Ac and Cry2Aa proteins using transit peptides was found to be 0.673 μg g−1 and 0.568 μg g−1 tissue, respectively (Muzaffar et al. 2015). In a recent report, the ats1A promoter-driven expression of truncated Cry1Ac protein was found to be relatively higher (30–79 μg g−1 FW) in transgenic Arabidopsis plants (Hazarika et al. 2019). In the present study, a higher accumulation of Cry protein (22–62 µg−1 FW) was observed in all leaf tissues due to the constitutive activity of the CaMV35S promoter, whereas protein accumulation was found to be restricted to the outermost epidermal regions in green tissue promoter containing ats-cry pigeon pea events. The histological analysis of pigeon pea leaflets demonstrated the presence of more lignified tissue in comparison to the leaves of N. tabacum and A. thaliana. This further justified the observation of lesser Cry1Ac accumulation under the influence of ats1A in pigeon pea. This is the first report where green-tissue-specific expression of Cry1Ac protein was analyzed in pigeon pea using the ats1A promoter conjugated with chloroplast transit peptide.
Nine constitutively expressing T1 Cry1Ac events exhibited 80–100% mortality of 2nd instar H. armigera larvae along with drastic weight loss of 3rd–4th instar larvae. This mortality data was found to be comparable to the previous studies based on cry genes. According to a previous report, the constitutive expression of Cry1Ac protein conferred insect resistance, and only 55% mortality was recorded in T0 transgenic lines (Krishna et al. 2011). Ramu et al. (2012) performed the insect bioassay in T1 and successive two generations of pigeon pea where constitutive expression of Cry1AcF protein showed 80–100% mortality of H. armigera (Ramu et al. 2012). Through tissue culture-based transgenic development of pigeon pea, the current group reported a high level of insect-resistant activity of Cry1Ac and Cry2Aa proteins through mortality and weight loss bioassay of H. armigera larvae in successive T1 and T2 generations, although there was no provision of SMG elimination from those transgenic events (Ghosh et al. 2017). On contrary, the insecticidal activity of Cry1Ac protein under the control of the green-tissue-specific promoter was found to be lower, and thus these events were not continued for marker elimination studies. These results clearly illustrated that use of constitutive promoter will be more suitable for cry-transgenic development in pigeon pea.
The use of the Cre/lox system was found to be effective on a wide range of plants (Kerbech et al. 2005). Cre recombinase events were independently established to generate marker-free transgenic pigeon pea plants, following the crossing between these cre- and lox-transgenic plants. The crossing-mediated marker elimination strategy was reported to be more convenient than the cre-mediated auto-excision method (Konig 2003). This is the first report in a legume system where detailed analyses of cre-transgenics were performed at both integration and expression levels. According to a previous study, the highly expressive Cre-recombinase protein is required for precise and complete excision of the lox-flanked marker gene (Bayley et al. 1992).
Reciprocal crossing between single-copy 35S-cry and 35S-cre events was performed, and T2 hybrid events were found to be nptII marker negative. The T1 transgenic plants were mixtures of homozygous and hemizygous progenies, which were utilized for crossing. Therefore, the segregation pattern could not be predicted in the T2 generation and hence no χ2 test is presented for T2 segregation. All the marker-free events were allowed to self-pollinate for the segregation of cre, and linked bar genes in the T3 generation, and molecular analyses confirmed the absence of cre and linked bar genes in all those events. Previously, in a study by Sarkar et al. (2021), the elimination of the bar selectable marker was accomplished in transgenic pigeon pea, which was expressing Cry1Ab under the green-tissue-specific rubisco small subunit promoter. However, the expression level of Cry1Ab protein was not monitored, and the study demonstrated marker elimination of a sole transgenic event with partial resistance in larval bioassay. This finding could be explained by the limited activity of the green-tissue-specific promoter to accumulate the inadequate amount of Cry toxin in pigeon pea. This was similar to our observation that the rubisco small subunit promoter-mediated Cry toxin expression was unable to provide desired resistance against H. armigera in pigeon pea.
This is the first report in pigeon pea where constitutive and tissue-specific expressions of the cry1Ac gene and their insecticidal activities were compared. Bioassay and protein expression study indicated that constitutive expression of Cry1Ac in transgenic pigeon pea plants conferred complete resistance against H. armigera, whereas ats1A promoter-driven Cry1Ac demonstrated partial resistance. The reason for this partial resistance could be attributed to the lesser accumulation of this Cry toxin as a whole in lignified, leathery leaves of ats-cry pigeon pea. This information could influence the choice of promoters in the future for transgenic pigeon pea development. The key feature of this research work was the establishment of transgenic events by a tissue culture-independent transformation protocol to overcome the recalcitrant nature of this particular legume along with a kanamycin-based T1 selection procedure to avoid chimeric interference. The application of appropriate Cre/lox binary vectors was proven to be successful for the precise elimination of the marker gene. The manifestation of the insect-resistant ability of integrated the cry1Ac gene product was effectively maintained up to the T3 generation in 5 marker-free events. The implementation of such a marker-free transgenic development could be extended to a multi-toxin approach using both cry1Ac and cry2Aa for durable resistance. This strategy has the potential to increase commercial acceptance of genetically modified crops addressing marker gene-related environmental and food safety issues.
Data availability
All data generated or analyzed during this study are included in this published article [and its Supplementary Information files].
References
Acharjee S, Sarmah BK, Kumar PA, Olsen K, Mahon R, Moar WJ, Moore A, Higgins TJ (2010) Transgenic chickpeas (Cicer arietinum L) expressing a sequence-modified cry2Aa gene. Plant Sci 178(3):333–339
Bala A, Roy A, Das A, Chakraborti D, Das S (2013) Development of selectable marker free, insect resistant, transgenic mustard (Brassica juncea) plants using Cre/lox mediate recombination. BMC Biotechnol 13(1):88
Bakhsh A, Qayyum RA, Shamim Z, Husnain T (2011) A mini review: RuBisCo small subunit as a strong, green tissue-specific promoter. Arch Biol Sci 63(2):299–307
Bayley CC, Morgan M, Dale EC, Ow DW (1992) Exchange of gene activity in transgenic plants catalyzed by the Cre-lox site-specific recombination system. Plant Mol Biol 18:353–362
Bradford MM (1976) A rapid and sensitive method for the quantitation of proteins using the principle of protein dye binding. Anal Biochem 72:248–254
Chakraborti D, Sarkar A, Gupta S, Das S (2006) Small and large scale genomic DNA isolation protocol for chickpea (Cicer arietinum L) suitable for molecular marker and transgenic analyses. Afr J Biotechnol 5(8):585–589
Chakraborti D, Sarkar A, Mondal H, Schuermann D, Hohn B, Sarmah B, Das S (2008) Cre/lox system to develop selectable marker free transgenic tobacco plants conferring resistance against sap sucking hemipteran insect. Plant Cell Rep 27:1623–1633
Chanda Venkata SK, Nadigatla Veera Prabha Rama GR, Saxena RK, Saxena K, Upadhyaya HD, Siambi M, Silim SN, Reddy KN, Hingane AJ, Sharma M, Sharma S (2019) Pigeonpea improvement: an amalgam of breeding and genomic research. Plant Breed 138(4):445–454
Choudhary AK, Raje RS, Datta S, Sultana R, Ontagodi T (2013) Conventional and molecular approaches towards genetic improvement in pigeonpea for insect resistance. Amer J Plant Sci 4:372–385
Dale EC, Ow DW (1991) Gene transfer with subsequent removal of the selection gene from the host genome. P Natl Acad Sci USA 88:10558–10562
Das A, Datta S, Sujayanand GK, Kumar M, Singh AK, Shukla A, Ansari J, Faruqui L, Thakur S, Kumar PA, Singh NP (2016) Expression of chimeric Bt gene, Cry1Aabc in transgenic pigeonpea (cv Asha) confers resistance to gram pod borer Helicoverpa armigera Hubner. Plant Cell Tissue Organ Cult 127(3):705–15
FAO (2019) Food and Agricultural Organization of the United Nation, FAO Statistical Database. http://faostat.fao.org. Accessed 1st May 2021
Ganguly S, Ghosh G, Purohit A, Sreevathsa R, Chaudhuri RK, Chakraborti D (2017) Effective screening of transgenic pigeonpea in presence of negative selection agents. Proc Natl Acad Sci India Sect B Biol Sci 88(4):1565–1571
Ganguly S, Ghosh G, Purohit A, Chaudhuri RK, Chakraborti D (2018) Development of transgenic pigeonpea using high throughput plumular meristem transformation method. Plant Cell Tissue Organ Cult 135(1):73–83
Ghosh G, Purohit A, Ganguly S, Chaudhuri RK, Chakraborti D (2014) In vitro shoot grafting on rootstock: an effective tool for Agrobacterium-mediated transformation of pigeonpea (Cajanus cajan (L.) Millsp.). Plant Biotechnol 31:301–308
Ghosh G, Ganguly S, Purohit A, Chaudhuri RK, Das S, Chakraborti D (2017) Transgenic pigeonpea events expressing Cry1Ac and Cry2Aa exhibit resistance to Helicoverpa armigera. Plant Cell Rep 36(7):1037–1051
Hazarika N, Acharjee S, Boruah RR, Baba K, Parimi S, Char B, Armstrong J, Moore A, Higgins TJ, Sarmah BK (2019) Enhanced expression of Arabidopsis rubisco small subunit gene promoter regulated Cry1Ac gene in chickpea conferred complete resistance to Helicoverpa armigera. J Plant Biochem Biotechnol 1–11
Hoa TTC, Bong BB, Huq E, Hodge TK (2002) Cre/lox site specific recombination controls the excision of a transgene from the rice genome. Theor Appl Genet 104:518–525
Jaiwal PK, Sahoo L, Singh ND, Singh RP (2002) Strategies to deal with the concern about marker genes in transgenic plants: some environment-friendly approaches. Curr Sci 83(2):128–136
Kaur A, Sharma M, Sharma C, Kaur H, Kaur N, Sharma S, Arora R, Singh I, Sandhu JS (2016) Pod borer resistant transgenic pigeon pea (Cajanus cajan L) expressing cry1Ac transgene generated through simplified Agrobacterium transformation of pricked embryo axes. Plant Cell Tissue Organ Cult 127(3):717–727
Kerbach S, Lorz H, Becker D (2005) Site-specific recombination in Zea mays. Theor Appl Genet 111:1608–1616
Konig A (2003) A framework for designing transgenic crops—science, safety and citizen’s concerns. Nat Biotechnol 21(11):1274–1279
Kopertekh L, Jüttner G, Schiemann J (2004) PVX-Cre-mediated marker gene elimination from transgenic plants. Plant Mol Biol 55(4):491–500
Krishna G, Reddy PS, Ramteke PW, Rambabu P, Tawar KB, Bhattacharya P (2011) Agrobacterium-mediated genetic transformation of pigeon pea [Cajanus cajan (L) Millsp] for resistance to legume pod borer Helicoverpa armigera. J Crop Sci Biotechnol 14(3):197–204
Kumar S, Timko MP (2004) Enhanced tissue-specific expression of the herbicide resistance bar gene in transgenic cotton (Gossypium hirsutum L cv Coker 310FR) using the Arabidopsis rbcS ats1A promoter. Plant Biotechnol J 21(4):251–259
Kumar S, Chandra A, Pandey KC (2008) Bacillus thuringiensis (Bt) transgenic crop: an environment friendly insect-pest management strategy. J Environ Biol 29(5):641–653
Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation–competent Arabidopsis genomic library in Agrobacterium. Bio/technol 9(10):963–7
Miki B, McHugh S (2004) Selectable marker genes in transgenic plants: applications, alternatives and biosafety. J Biotechnol 107(3):193–232
Miklos JA, Alibhai MF, Bledig SA, Connor-Ward DC, Gao AG, Holmes BA, Kolacz KH, Kabuye VT, MacRae TC, Paradise MS, Toedebusch AS (2007) Characterization of soybean exhibiting high expression of a synthetic Bacillus thuringiensis cry1A transgene that confers a high degree of resistance to lepidopteran pests. Crop Sci 47(1):148–157
Mishra RK, Bohra A, Kamaal N, Kumar K, Gandhi K, Sujayanand GK, Saabale PR, SN SJ, Sarma BK, Kumar D, Mishra M (2018) Utilization of biopesticides as sustainable solutions for management of pests in legume crops: achievements and prospects. Egypt J Biol Pest Co 28(1):3
Mondal HA, Chakraborti D, Majumder P, Roy P, Roy A, Gupta Bhattacharya S, Das S (2011) Allergenicity assessment of Allium sativum leaf agglutinin (ASAL) a potential candidate protein for developing sap sucking insect resistant food crops. PLoS One 6(11):e27716
Muzaffar A, Kiani S, Khan MAU, Rao AQ, Ali A, Awan MF, Iqbal A, Nasir IA, Shahid AA, Husnain T (2015) Chloroplast localization of Cry1Ac and Cry2A protein-an alternative way of insect control in cotton. Biol Res 48(1):1–11
Perlak FJ, Deaton RW, Armstrong TA, Fuchs RL, Sims SR, Greenplate JT, Fischhof DA (1990) Insect resistant cotton plants. Bio/technology 8:939–943
Potenza C, Aleman L, Sengupta-Gopalan C (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. In Vitro Cell Dev Biol 40(1):1–22
Ramessar K, Peremarti A, Go´mez-Galera S, Naqvi S, Moralejo M, Munoz P, Capell T, Christou P (2007) Biosafety and risk assessment framework for selectable marker genes in transgenic crop plants: a case of the science not supporting the politics. Transgenic Res 16:261–280
Ramu SV, Rohini S, Keshavareddy G, Neelima MG, Shanmugam NB, Kumar ARV, Sarangi SK, Ananda Kumar P, Udayakumar M (2012) Expression of a synthetic cry1AcF gene in transgenic pigeonpea confers resistance to Helicoverpa armigera. J Appl Entomol 136:675–687
Sarkar S, Roy S, Ghosh SK (2021) Development of marker-free transgenic pigeon pea (Cajanus cajan) expressing a pod borer insecticidal protein. Sci Rep 11:10543
Saxena RK, Saxena KB, Pazhamala LT, Patel K, Parupalli S, Sameerkumar CV, Varshney RK (2015) Genomics for greater efficiency in pigeonpea hybrid breeding. Front Plant Sci 6:793
Schenk ST, Schikora A (2015) Staining of callose depositions in root and leaf tissues. Bio-Protoc 5(6):1429
Sengupta S, Chakraborti D, Mondal HA, Das S (2010) Selectable antibiotic resistance marker gene-free transgenic rice harbouring the garlic leaf lectin gene exhibits resistance to sap-sucking planthoppers. Plant Cell Rep 29(3):261–271
Sharma KK, Lavanya K, Anjaiah A (2006) Agrobacterium tumefaciens-mediated production of transgenic pigeonpea (Cajanus cajan L. Millsp.) expressing the synthetic Bt Cry1AB gene. In Vitro Cell Dev Biol Plant 42:165–173
Sharma HC, Sujana G, Rao DM (2009) Morphological and chemical components of resistance to pod borer, Helicoverpa armigera in wild relatives of pigeonpea. Arthropod Plant Interact 3(3):151–161
Singh S, Kumar NR, Maniraj R, Lakshmikanth R, Rao KY, Muralimohan N, Arulprakash T, Karthik K, Shashibhushan NB, Vinutha T, Pattanayak D (2018) Expression of Cry2Aa, a Bacillus thuringiensis insecticidal protein in transgenic pigeonpea confers resistance to gram pod borer. Helicoverpa Armigera Sci Rep 8(1):1–2
Sreekala C, Wu L, Gu K, Wang D, Tian D, Yin Z (2005) Excision of a selectable marker in transgenic rice (Oryza sativa L.) using a chemically regulated Cre /loxP system. Plant Cell Rep 24:86–89
Sreekanth M, Lakshmi MSM, Rao YK (2014) Bio-efficacy and economics of certain new insecticides against gram pod borer Helicoverpa armigera (Hubner) infesting pigeonpea (Cajanus cajan L). Int J Plant Animal Env Sci 4(1):11–15
Srivastava V, Ow DW (2003) Rare instances of Cre-mediated deletion product maintained in transgenic wheat. Plant Mol Biol 52:661–668
Surekha C, Beena MR, Arundhati A, Singh PK, Tuli R, Dutta-Gupta A, Kirti PB (2005) Agrobacterium-mediated genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) using embryonal segments and development of transgenic plants for resistance against Spodoptera. Plant Sci 169:1074–1080
Tabe LM, Wardley-Richardson T, Ceriotti A, Aryan A, McNabb W, Moore A, Higgins TJ (1995) A biotechnological approach to improving the nutritive value of alfalfa. J Anim Sci 73(9):2752–2759
Thu TT, Dewaele E, Trung LQ, Claeys M, Jacobs M, Angenon G (2007) Increasing lysine levels in pigeonpea (Cajanus cajan (L) Millsp) seeds through genetic engineering. Plant Cell Tissue Organ Cult 91:35–143
Upadhyaya CP, Nookaraju A, Gururani MA, Upadhyaya DC, Kim DH, Chun SC, Park SW (2010) An update on the progress towards the development of marker-free transgenic plants. Bot Stud 51(3):277–292
Varshney RK, Penmetsa RV, Dutta S, Kulwal PL, Saxena RK, Datta S, Sharma TR, Rosen B, Carrasquilla-Garcia N, Farmer AD, Dubey A (2010) Pigeonpea genomics initiative PGI an international effort to improve crop productivity of pigeonpea (Cajanus cajan L). Mol Breed 26(3):393–408
Varshney RK, Chen W, Li Y, Bharti AK, Saxena RK, Schlueter JA (2012) Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource-poor farmers. Nat Biotechnol 30:83–89
Wong EY, Hironaka CM, Fischhoff D (1992) Arabidopsis thaliana small subunit leader and transit peptide enhance the expression of Bacillus thuringiensis proteins in transgenic plants. Plant Mol Biol 20(1):81–93
Yau YY, Stewart CN (2013) Less is more: strategies to remove marker genes from transgenic plants. BMC Biotechnol 13(1):36
Zhang W, Subbarao S, Addae P, Shen A, Armstrong C, Peschke V, Gilbertson L (2003) Cre/lox-mediated marker gene excision in transgenic maize (Zea mays L.) plants. Theor Appl Genet 107:1157–1168
Acknowledgements
The authors acknowledge the Indian Council of Agricultural Research for the financial support (grant number NFBSFARA/PB2010/2010-11); DST-PURSE, University of Calcutta, St. Xavier’s College, and Bose Institute, Kolkata, India, for the infrastructure support. S. Ganguly A. Purohit and S. Ghosh thank West Bengal Higher Education Department-Swami Vivekananda Merit cum Means Scholarship (No. 52-Edn (B) l 5B-l s/2017), Council of Scientific and Industrial Research, India (File No. 08/548(0007)/2018 EMR-I), and the Department of Science and Technology, Govt. of India, INSPIRE fellowship (DST/INSPIRE Fellowship/2018/IF180158), respectively, for providing the fellowship.
Funding
Indian Council of Agricultural Research,NFBSFARA/PB2010/2010-11,Dipankar Chakraborti
Author information
Authors and Affiliations
Contributions
SG, AP, SG, and DC conceived and designed all of the experiments. SG, AP, and SG conducted all the experiments. RKC, SD, and DC were responsible for the data analysis and supervision of the work. SG and DC drafted and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants. The authors did not perform any animal-based experiments for this work.
Consent for publication
Review work is presented in this manuscript with the consent of all authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ganguly, S., Purohit, A., Ghosh, S. et al. Clean gene technology to develop selectable marker-free pod borer-resistant transgenic pigeon pea events involving the constitutive expression of Cry1Ac. Appl Microbiol Biotechnol 106, 3051–3067 (2022). https://doi.org/10.1007/s00253-022-11922-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11922-1